Abstract
Background and purpose
Exposure to vascular risk factors has a gradual deleterious effect on brain MRI and cognitive measures. We explored whether a pattern of these measures exists that predicts stroke and Alzheimer’s disease (AD) risk.
Methods
A cognitive battery was administered to 1,679 dementia and stroke-free Framingham Offspring (age>55; mean=65.7±7.0) between 1999 and 2004; participants were also free of other neurological conditions that could affect cognition and >90% also had brain MRI examination. We related cognitive and MRI measures to risks of incident stroke and AD during up to 10 years of follow-up. As a secondary analysis, we explored these associations in the FHS Original cohort (mean age 67.5±7.3 and 84.8±3.3 at the cognitive assessment and MRI examination, respectively).
Results
A total of 55 Offspring participants sustained strokes and 31 developed AD. Offspring who scored <1.5 standard-deviations below predicted mean scores, for age and education, on an executive function test, had a higher risk of future stroke (HR=2.27;95%CI:1.06–4.85) and AD (3.60;95%CI:1.52–8.52); additional cognitive tests also predicted AD. Participants with low (<20%ile) total brain volume and high (>20%ile) white matter hyperintensity volume had a higher risk of stroke (HR=1.97;95%CI:1.03–3.77 and HR=2.74;95%CI:1.51–5.00, respectively) but not AD. Hippocampal volume at the bottom quintile predicted AD in the Offspring and Original cohorts (HR=4.41;95%CI:2.00–9.72 and HR=2.37;95%CI:1.12–5.00, respectively). A stepwise increase in stroke risk was apparent with increasing numbers of these cognitive and imagingmarkers.
Conclusions
Specific patterns of cognitive and brain structural measures observed even in early aging predict stroke risk and may serve as biomarkers for risk prediction.
Keywords: stroke, Alzheimer’s disease, cognitive function, brain MRI
Introduction
Stroke and dementia are the most common neurological diseases in the elderly.1, 2 They frequently coexist3, 4 and certain vascular risk factors are associated with both.5 A prodromal phase exists in Alzheimer’s disease (AD) which is well characterized. People with mild cognitive impairment have a greater risk of developing AD in the future6 and changes in cognitive performance as early as 22 years before any clinical manifestations of AD have been demonstrated.7 Clinical AD may also be preceded by alteration in structural brain volumes, mainly in the hippocampus8, 9 but also in other brain regions such as temporal,10, 11 parietal,10, 11 posterior12 and frontal.12
Unlike AD, stroke often occurs without prior warning. Multiple risk factors for stroke have been recognized,13 and an individual’s risk of future stroke can be estimated based on information regarding one’s vascular risk factors.14 In addition, stroke risk factors have been related to brain magnetic resonance imaging (MRI) changes15, 16 and to poor cognitive function.5, 17 Nevertheless, it is not clear whether such structural and cognitive changes precede the occurrence of clinical stroke and therefore can serve as intermediate markers between one’s vascular burden and the risk of clinical stroke.
We hypothesized, that the effect of vascular risk factors on MRI and cognitive measures would be detectable as a specific structural and cognitive pattern which, in turn, predicts incident stroke. We used AD as an outcome in order to examine whether these patterns are specific for stroke rather than reflective of generalized brain aging.
Methods
The Framingham Heart Study (FHS) is a longitudinal community-based cohort study which was initiated in 1948 with the enrollment of 5,209 participants aged 28 to 62 years (Original Cohort). In 1971 the offspring and spouses of the offspring were enrolled as the Offspring cohort. Since the study’s inception, participants have had serial examinations including standardized interviews, physician examinations, and laboratory testing. More details have been provided elsewhere.18, 19
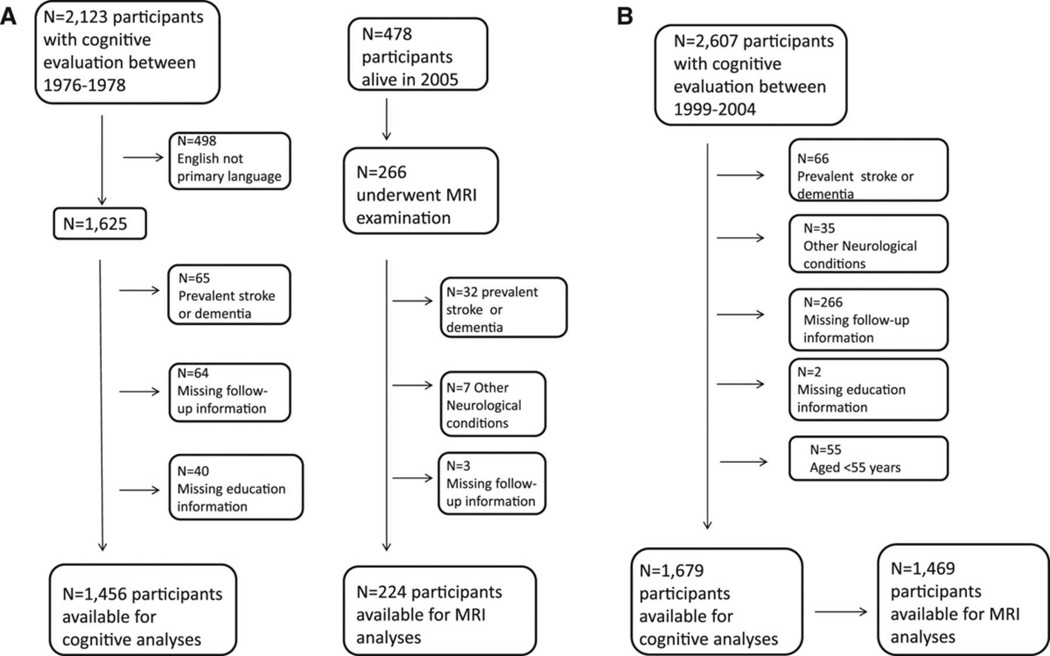
The study design is described in figure 1. Among the Offspring cohort, 2,607 had cognitive testing between 1999 and 2004.20 After exclusion of participants with prevalent stroke or dementia, and those with neurological conditions that could affect their cognition, with incomplete follow-up or education information and persons under 55 years of age, 1,679 persons above age 55 years were available for cognitive analysis, and among them, 1,469 also had brain MRI measures.
Figure 1.
Flow diagram of study participants. A. Original cohort, B. Offspring cohort.
A cognitive test battery was administered to the Original cohort participants between 1976 and 1978; most of these tests were included in the subsequently designed Offspring battery.21 Of the participants who were tested (N=2,123), 1,456 were native English speakers, had complete follow-up information and did not have prevalent stroke or dementia, meeting an inclusion criteria for the cognitive analyses. Approximately 23 years later, surviving participants from the Original cohort were invited to undergo brain MRI. Among the 478 alive in 2005, 266 participants had MRI information, and after excluding individuals with prevalent stroke or dementia or with neurological conditions that might confound the assessment of cognitive function and structural brain measurement, 224 persons were available for these MRI analyses.
Prospective surveillance for incident stroke and AD is ongoing and for these analyses data were used for the period extending 10 years from the date of the MRI and cognitive testing or until December 2009, whichever occurred earlier.
Data was obtained under a protocol approved by the Institutional Review Board of the Boston University Medical Center and informed consent was obtained from all participants.
Methodology for Volumetric Brain MRI
Participants were evaluated with a 1 or 1.5-Tesla Siemens Magnetom scanner. 3D T1 and double echo proton density (PD) and T2 coronal images acquired in 4-mm contiguous slices were performed. All images were transferred to the centralized reading center at the University of California–Davis Medical Center and analyses were performed on QUANTA 6.2, a custom-designed image analysis package. All images were read centrally, blind to the subject’s identity, age, sex, exposure to stroke risk factors, and cognitive performance on cognitive testing. Total cerebral brain volume (TCBV), hippocampal volume (HPV) and white matter hyperintensity volume (WMHV) assessments as well as their inter-rater reliability have been described previously.22–26 Brain volume calculation was performed using semiautomated analysis of pixel distributions based on mathematical modeling of MRI pixel intensity histograms for cerebrospinal fluid (CSF) and brain matter (white matter and gray matter) to determine the optimal threshold of pixel intensity to best distinguish CSF from brain matter.25 TCBV was computed as the ratio of total brain parenchymal volume to total cranial volume to correct for differences in head size.
WMHV was expressed as a proportion of total cranial volume to correct for head size, and was log transformed for all regression analyses. HPV was measured as the sum of right and left hippocampal volumes assessed using in-house software that allowed for 3-dimensional visualization at three-times magnification of the image. Areas included were the CA1– CA4 fields, dentate gyrus, and the subicular complex. On a coronal 3D MRI dataset re-sliced to be aligned perpendicular to the long axis of the hippocampus, borders were traced on contiguous 1.5 mm coronal slices and these slice specific volumes were summed to give hippocampal volume for each side.
Finally, the presence or absence of silent cerebral infarcts (SCI) was determined manually by the operator, based on the size (≥3 mms), location and imaging characteristics of the lesion.27
Cognitive tests
Subjects were administered a cognitive test battery using standard administration protocols and trained examiners. Description of the tests administered and the cohorts in which they were administered to are presented in table 1. Additional details of tests administered and normative values for the FHS Original21 and Offspring20 cohorts have been published. The cognitive test battery administered between 1999 and 2004 to the Offspring cohort and the battery administered previously to the Original cohort both included the following tests: Logical memory (LM), visual memory (VR), Paired associate (PAS) immediate recall and Similarities (SIM). The Offspring’s cognitive battery also included: Hooper Visual Organization test (HVOT), Trail Making Test A (TrA) Trail Making Test B (TrB) and Boston Naming Test (BNT), and the Original cohort battery included the following tests in addition: Digit span forward (DSF), Digit Span Backward (DSB) and Controlled Word Association Fluency Test (COWAT).HVOT, BNT, TrA and TrB were log-transformed to normalize for skewed distribution.
Table 1.
Description of the neuropsychological tests
| Neuropsychological Tests |
Cohort in which test was administered |
Cognitive Domain Assessed | Origin of test | Range |
|---|---|---|---|---|
| LM | Original and Offspring | verbal memory – delayed recall | WMS | 0–24 |
| PAS | Original and Offspring | verbal learning | WMS | 0–21 |
| VR | Original and Offspring | visual memory–immediate recall | WMS | 0–14 |
| HVOT* | Offspring | visuoperception | 30 | |
| SIM | Original and Offspring | abstract reasoning | WAIS | 0–26 |
| DSF | Original | short-term memory capacity | WAIS | 0–9 |
| DSB | Original | executive function | WAIS | 0–8 |
| COWAT | Original | Verbal fluency, executive function | ||
| Trail Making Test A | Offspring | Perceptuo-motor speed | Halstad-Reitan | 0–420 (time to completion in seconds) |
| Trail Making Test B | Offspring | executive function | Halstad-Reitan | 0–600 (time to completion in seconds) |
| BNT | Offspring | Language | 30 |
LM = Logical Memory; PAS = Paired Associates; VR = Visual Reproductions; HVOT = Hooper Visual Organization Test; SIM = Similarities test; DSF = Digit Span Forward; DFB = Digit Span Backward; COWAT = Controlled word association test; TrA=Trail Making Test A; TrB=Trail Making Test B; BNT = Boston Naming Test; WMS= Wechsler Memory Scale; WAIS=Wechsler Adult Intelligence Scale
A validated measure of visuoperceptual skills (Boyd, 1981; Gerson, 1974)
Outcomes
All participants of the FHS are under continuous surveillance for stroke and impairment in cognitive function. We have previously outlined our screening and surveillance methods for the development of stroke14 or dementia.28 In brief, stroke was defined as an acute-onset focal neurological deficit of presumed vascular etiology lasting ≥24 hours. All stroke subtypes were included in the analyses. Ischemic stroke was diagnosed if a focal neurological deficit was documented, imaging showed an infarct that correlated with the clinical deficit, or an infarct was documented at autopsy. Hemorrhagic stroke was defined based on brain imaging, lumbar puncture or autopsy.
AD was diagnosed based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) for definite, probable, or possible AD.29
Statistical analysis
We used multivariable Cox regression to examine the associations of MRI and cognitive measures with incident stroke and AD among the Offspring cohort. Independent variables were defined as continuous or dichotomous. To allow comparisons between performance in different cognitive tests and between different regional brain volumes, we used continuous variables transformed using standardized residuals (Z-scores) from cohort-specific regressions onto age (and education for the cognitive outcomes). Cognitive measures were dichotomized with a cut-off set at z=−1.5 and cognitive deficit in a specific domain was defined as a z-score below that cut-off. The cut-off of 1.5 SDs below the mean is in accordance with previous studies.30–33 Brain MRI measures were divided into cohort-specific quintiles, and the most abnormal quintile (for TCBV and HPV this is the bottom quintile and for WMHV, the top quintile) was compared to the rest. The models were adjusted for age and sex and in a subsequent model additionally for hypertension, smoking, prevalent diabetes, body mass index and ApoEε4 genotype status. In secondary analyses we examined these associations in the Original cohort. ApoEε4 genotype information was not available as a covariate in all persons in the Original cohort. In addition, we ran sensitivity analyses using only the outcomes of probable AD.
We ran Cox-proportional hazards models relating the number of identified predictor variables for stroke to risk of subsequent stroke and AD and illustrate these using comulative hazard function.
Lastly, we estimated net reclassification improvement (NRI), using continuous Cox proportional hazards based models,34 observed on adding each of the statistically significant predictors of stroke risk in a stepwise regression model to the Framingham stroke risk profile (FSRP). The FSRP provides an estimate of the 10-year risk of stroke for a given individual based on age, sex and measurements of systolic blood pressure, antihypertensive therapy, diabetes, smoking status, history of cardiovascular disease and the presence of atrial fibrillation or the left ventricular hypertrophy on the electrocardiogram (EKG-LVH). It has been previously described and validated for stroke prediction risk both at Framingham and in other populations.35–37
Analyses were performed using Statistical Analyses System software Version 9.2 (SAS Institute, Cary, NC).
Results
The baseline characteristics of study participants from the Offspring cohort and the Original cohort (both at the time of the cognitive assessment and at the MRI examination) are presented in Table 2. The mean age of the Offspring was 65.7±7.0 years. The mean ages of the Original cohort at the cognitive assessment and at the later MRI examination were 67.5±7.3 and 84.8±3.3 years, respectively. The mean duration of follow-up for stroke was 7.4±1.7 years for the Offspring cohort, 8.7±2.5 years for the Original cohort and 5.9±2.3 for the Original cohort survivors who underwent MRI (figure 1).
Table 2.
Baseline Characteristics of study participants from the Offspring and Original Cohorts
| Offspring cohort | Original cohort | ||
|---|---|---|---|
| cognitive assessment | MRI examination | ||
| N | 1,679 | 1,456 | 224 |
| age | 65.7±7.0 | 67.5±7.3 | 84.8±3.3 |
| Women | 895 (53.3) | 869 (59.7) | 139 (62.0) |
| High-school graduate | 1,606 (95.7) | 1,042 (72) | 187 (83.5) |
| Hypertension* | 806 (48.8) | 768 (52.8) | 185 (76.8) |
| Body Mass Index (kg/m2) | 28.2±5.2 | 27.1±4.5 | 27.2±4.4 |
| Current smokers | 166 (10.1) | 363 (25.6) | 7 (3.5) |
| Diabetes† | 211 (13.2) | 108 (8.1) | 26 (14.7) |
| ApoEε4 | 367 (22.5) | 46 (20.6) | |
Values are n (%) or mean ± SD.
Hypertension was defined as either systolic blood pressure ≥140 mm Hg or diastolic blood pressure≥90 mm Hg or the use of antihypertensive medication
Diabetes mellitus as a recorded random blood glucose level ≥200 mg/dL (11.1 mmol/L), a previous diagnosis of diabetes mellitus, or the use of insulin or a hypoglycemic agent.
Predictors of Stroke
Cognitive measures
Among the Offspring, 55 out of 1,679 developed a stroke. Poorer performance on TrB, a measure of executive function, was associated with a higher risk of stroke (HR=1.27, 95% CI:1.01–1.59), and this association did not attenuate after additional adjustments (table 3). The risk of future stroke more than doubled in individuals whose executive function performance fell below a pre-defined threshold (using TrB; cognitive z-scores<−1.5) compared to those with intact executive function (HR=2.27, 95% CI:1.06 to 4.85), even after adjustment for concomitant levels of vascular risk factors (HR=2.25, 95% CI:1.05 to 4.86) (table 4). Seventeen percent of people with poor performance on TrB and 12% of people with better performance had SCI (p=0.200). Adjustment for SCI also did not attenuate the strength of the association (HR=2.28, 95% CI:0.96 to 5.39); however the association was no longer significant, possibly due to lack of statistical power since information on SCI was available only on 86% of the study sample. In the Original cohort, 95 out of 1,445 persons sustained a clinical stroke during the follow-up period. As in the Offspring, a test measuring executive function (COWAT) was associated with risk of stroke (HR=1.28, 95% CI: 1.03–1.58), although this association attenuated after additional adjustments (supplementary table I).
Table 3.
Hazard ratios (95% CI) for stroke and AD associated with 1SD decrease in cognitive/MRI measure among the Offspring cohort
| Model A* | Model B† | ||||
|---|---|---|---|---|---|
| Incident stroke | Incident AD | Incident stroke | Incident AD | ||
| No. of events /max sample size | 55/1,679 | 32/1,679 | 54/1,592 | 31/1,592 | |
| Cognitive tests | LM | 1.10 (0.84–1.42) | 2.75 (1.95–3.90) | 1.12 (0.85–1.46) | 2.67 (1.85–3.86) |
| PAS | 1.09 (0.83–1.45) | 1.51 (1.02–2.23) | 1.06 (0.80–1.41) | 1.41 (0.92–2.17) | |
| VR | 1.02 (0.78–1.32) | 2.58 (1.76–3.79) | 0.97 (0.75–1.27) | 2.66 (1.73–4.10) | |
| HVOT§ | 1.03 (0.77–1.37) | 1.25 (0.86–1.82) | 1.05 (0.79–1.39) | 1.16 (0.78–1.74) | |
| SIM | 1.13 (0.87–1.46) | 1.52 (1.13–2.04) | 1.07 (0.82–1.40) | 1.64 (1.20–2.24) | |
| TrA‡§ | 1.09 (0.84–1.42) | 1.61 (1.18–2.19) | 1.07 (0.82–1.40) | 1.63 (1.17–2.27) | |
| TrB‡§ | 1.27 (1.01–1.59) | 1.86 (1.44–2.41) | 1.28 (1.01–1.64) | 1.75 (1.35–2.28) | |
| BNT§ | 1.00 (0.76–1.30) | 1.86 (1.25–2.76) | 0.97 (0.74–1.28) | 1.95 (1.24–3.06) | |
| MRI measure | No. of events /max sample size | 45/1,469 | 28/1,469 | 45/1,414 | 28/1,414 |
| TCBV | 1.61 (1.21–2.13) | 1.15 (0.78–1.68) | 1.51 (1.13–2.01) | 1.28 (0.89–1.83) | |
| HPV | 1.02 (0.76–1.38) | 1.62 (1.13–2.33) | 1.04 (0.77–1.41) | 1.46 (0.98–2.17) | |
| WMHV‡§ | 1.42 (1.10–1.83) | 1.34 (0.98–1.84) | 1.45 (1.11–1.90) | 1.42 (1.05–1.94) | |
CI=Confidence Interval; MRI= Magnetic Resonance Imaging; LM= Logical Memory; PAS = Paired Associates; VR = Visual Reproductions; HVOT = Hooper Visual Organization Test; SIM = Similarities test; TrA=Trail Making Test A; TrB=Trail Making Test B; BNT = Boston Naming Test; TCBV = Total Cerebral Brain Volume; HPV = Hippocampal Volume; WMHV = White Matter Hyperintensity Volume.
Model A: Adjusted for age and sex
Model B: Adjusted for age, sex, education, hypertension, current smoking, history of DM, BMI and ApoEε4.
HRs are for 1SD increase in these measures
Variables are log-transformed
Table 4.
Hazard ratios (95% CI) for stroke and AD associated with cognitive deficit (z-score of cognitive measure≤1.5) and with quintiles of MRI measures (bottom vs. others for TCBV, and HPV and top vs. others for WMHV) among the Offspring cohort
| Model A* | Model B† | ||||
|---|---|---|---|---|---|
| Incident stroke | Incident AD | Incident stroke | Incident AD | ||
| Cognitive tests | LM | 1.31 (0.52–3.29) | 8.43 (4.02–17.70) | 1.42 (0.56–3.58) | 7.37 (3.30–16.46) |
| PAS | 1.24 (0.44–3.48) | 1.28 (0.17–9.75) | 1.18 (0.42–3.37) | 1.50 (0.19–12.03) | |
| VR | 1.80 (0.81–4.02) | 4.67 (2.00–10.87) | 1.75 (0.77–3.96) | 4.28 (1.74–10.56) | |
| HVOT‡ | 1.80 (0.72–4.53) | 1.48 (0.35–6.24) | 1.79 (0.71–4.53) | 1.54 (0.35–6.73) | |
| SIM | 0.84 (0.26–2.70) | 0.99 (0.24–4.16) | 0.56 (0.14–2.33) | 0.85 (0.19–3.81) | |
| TrA‡ | 1.78 (0.76–4.17) | 6.44 (2.82–14.69) | 1.74 (0.74–4.11) | 5.72 (2.33–14.06) | |
| TrB‡ | 2.27 (1.06–4.85) | 3.60 (1.52–8.52) | 2.25 (1.05–4.86) | 3.52 (1.37–9.06) | |
| BNT‡ | 1.83 (0.73–4.60) | 1.85 (0.56–6.11) | 1.93 (0.76–4.91) | 2.45 (0.69–8.72) | |
| MRI measure | TCBV | 1.97 (1.03–3.77) | 1.57 (0.61–4.04) | 1.78 (0.92–3.45) | 1.47 (0.54–4.04) |
| HPV | 0.90 (0.42–1.94) | 4.41 (2.00–9.72) | 0.89 (0.41–1.93) | 3.53 (1.53–8.13) | |
| WMHV§ | 2.74 (1.51–5.00) | 1.36 (0.60–3.10) | 2.73 (1.48–5.02) | 1.43 (0.61–3.36) | |
CI=Confidence Interval; MRI= Magnetic Resonance Imaging; LM= Logical Memory; PAS = Paired Associates; VR = Visual Reproductions; HVOT = Hooper Visual Organization Test; SIM = Similarities test; TrA=Trail Making Test A; TrB=Trail Making Test B; BNT = Boston Naming Test; TCBV = Total Cerebral Brain Volume; HPV = Hippocampal Volume; WMHV = White Matter Hyperintensity Volume.
Model A: Adjusted for age and sex
Model B: Adjusted for age, sex, education, hypertension, current smoking, history of DM, BMI and ApoEε4.
Variables are log-transformed
MRI measures
Among the 1,469 participants from the Offspring cohort who had brain MRI measures, 45 were diagnosed with stroke during the follow-up period. Each 1 SD decrease in TCBV, and increase in WMHV, was associated with a statistically significant higher risk of incident stroke (HR=1.61, 95% CI:1.21–2.13; HR=1.42, 95% CI:1.10–1.83, respectively) (table 3, model A). The association with TCBV but not with WMHV was slightly attenuated by adjusting for vascular risk factors (table 3, model B). Using the threshold models, we had similar findings with the bottom quintile of TCBV and the top quintile of WMHV being associated with a higher risk of incident stroke (HR=1.97, 95% CI:1.03–3.77; HR=2.74, 95% CI:1.51–5.00, respectively) (table 4, model A), however the association with TCBV was no longer significant after additional adjustments for potential risk factors (table 4, model B). While TCBV was not significantly associated with the presence of SCI (15% vs. 12% for top TCBV quintile compared to others; p-value=0.150), people who had WMHV at the top quintile also had more SCI compared to those with lower WMHV (22% vs. 10%; p-value<0.001). The associations of both TCBV and WMHV with incident stroke attenuated after adjustment for SCI, however the association of WMHV (HR=2.41, 95% CI:1.30–4.54) but not TCBV (HR=1.85, 95% CI:0.96–3.57) remained statistically significant. Of the 224 older Original cohort participants who had MRI measures, 29 sustained a stroke during the follow-up. None of the MRI measures was associated with future stroke in the Original cohort (supplementary table I and II).
Predictors of AD
Cognitive measures
Thirty-two out of 1,679 Offspring participants developed AD (all were probable AD). Performance in all cognitive domains, except visual perception (HVOT) was significantly associated with a higher risk of developing AD. The most pronounced associations were between verbal and visual memory and AD (HR=2.75 95% CI:1.95–3.90; HR=2.58, 95% CI:1.76–3.79, respectively) (table 3, model A). Most associations remained significant and robust after controlling for risk factors, although the association with PAS was no longer significant (table 3, model B). In the threshold model, impairment in LM, VR, TrA and TrB were associated with incident AD.
Of the 1,445 participants from the Original cohort, 72 were classified as having at least mild AD during the follow-up (7 were possible AD, 3 of whom had suffered a clinical stroke and the rest were probable AD). The findings here were comparable to those in the Offspring cohort. Of the tests which were administered only in this sample, a measure of executive function (COWAT) was associated with AD (HR=1.47 95% CI: 1.12–1.92), however the association was not significant after additional adjustments or when a threshold model was applied. In addition, another test that measures executive function, DSB, was not associated with AD (supplementary table I and II).
MRI measures
During the follow-up period, 28 out of 1,469 Offspring participants who underwent MRI developed AD (all were probable AD). Lower HPV predicted increased risk of AD (tables 2 and 4), with more than 4 times the risk of AD for those at the bottom HPV quintile (table 4). The association with continuous HPV attenuated after additional adjustment for vascular risk factors and ApoEε4 genotype (table 3), but remained significant in the threshold model (table 4).
Thirty-seven participants of the Original cohort out of 224 persons who underwent brain MRI (22% of the Original cohort participants who survived until 1999 when the MRI evaluations began) were diagnosed with AD (2 were possible AD, none of whom had a clinical stroke and the rest were probable AD). In this sample of elderly persons, HPV predicted future AD as in the Offspring, however in addition, low TCBV was a strong predictor of AD (HR=6.69 95% CI 2.62–17.09) (table S3, model B).
The results were similar when we used probable AD as an outcome rather than possible AD in the Original cohort.
Overall patterns
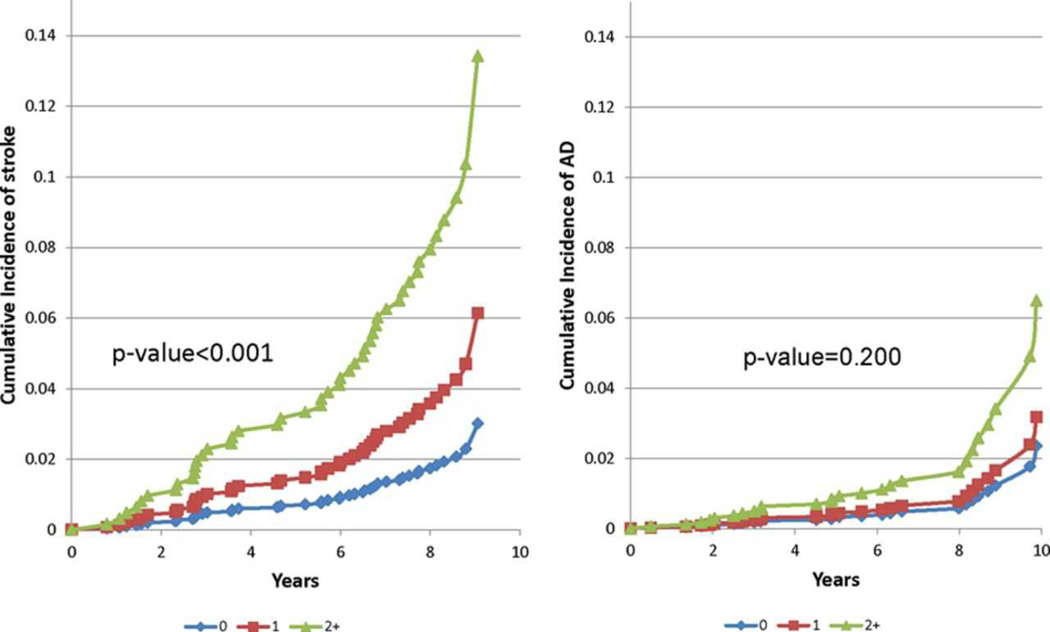
The age-adjusted bivariate inter-correlations were 0.063, 0.047 and 0.019 for the following pairs of stroke-associated indicator variables, respectively: TCBV and TrB, WMHV and TrB and TCBV and WMHV. There was a dose dependent relationship between the number of these three stroke predictors and the risk of developing stroke (p<0.001), but not AD (figure 2).
Figure 2.
Cumulative incidence of stroke (left) and AD (right) in the Offspring cohort based on age- and gender-adjusted Cox models by number of indicators among: poor performance on Trail making B test, top WMHV quintile and bottom TCBV quintile.
The NRI on adding WMHV to the FSRP was estimated at 0.37 and was statistically significant (95% CI: 0.02, 0.85). The NRI on adding TCBV or TrB to the FSRP was not statistically significant.
Discussion
Our findings demonstrate a specific pattern of cognitive deficits and brain MRI measures that precede the occurrence of stroke. Hence we suggest that vascular risk factors may have a continuous subclinical deleterious effect on the brain, which in turn may manifest as a clinical stroke. Individuals with a deficit in executive function, with low total brain volume or large white matter hyperintensity volumes had twice or more the risk of future stroke, and having more of these markers was associated with a gradual increase in stroke risk. This pattern was specific to stroke and was not demonstrated in AD.
Previous literature has suggested that people with cognitive impairment may have a higher risk of subsequent stroke, independently of vascular risk factors, and emphasized the role of cerebrovascular disease in cognitive impairment.38, 39 Our finding that in both the Offspring and Original cohorts measures of executive function were associated with incident stroke is reasonable considering that these measures reflect the integrity of the frontal lobe which is more prone to subclinical vascular injury.40, 41 However only TrB, a measure of executive function, predicted stroke independently of vascular risk factors. This is in accordance with another study that demonstrated an independent association of TrB (and not TrA or the mimi-mental-state-examination) to subsequent risk of brain infarction in a sample of 930 elderly men followed for 13 years.39 The presence of SCI could not explain, in our analysis, the association between TrB and risk of incident stroke.
While TrB also predicted AD, total brain volume and white matter hyperintensity volume were specific for stroke prediction. We and others have previously shown that white matter hyperintensity volume is related to risk of incident stroke,42, 43 however to our knowledge, we are demonstrating for the first time an association between total brain volume and risk of incident stroke. Unlike white matter hyperintensity volume, which was an independent predictor, the association of total brain volume with risk of stroke attenuated after controlling for vascular risk factors, and in the threshold model was no longer significant. Moreover, white matter hyperintensity volume significantly improved the predictive value over the Framingham stroke risk profile, a finding that is supported by a recent study,44 while total brain volume did not. Therefor we conclude that total brain volume reflects current vascular burden while white matter hyperintensity volume may represent cumulative vascular risk or, albeit less likely, a distinct, non-vascular mechanism. Keeping this in mind, the fact that total brain volume was related to risk of stroke in the younger Offspring sample and to risk of AD in the elderly survivors from the Original cohort is plausible, since vascular burden is associated with both conditions but AD occurs later in life and the changes preceding it might be delayed till the 8th and subsequent decades.45, 46 Similarly, the absence of associations between any brain MRI measure and risk of incident stroke among the survivors of the Original cohort may be explained by a healthy survivor bias. Prior studies have also demonstrated a negative association between total brain volume and vascular burden as assessed by the Framingham stroke risk profile.24 Moreover, presence and progression of lacunar infarcts,47 as well as white matter hyperintensity volume48, 49 were shown to be associated with a greater decrease in total brain volume. In the present analysis, the associations of both total cerebral brain volume and white matter hyperintensity volume with incident stroke could be only partially explained by the concomitant presence of silent cerebral infarcts.
Previous studies have demonstrated the existence of a long preclinical phase for AD, which involves impairment in multiple cognitive domains7, 50 as well as changes in brain MRI measures.8–12 Accordingly, in the current study, multiple tests such as verbal and visual memory, abstract reasoning and language were predictive of AD risk. Interestingly, measures of executive function (TrB) in the Offspring but not in the Original cohort (DFB, COWAT) strongly predicted risk of incident AD. This finding is especially intriguing considering the fact that all cases of AD in the Offspring were classified as probable AD and thus the association between TrB and AD was less likely to be driven by cases of mixed AD and vascular pathology. Inconsistency regarding the ability of measures of executive function to predict AD exists also in the literature7, 51–53 and may be due to differences in tests (Digit span test is less demanding than Trail making), in sample characteristics, or due to different time courses between the baseline assessment and onset of clinical dementia.53 Our study demonstrates a strong association between hippocampal volume and future AD risk which is in line with prior studies,8, 9 however our observation that low hippocampal volume is related to AD risk more strongly among those at late middle-age as compared to the elderly (bottom quintile of hippocampal volume confers 4 times the risk in the Offspring compared to a doubling of the risk in the Original cohort) is intriguing and needs further exploration.
The strengths of this study are its large sample and its prospective, population-based design, with a comprehensive cognitive battery and volumetric MRI measures at baseline, and a careful surveillance for clinical outcomes. The fact that the same methods and surveillance were applied to multiple generations enabled us to compare the two samples which were, for the MRI measures, also of different ages. Also, compared with previous publications, this study included a younger sample with a mean age of only 66 years. Limitations include the potential for healthy survivor bias among participants from the Original cohort who underwent MRI and the predominantly European origin and highly educated status of our samples that limits the study generalizability.
Stroke often occurs without prior warning, and when clinical symptoms appear, it is often too late for an effective treatment. Thus, it is of great importance to define biomarkers for early detection of people who are at a higher risk. These biomarkers can then help identify candidates who would benefit maximally from preventive interventions, help define endpoints for clinical trials and endophenotypes for genetic studies and contribute to the creation of a composite risk score for stroke. Future studies are warranted to validate these markers, to study the time points in one’s lifespan at which they would be most useful and to integrate these with other demographic, circulating and imaging biomarkers, both established and novel, to improve risk prediction.
Supplementary Material
Acknowledgments
This work was supported by the dedication of the Framingham Heart Study participants, the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and by grants from the National Institute of Neurological Disorders and Stroke (NS17950), the National Heart, Lung and Blood Association (HL93029, U01HL 096917) and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, P30AG013846). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, the National Heart Lung and Blood Institute, the National Institute of Aging or the National Institutes of Health.
Study funding: Supported by National Institute of Neurological Disorders and Stroke (NS17950), the National Heart, Lung and Blood Association (HL93029, U01HL 096917) and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, P30AG013846).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
none
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 3.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, et al. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 4.Jin YP, Di Legge S, Ostbye T, Feightner JW, Hachinski V. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement. 2006;2:171–178. doi: 10.1016/j.jalz.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 6.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 7.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch. Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 10.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 11.Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang S, et al. Identifying cognitively healthy elderly individuals with subsequent memory decline by using automated MR temporoparietal volumes. Radiology. 2011;259:844–851. doi: 10.1148/radiol.11101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol. Aging. 2012;33:825, e825–e836. doi: 10.1016/j.neurobiolaging.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 17.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am. J. Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev. Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 20.Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp. Aging Res. 2004;30:333–358. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 21.Farmer ME, White LR, Kittner SJ, Kaplan E, Moes E, McNamara P, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol. Rep. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 22.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol. Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 25.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J. Comput. Assist. Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch. Neurol. 2008;65:642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 28.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 31.Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement. Geriatr. Cogn. Disord. 2010;29:164–175. doi: 10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- 32.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch. Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 33.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch. Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 34.Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am. J. Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 36.Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- 37.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 38.Elkins JS, Knopman DS, Yaffe K, Johnston SC. Cognitive function predicts first-time stroke and heart disease. Neurology. 2005;64:1750–1755. doi: 10.1212/01.WNL.0000161850.01792.77. [DOI] [PubMed] [Google Scholar]
- 39.Wiberg B, Lind L, Kilander L, Zethelius B, Sundelof JE, Sundstrom J. Cognitive function and risk of stroke in elderly men. Neurology. 2010;74:379–385. doi: 10.1212/WNL.0b013e3181ccc516. [DOI] [PubMed] [Google Scholar]
- 40.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch. Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 41.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 42.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poels MM, Steyerberg EW, Wieberdink RG, Hofman A, Koudstaal PJ, Ikram MA, et al. Assessment of cerebral small vessel disease predicts individual stroke risk. Journal of neurology, neurosurgery, and psychiatry. 2012;83:1174–1179. doi: 10.1136/jnnp-2012-302381. [DOI] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kloppenborg RP, Nederkoorn PJ, Grool AM, Vincken KL, Mali WP, Vermeulen M, et al. Cerebral small-vessel disease and progression of brain atrophy: the SMART-MR study. Neurology. 2012;79:2029–2036. doi: 10.1212/WNL.0b013e3182749f02. [DOI] [PubMed] [Google Scholar]
- 48.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann. Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 50.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J. Intern. Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 51.Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE) The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2005;13:134–141. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- 52.Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch. Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 53.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J. Int. Neuropsychol. Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.