Abstract
Objective
Aggressive care interventions at the end of life (ACE) are reported metrics of sub-optimal quality of end of life care that are modifiable by palliative medicine consultation. Our objective was to evaluate the association of inpatient palliative medicine consultation with ACE scores and direct inpatient hospital costs of patients with gynecologic malignancies.
Methods
A retrospective review of medical records of the past 100 consecutive patients who died from their primary gynecologic malignancies at a single institution was performed. Timely palliative medicine consultation was defined as exposure to inpatient consultation ≥30 days before death. Metrics utilized to tabulate ACE scores were ICU admission, hospital admission, emergency room visit, death in an acute care setting, chemotherapy at the end of life, and hospice admission <3 days. Inpatient direct hospital costs were calculated for the last 30 days of life from accounting records. Data were analyzed using Fisher's Exact, Mann–Whitney U, Kaplan–Meier, and Student's T testing.
Results
49% of patients had a palliative medicine consultation and 18% had timely consultation. Median ACE score for patients with timely palliative medicine consultation was 0 (range 0–3) versus 2 (range 0–6) p = 0.025 for patients with untimely/no consultation. Median inpatient direct costs for the last 30 days of life were lower for patients with timely consultation, $0 (range 0–28,019) versus untimely, $7729 (0–52,720), p = 0.01.
Conclusions
Timely palliative medicine consultation was associated with lower ACE scores and direct hospital costs. Prospective evaluation is needed to validate the impact of palliative medicine consultation on quality of life and healthcare costs.
Keywords: Palliative medicine, Gynecologic malignancies, Aggressiveness of care, End of life care, Quality-of-life, Hospital costs
Introduction
Palliative care is defined by the World Health Organization as “an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual. Palliative care is applicable early in the course of illness, in conjunction with other therapies that are intended to prolong life, such as chemotherapy or radiation therapy, and includes those investigations needed to better understand and manage distressing clinical complications.” [1] Palliative care is often confused with hospice care. The important difference is that palliative care is appropriate at any age and any stage in a serious illness and can be provided along with curative treatment [2]. The multidisciplinary palliative care team (physician, nursing, social work, chaplaincy) focuses on the patient and family throughout the trajectory of illness from diagnosis to death [3,4].
In 2012 the American Society of Clinical Oncology asserted that “combined standard oncology care and palliative care should be considered early in the course of illness for any patient with metastatic cancer and/or high symptom burden.” [5] The provisional clinical opinion cited seven randomized controlled trials (RCTs) demonstrating improvement in symptoms, quality-of-life (QOL), patient satisfaction, reduced caregiver burden, more appropriate referral and use of hospice, reduced use of futile intensive care and other invasive care and improved survival [5–12]. The most compelling of these trials, by Temel et al., found improved QOL and mood for patients with metastatic lung cancer who had early as opposed to usual palliative care. As a secondary finding, these authors proved that early consultation resulted in less intensive oncologic interventions at the end of life with prolonged survival [12]. The impact of combined standard oncology care and palliative care on metrics of QOL and cost has not been previously reported for women with gynecologic malignancies.
Evidence suggests that palliative care consultations in patients at the end of life decrease costs while improving QOL. In a report of palliative care consultation team hospital cost savings, projected savings in New York State alone for Medicaid beneficiaries are up to $252 million annually if every hospital with 150 or more beds had a fully operational palliative care consultation team (defined as multidisciplinary, operating for more than 5 years, and trained in preferred practices for palliative and hospice care recommended by the National Quality Forum) [13]. However, there is a paucity of data on the impact of a palliative medicine consultation on these costs for women with gynecologic malignancies.
A composite metric of aggressiveness of care at the end-of-life (ACE) reported by Earle et al. has been used as a point of reference for many palliative care studies [14]. Increased ACE scores are indicative of poor end of life care [15]. These metrics include admission to the intensive care unit (ICU) within 30 days of death, hospital admission more than 14 days in the last 30 days of life, more than one hospital admission during the past 30 days of life, more than one emergency room visit during the last 30 days of life, death in an acute care setting, initiation of a new chemotherapy during the last 30 days of life, last chemotherapy within 14 days of death, and hospice admission less than 3 days before death. These aggressive interventions were not associated with improvement in survival for women with ovarian cancer according to a report by Von Gruenigen et al. [16] However, timely palliative medicine, as defined by two weeks of exposure, was reported to decrease ACE scores in a Veteran's Affairs cancer population [17].
While the evidence from RCTs integrating standard oncology practice and palliative care is promising, the applicability of these trials to general gynecologic oncology practice is yet to be tested, reproduced, or proven. In particular the application of early consultation for ethnically and racially diverse women with poor socioeconomic resources has not been investigated. The optimal method of integration of palliative medicine into standard oncology care is unknown, and the intensiveness or “dose” has not yet been defined for optimal clinical impact with minimal resource utilization. The objective of our study was to retrospectively evaluate the impact of palliative medicine consultation on cost and quality of end of life care as measured by ACE for women with gynecologic malignancies.
Methods
Montefiore Medical Center is the largest hospital center in the Bronx, which has approximately 1.4 million persons. It is a 1062 bed, urban community academic medical center. Over 27% of Bronx residents have incomes below the poverty level and 32% of the Bronx population is foreign born. Montefiore Medical Center provides medical care to a highly diverse population: 48% of its patients are identified as Latino/Hispanic, 31% as African American. English is the second language for more than half of all the inhabitants of the Bronx. The Montefiore Medical Center Palliative Care Service was established in 2000 and currently provides care to nearly 40% of the adult patients who die at Montefiore Medical Center each year. On average, there are 1800 new in-patient consultations, more than 600 in-patient unit admissions and 2000 outpatient clinic visits to the palliative care service each year [18,19].
After institutional review board approval was obtained, 100 consecutive patients who were treated during the last year at a single institution and died from their primary gynecologic malignancy were identified from the Gynecologic Oncology Tumor Board Registry. Data were abstracted from inpatient as well as outpatient medical records for the last year of life. These data included age at death, date of consultation, disease site, stage, self-reported race/ethnicity, marital status, provider and payer (private insurance versus Medicare/Medicaid). Providers were defined as “junior gynecologic oncology faculty” if in sub-specialty practice for less than 15 years, and senior “gynecologic oncology faculty” if in sub-specialty practice for more than 15 years. Tumor stage was determined by the 1988 International Federation of Gynecology and Obstetrics (FIGO) criteria [20]. Patients who had a formal inpatient palliative medicine consultation ≥30 days from death were considered to have timely consultation. Patients with less than 30 days from consultation until death were considered to have inadequate time of exposure and were evaluated as a group with patients who received no consultation. Palliative medicine consultations were identified by manual review of all inpatient medical records in the last year of life by trained personnel. Criteria for defining consultation included i) consultation request by an attending physician ii) the patient was seen and evaluated by the palliative care team for one or more visits and iii) at least one set of recommendations was made by the palliative care team for the primary team caring for the patient. The rationale to define timely consultation as at least 30 days before death was to allow a minimum amount of time for the consulting team to establish a rapport and to ensure that exposure time encompassed the longest time frame inherent in ACE criteria.
ACE scores were computed for each patient by addition of 1 point for each of the following metrics: admission to ICU within 30 days of death, hospital admission more than 14 days in the last 30 days of life, more than one hospital admission during the past 30 days of life, more than one emergency room visit during the last 30 days of life, death in an acute care setting, initiation of a new chemotherapy during the last 30 days of life, last chemotherapy within 14 days of death, and hospice admission less than 3 days before death [15–17]. Inpatient direct hospital costs were calculated in dollars for the last 30 days of life from hospital accounting records. Direct hospital cost was defined as combined cost for hospital stay, blood bank, medications, intravenous infusions, laboratory tests, intensive care unit stay, procedures, physical therapy, diagnostic radiology and respiratory therapy.
Baseline patient characteristics were compared using Fisher's Exact and Student's T testing., ACE scores were compared using Mann–Whitney U. Direct hospital costs for the last 30 days of life and the last 14 days of life were compared between patients having timely verses late/no consultation using Mann–Whitney U testing. Patients were included in cost analysis regardless of admission status during the last 30 and 14 days of life. Exploratory analysis was conducted of costs for the last 30 and 14 days of life for patients who had 14 or more days of exposure to palliative medicine consultation. Additional chi-square analysis was made of admission status of patients in both 14 and 30 day exposure groups. Overall survival was compared using Kaplan–Meier Statistics. All analyses were two sided and performed utilizing SPSS Statistics Version 20 (IBM SPSS Statistics, Armonk, NY).
Results
Data were collected from patients who died from June 5, 2005 until February 7, 2010. 49% of patients had an inpatient palliative medicine consultation, and the median number of days from consultation to death was 16 days (range 0–159 days) (Table 1). 18% of patients had palliative medicine consultation more than 30 days before death, with the median number of days from consultation until death being 63 days (33–159) in this group. The shortest time from diagnosis until consultation was 24 days, with the majority of consultations made from 100 to 1000 days from diagnosis (59%).
Table 1.
Characteristics of palliative medicine consultation.
| Days from consultation to death | N (%) |
|---|---|
| Median days (range) | 16 (0–159) |
| >60 days | 10 (20%) |
| >40–60 days | 4 (8%) |
| >30–40 days | 4 (8%) |
| >20–30 days | 6 (12%) |
| >10–20 | 6 (12%) |
| >1–10 days | 14 (29%) |
| ≤1 day | 5 (10%) |
| Days from diagnosis to consultation | N (%) |
| Median (range) | 666 (24–5718) |
| >1000 days | 14 (29%) |
| >100–1000 days | 29 (59%) |
| 10–100 days | 6 (12%) |
| <10 days | 0 (0%) |
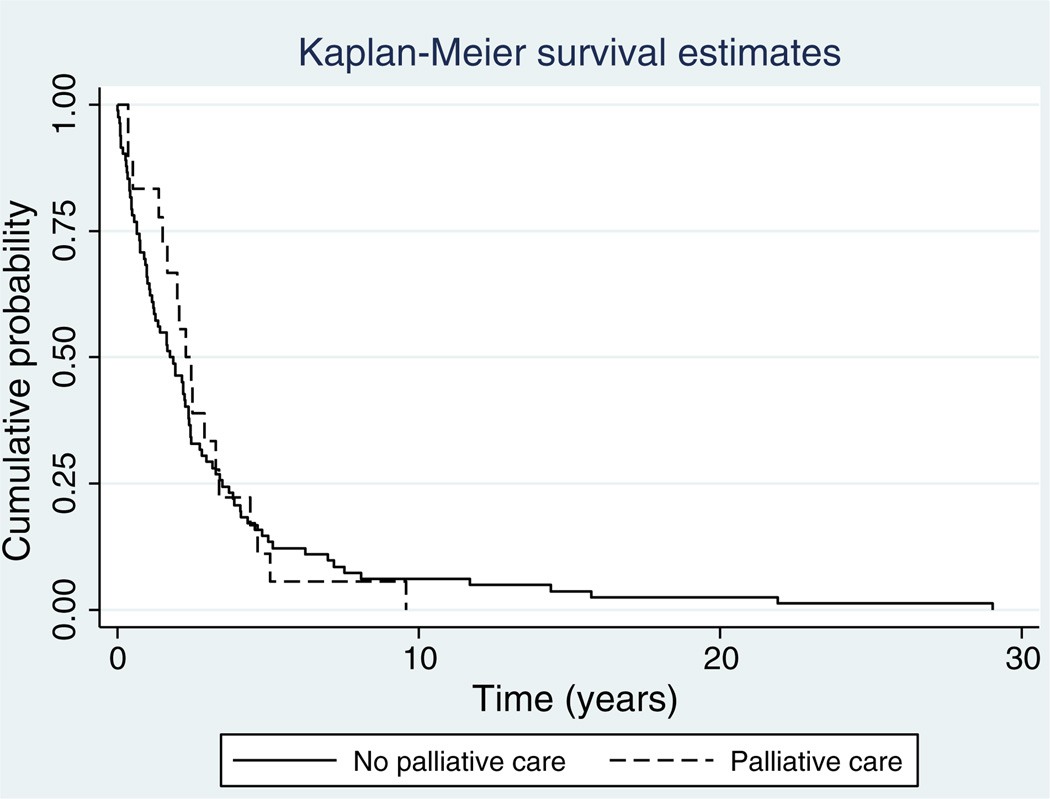
Mean age of death was 64, the main disease site was the uterus (42%) and the most common stage was 3 (32%) (Table 2). Self-identified race/ethnicity was white (39%), black (34%), Hispanic (14%) and other (13%). There were no differences between patients who had timely versus no/untimely consultation in disease site, age, stage, race or payer. Patients who had timely consultation were more likely to be married (63%) than those who had no/untimely consultation (p = 0.007). The provider group consisted of five gynecologic oncologists, of whom two were of senior level and three were of junior level as per the definition of sub-specialty practice for more and less than 15 years. There were no differences in referral patterns between junior and senior level gynecologic oncologists. Mean survival estimates were 145 weeks for patients with timely palliative medicine consultation and 162 weeks for patients with no/untimely palliative medicine consultation, p = 0.816 (Fig. 1).
Table 2.
Patient demographics/characteristics.
| Patient characteristics |
Total patient cohort N (%) |
Timely consultation N (%) |
Untimely/no consultation N (%) |
p |
|---|---|---|---|---|
| Mean age at death (range) | 63.5 +/− 12.3 (30–94) | 57.8 +/− 10.1 | 64.7 +/− 12 | |
| Disease site | ||||
| Cervix | 19 | 7 (39) | 12 (15) | |
| Ovary | 30 | 4 (22) | 29 (35) | |
| Uterus | 42 | 7 (39) | 35 (43) | |
| Other | 9 | 0 (0) | 6 (7) | |
| Stage | ||||
| 1 | 9 | 3 (17) | 12 (15) | |
| 2 | 15 | 2 (11) | 11 (13) | |
| 3 | 32 | 5 (28) | 27 (33) | |
| 4 | 21 | 5 (28) | 16 (20) | |
| Recurrent | 10 | 1 (6) | 9 (11) | |
| Unknown | 13 | 2 (11) | 7 (9) | |
| Race | ||||
| Black | 34 | 7 (39) | 27 (33) | |
| Hispanic | 14 | 3 (17) | 11 (13) | |
| White | 39 | 6 (33) | 33 (40) | |
| Other | 13 | 2 (11) | 11 (13) | |
| Marital status | 0.007 | |||
| Married | 33 | 12(67) | 21 (26) | |
| Single | 36 | 5 (28) | 31 (38) | |
| Divorced | 10 | 0 (0) | 10 (12) | |
| Widowed | 17 | 1 (6) | 3 (4) | |
| Unknown | 4 | 0 (0) | 17 (21) | |
| Provider | ||||
| Senior gyn | 65 | 32 (65) | 33 (65) | |
| Oncologist | ||||
| Junior gyn | 32 | 17 (35) | 15 (29) | |
| Oncologist | ||||
| Other | 3 | 0 (0) | 3 (6) | |
| Insurance status | ||||
| Medicare/Medicaid | 47 | 6 (33) | 45 (55) | |
| Private insurance | 49 | 12 (67) | 37 (45) |
Fig. 1.
Kaplan–Meier survival curve comparing patients with timely versus no/untimely consultation (median survival timely consultation 145 weeks versus 161 weeks no/untimely consultation, log rank p = 0.81).
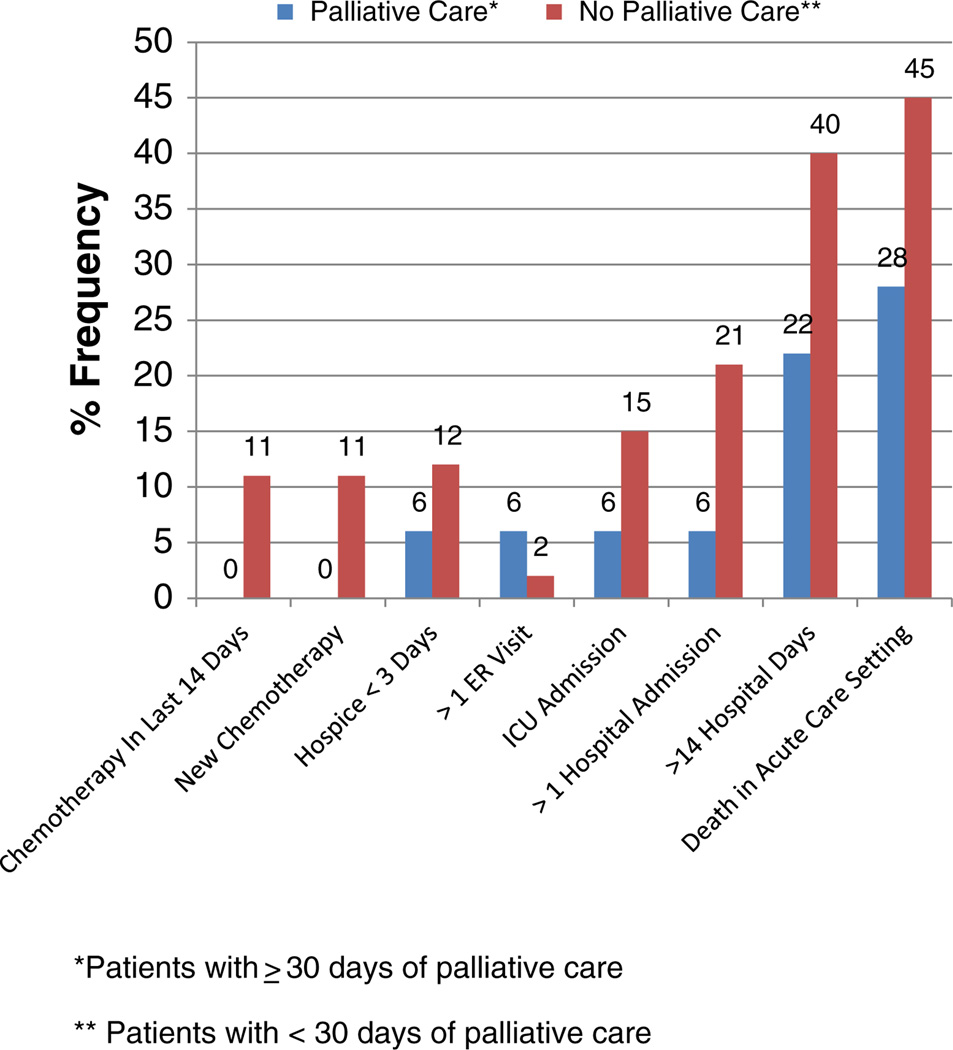
The median composite ACE score for patients with palliative medicine consultation ≥30 days was 0 (range 0–3) versus 2 (range 0–6) p = 0.025. The most common aggressive measure at the end-of-life was death in an acute care setting for both patients with timely (28%) versus no/untimely (45%) consultation (Fig. 2). Aggressive measure interventions were lower in every category for those with timely intervention except >1 emergency department visit (6% versus 2%). Metrics with the largest differences were death in an acute care setting, greater than 14 days in the hospital during the last 30 days of life and more than 1 hospital admission during the last 30 days of life (Fig. 2).
Fig. 2.
Comparison of indicators of aggressive care at the end of life between patients receiving timely palliative care versus no/untimely palliative care.
Cost data were evaluable for 97 patients of which 18 had consultation ≥30 days before death, and 79 had <30 day exposure to palliative medicine consultation. Records of three patients were missing from the hospital accounting database. The median direct hospital cost for the last 30 days of life for patients with timely consultation was significantly less, $0 (range $0–$28,019) than for patients who had no/untimely consultation $7729 (range $0–52,720), p = 0.01 (Table 3). This difference persisted in comparison of median direct costs for the last 14 days of life $0 (range $0–$11,008) versus $3935 (range $0–$26,794), p = 0.01. There was a significant difference in the percentage of patients with timely palliative consultation admitted to the hospital within the last 30 days of life when compared with those with no/untimely consultation (44% vs 80%) p = 0.004. When timely consultation was evaluated as 14 days or more of exposure there was a decreased median direct inpatient hospital cost compared to no/untimely consultation $0 (range $0–$18,184) versus $5106 (range $0–26,794), p = 0.01 for the last 14 days and a trend for the last 30 days $3246 (range 0–36,749) versus $8098 (0–52,720), p = 0.06. There was a significant difference in hospital admissions in the last 14 days of life for patients with more than 14 days of exposure to palliative medicine consultation (31% versus 67%) p = 0.01.
Table 3.
Direct hospital cost savings associated with palliative medicine consultation for 30 and 14 day exposure to palliative medicine consultation.
| Palliative care ≥ 30 days (N = 18) |
No palliative care/ palliative care < 30 days (N = 79) |
p | |
|---|---|---|---|
| Median direct hospital costs for last 30 days (range) | $0 ($0–$28,019) | $7729 ($0–$52,720) | 0.01 |
| Median direct hospital costs for last 14 days (range) | $0 ($0–$11,008) | $3935 ($0–$26,794) | 0.01 |
| Palliative care ≥ 14 days (N = 29) | No palliative care/palliative care < 14 days (N = 68) | ||
| Median direct hospital costs for last 30 days (range) | $3246 ($0–36,749) | $8098 (0–52,720) | 0.06 |
| Median direct hospital costs for last 14 days (range) | $0 ($0–$18,184) | $5106 ($0–$26,794) | <0.01 |
Discussion
Palliative medicine consultation resulted in lower ACE scores when compared to patients who did not receive a timely palliative medicine consultation. Patients who had timely consultations had decreased interventions in all domains except for emergency department visits. This may implicate a deficit in adequate outpatient palliative medicine resources for attention to symptoms. While ACE scores have been utilized as benchmark metrics for evaluation of healthcare systems' resources for transition to end-of-life care, they should not be applied to the quality of care given to a particular patient or by a particular physician. Outpatient palliative care consultation has been associated with decreased symptom burden as measured by the Edmonton Symptom Assessment Scale (ESAS) [21]. However, no studies have reported, patient-oriented perception of quality of life in association with either decreased metrics of aggressive care at the end-of-life, ACE, or ESAS. Future research is needed to validate whether there are improvements in patient and family QOL following palliative medicine consultation with prospective testing of QOL assessments in patients with gynecologic malignancies at the end of life.
At our institution a palliative medicine consultation was associated with lower inpatient overall direct costs when compared to patients who did not receive a timely consultation. Decreases in direct costs were seen even if consultation occurred at a minimum of 14 days before death. It would be important to validate cost savings following palliative medicine consultation with prospective testing in patients with gynecologic malignancies. Importantly, there was a significant difference in the percentage of patients admitted to the hospital associated with palliative medicine consultation, which probably accounts for the majority of difference in cost. The financial implications of cost-shifting and burden upon family savings and non-hospital costs should also be considered in the cost-effectiveness of inpatient/outpatient palliative medicine teams. Outpatient palliative medicine consultation has been reported to be cost-effective for patients with cervical and ovarian cancer [22,23]. As in our data, exposure to hospice discussions resulted in decreased lengths of stay for women with gynecologic malignancies as reported by Doll et al. [24].Despite these reports, no studies have undertaken to incorporate measurement of these metrics, costs (both to the hospital system and patient/family), and patient oriented quality of life.
These data are consistent with the report of high utilization of aggressive interventions at the end of life by Von Gruenigen et al. for women with ovarian cancer without improved survival [16]. However, our data are unique in the representation of multiple disease sites in an ethnically and racially heterogeneous population, and association of an intervention, palliative medicine consultation, that may reduce unnecessary interventions at the end of life. These data also concur with previous report by Lewin et al., which associated reduction of resource utilization at the end of life for women with ovarian cancer with hospice enrollment without detriment to survival [25]. The optimal timing for palliative medicine consultation/hospice enrollment remains unknown, however, our data suggest that short lead time from diagnosis is not the explanation, given that less than 12% of patients had consultation less than 100 days from diagnosis.
Shortcomings of our study include the retrospective collection of data. Patients were not included unless they were identified by our Gynecologic Oncology Tumor Board Registry, therefore patients presenting to other medical services or to the emergency department who were not identified to the Gynecologic Oncology Service were not included. Although limited at our institution, we did not consider outpatient patient referrals. Additionally, the gold standard for optimal end of life care should include measure of quality-of-life for patients and families, which was not available in our retrospective review. Savings from inpatient direct hospital costs should be interpreted with caution as there was no accounting for outpatient costs to insurers and costs to the family. Future study of these factors should be conducted in a prospective fashion to better characterize the optimal standard of end-of-life care for women with gynecologic malignancies.
HIGHLIGHTS.
Timely palliative medicine consultation is associated with improved quality of end of life care.
Decreased direct hospital costs are associated with timely palliative medicine consultation.
Acknowledgments
This work was supported in part by the Albert Einstein Cancer Center through its NCI Cancer Center Support Grant (P30CA013330) and NIH (K12CA132783-03).
Footnotes
A portion of this work was presented at the 2013 Annual Meeting of the Society of Gynecologic Oncologists.
Conflict of interest statement
No conflict of interest.
References
- 1.Sepulvada C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organization's global perspective. J Pain Symptom Manage. 2002;24:91–96. doi: 10.1016/s0885-3924(02)00440-2. [DOI] [PubMed] [Google Scholar]
- 2.Ramchandran K, Von Roenn J. Palliative care always. Oncology. 2013:13–36. [PubMed] [Google Scholar]
- 3.Unger S, Payne S, Brearley S, Ploenes V, Radbruch L. Consensus building in palliative care: a Europe-wide Delphi study on common understandings and conceptual differences. J Pain Symptom Manage. 2012;44:192–205. doi: 10.1016/j.jpainsymman.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D. From margins to centre: a review of the history of palliative care in cancer. Lancet Oncol. 2007;8:430–438. doi: 10.1016/S1470-2045(07)70138-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith T, Temin S, Alesi E, Abernathy A, Balboni T, Basch E, et al. American Society of Clinical Oncology Provisional Clinical Opinion: The Integration of Palliative Care Into Standard Oncology Care. J Clin Oncol. 2012;30:1–9. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 6.Bakitas L, Hegel M, Balan S, Brokaw FC, Seville J, Hull JG, et al. Effects of palliative care intervention on clinical outcomes in patients with advanced cancer: the project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumely R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55:993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 8.Gade G, Venohr I, Conner D. Impact of an inpatient palliative care team: a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55:993–1000. [Google Scholar]
- 9.Meyers F, Carducci M, Loscalso M, Linder J, Greasby T, Beckett L. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: simultaneous care educational intervention (SCEI) — linking palliation and clinical trials. J Palliat Med. 2001;14:465–473. doi: 10.1089/jpm.2010.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantilat SZ, O'Riordan DL, Dibble SL, Landefeld CS. Hospital-based palliative medicine consultation: a randomized controlled trial. Arch Intern Med. 2010;170:2038–2040. doi: 10.1001/archinternmed.2010.460. [DOI] [PubMed] [Google Scholar]
- 11.Rabow M, Dibble S, Pantilat S, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83–91. doi: 10.1001/archinte.164.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Temel J, Greer J, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 13.Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff. 2011;30:454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 14.Earle C, Park E, Lai B, Weeks J, Ayanian J, Block S. Identifying potential indicators of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 15.Earle C, Nevilee B, Landrum M, Ayanian J, Block S, Weeks J. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 16.Von Gruenigen V, Daly B, Gibbons H, Hutchins J, Green A. Indicators of survival duration in ovarian cancer an implications for aggressiveness of care. Cancer. 2008;112(10):2221–2227. doi: 10.1002/cncr.23391. [DOI] [PubMed] [Google Scholar]
- 17.Gonsalves W, Tashi T, Krishnamurthy J, Davies T, Ortman S, Thota R, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran's Affairs cancer population. J Palliat Med. 2011;14(11):1231–1235. doi: 10.1089/jpm.2011.0131. [DOI] [PubMed] [Google Scholar]
- 18.O'Mahony S, McHugh M, Selwyn PA. Ventilator withdrawal: procedures and outcomes, a retrospective review of a collaboration between a critical care division and palliative care service. J Pain Symptom Manage. 2003;26(4):954–961. doi: 10.1016/s0885-3924(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 19.O'Mahony S, Blank A, Persaud J, Hutcheson A, Simpson J, Huvane B, et al. Report of a palliative care and case management project for elderly patients in the emergency department in an urban medical center. J Urban Health. 2008;85(3):443–451. doi: 10.1007/s11524-008-9257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecorelli S, Benedet J, Creasman W, Shepherd J. FIGO staging of gynecologic cancer. 1994–1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynecol Obstet. 1999;64(1):5–10. doi: 10.1016/s0020-7292(98)00234-3. [DOI] [PubMed] [Google Scholar]
- 21.Ruskin R, Rabow M, Alen I, Chen L. Outpatient palliative care consultation is associated with a decrease in symptom burden for women with gynecologic malignancies. Gynecol Oncol. 2013;31(1):255. [Google Scholar]
- 22.Lowery W, Lowery A, Barnett J, Lopez-Acevedo M, Lee P, Alvarez Secord A, et al. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol. 2013;130(3):426–430. doi: 10.1016/j.ygyno.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Phippen N, Leath C, Miller C, Lowery W, Havrilesky L, Barnett J. Is a home-based palliative care treatment strategy preferable to standard chemotherapy in recurrent cervical cancer? Gynecol Oncol. 2013;130(1):e30. doi: 10.1016/j.ygyno.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Doll K, Stine J, Van Le L, Moore D, Baejump V, Brewster W, et al. Outpatient end of life discussions shorten hospital admission in gynecologic oncology patients. Gyencol Oncol. 2013;130(1):e140. doi: 10.1016/j.ygyno.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Lewin S, Buttin B, Powell M, Gibb R, Rader J, Mutch D, et al. Resource utilization for ovarian cancer patients at the end of life: how much is too much? Gynecol Oncol. 2005;99:261–266. doi: 10.1016/j.ygyno.2005.07.102. [DOI] [PubMed] [Google Scholar]