Abstract
Background and objectives
One hypothesis states that IgA nephropathy (IgAN) is a syndrome with an autoimmune component. Recent studies strongly support the notion of shared genetics between immune-related diseases. This study investigated single-nucleotide polymorphisms (SNPs) reported to be associated with systemic lupus erythematosus (SLE) in a Chinese cohort of patients with IgAN and in controls.
Design, setting, participants, & measurements
This study investigated whether SNP markers that had been reported to be associated with SLE were also associated with IgAN in a Chinese population. The study cohort consisted of 1194 patients with IgAN and 902 controls enrolled in Peking University First Hospital from 1997 to 2008.
Results
Ninety-six SNPs mapping to 60 SLE loci with reported P values <1×10−5 were investigated. CFH (P=8.41×10−6), HLA-DRA (P=4.91×10−6), HLA-DRB1 (P=9.46×10−9), PXK (P=3.62×10−4), BLK (P=9.32×10−3), and UBE2L3 (P=4.07×10−3) were identified as shared genes between IgAN and SLE. All associations reported herein were corroborated by associations at neighboring SNPs. Many of the alleles that are risk alleles for SLE are protective alleles for IgAN. By analyses of two open independent expression quantitative trait loci (eQTL) databases, correlations between genotypes and corresponding gene expression were observed (P<0.05 in multiple populations), suggesting a cis-eQTL effect. From gene-expression databases, differential expressions of these genes were observed in IgAN. Additive interactions between PXK rs6445961and HLA-DRA rs9501626 (P=1.51×10−2), as well as multiplicative interactions between CFH rs6677604 and HLA-DRB1 rs9271366 (P=1.77×10−2), and between HLA-DRA rs9501626 and HLA-DRB1 rs9271366 (P=3.23×10−2) were observed. Disease risk decreased with accumulation of protective alleles. Network analyses highlighted four pathways: MHC class II antigen presentation, complement regulation, signaling by the B-cell receptor, and ubiquitin/proteasome-dependent degradation.
Conclusion
From this “systems genetics” perspective, these data provide important clues for future studies on pleiotropy in IgAN and lupus nephritis.
Introduction
Over the past two decades, considerable progress has been made in unraveling the complex pathogenesis of IgA nephropathy (IgAN). However, the exact pathogenesis remains poorly determined. Current data suggest that genetic factors combined with environmental factors lead to increased synthesis of aberrantly galactosylated IgA1, formation of glycan-specific antibodies to IgG and IgA, and mesangio-podocytic-tubular cross-talk in the occurrence and development of the disease (1–6). Whether IgAN should be termed an “autoimmune disease” is controversial. However, recent genome-wide association studies (GWAS) strongly indicate that many of its associated loci also affect other autoimmune and infectious diseases (5,7–9), further supporting the notion of shared genetics of immune-related diseases (10). Recent estimates suggest that the identified loci collectively explain <10% of the genetic risk for IgAN, highlighting the fact that much of the heritable basis for IgAN has yet to be identified.
Previously, we reported that genetic factors have an appreciable influence on the production of under-galactosylated IgA1 and that GWAS data strongly implicate new clues as to the pathogenesis of IgAN (7,8,11–15). We also reported on the overlap between several autoimmune diseases: systemic lupus erythematosus (SLE), rheumatoid arthritis, ANCA-associated small vasculitis, and anti–glomerular basement membrane disease (16–25). Thus, we hypothesized that refinement of GWAS data or identification of IgAN susceptibility genes could be underpinned by investigation of the genetic variants reported to be associated with other immune-related diseases. Identification of novel IgAN genes and shared genetic pathways could improve understanding of common genetic mechanisms and eventually the development of improved methods of diagnosis, prognosis, and targeted therapies.
SLE is an autoimmune disease. Lupus nephritis is characterized by multiple immune complexes depositing in the kidney, including IgA molecules. IgAN is an immune complex–mediated GN defined by the predominant IgA molecule that deposits in the kidney. A recent study showed the pathogenicity of anti-glycan antibodies in IgAN, which suggested that IgAN is a type of autoimmune disease (26). A new theory suggests that most types of GN are primarily autoimmune diseases. Certain pathogenic similarities between autoimmune diseases (e.g., greater prevalence among Asians than Europeans, chronic course, renal involvement, circulating immune complexes, complement activation, morphologic similarities, certain pathways being involved in ESRD) prompted us to investigate the overlap in genetic susceptibility between SLE and IgAN. Well established co-occurrences of SLE with IgAN suggest common etiologic factors (27–29). Little progress has been made regarding the identification of genetic factors specific to lupus nephritis, but a genetic cause in SLE has been substantiated. More than 40 genes have been robustly associated with SLE.
We investigated whether single-nucleotide polymorphism (SNP) markers that had been reported to be associated with SLE were also associated with IgAN in a Chinese population.
Materials and Methods
The protocol of this study complied with the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Peking University First Hospital (Beijing, China). Written informed consent was obtained from each patient.
Study Population
The samples used in the present study have been described previously. Briefly, exclusion of duplicates and first-degree relatives yielded 1194 IgAN cases and 902 healthy controls recruited in the Renal Division of Peking University First Hospital from 1997 to 2008 (8). All the cases were confirmed by renal biopsy, and all the controls were healthy blood donors without indicators of renal disease. Quality control was undertaken as described (8). Unexpected relatedness was excluded with a PLINK pi-hat cutoff of 0.125. We included men and women of Northern Chinese Han ancestry.
Selection and Genotyping of SNPs
We systemically examined data from GWAS, as well as large-scale replications conducted in SLE genetics through December 1, 2012. The reported SNPs associated with SLE in the GWAS context with a P value <1×10−5 were selected for analysis (30–42). The reported risk variants for SLE using data from the Catalog of Published Genome-Wide Association Studies from the National Human Genome Research Institute (http://www.genome.gov/gwastudies) were also checked. Finally, a panel of 96 SNPs representative of 60 genes or loci was selected (Supplemental Table 1). Genotyping was undertaken using the Illumina Human 610-Quad BeadChip, which involved 498,322 SNPs with a mean call rate of 0.9992.
Statistical Analyses
Only SNPs meeting the quality-control criteria of <1% overall missing data as well as consistency with Hardy–Weinberg equilibrium genotype frequency expectations (P<0.05) were included. As reported previously, after adjustment for population substructure, the inflation factor using all SNPs was λ=1.02, indicating a minimal effect of residual population structure. Thus, no further genomic control corrections were applied.
Genotype frequencies between IgAN cases and controls were compared using the chi-squared trend test implemented in PLINK software to determine whether individual SLE susceptibility loci were also associated with IgAN. Genetic models were defined relative to the minor allele. To reduce the risk of false-positive findings, all positive associations were checked further by associations at neighboring SNPs.
To test for additive interactions, the methods were taken using a 2×2 factorial design to calculate the attributable proportion due to interaction, the relative excess risk due to interaction, and the synergy index (20,43). P values <0.05 for attributable portion due to interaction were considered to be indicators of additive interactions. Ninety-five percent confidence intervals (95% CIs) were calculated using the delta method (44). Multiplicative interaction was assessed by adding an interaction variable (SNP×SNP) to the regression models. P<0.05 was considered to be evidence for multiplicative interactions.
Analyses of carriage of SLE alleles in patients with IgAN were carried out to determine whether there was an overall enrichment of SLE susceptibility variants in IgAN cases. Analyses were also undertaken to determine whether combining those risk alleles conferred a higher risk of disease.
Analyses of Bioinformatics
To explore whether the identified SNPs had expression quantitative trait loci (eQTLs) effects, Genevar software was used to determine associations between sequence variation and gene expression (http://www.sanger.ac.uk/resources/software/genevar). The sequence variation and gene-expression profiling data were from lymphoblastoid cell lines of 726 HapMap3 individuals. Another global map of the effects of polymorphism on gene expression in 400 children from families recruited through a proband with asthma was also investigated to associate gene expression on the basis of imputed genotypes (45).
The differential expressions of suspected IgAN candidate genes were compared with those of healthy controls using publically available data from the ArrayExpress Archive database (http://www.ebi.ac.uk/arrayexpress/) using “IgA nephropathy” as the search term. Three experiments (E-GEOD-37460, E-GEOD-35489, and E-GEOD-14795) involving comparatively large samples were included in the current analysis. The former two experiments took kidney biopsy samples and the latter experiment took whole-blood samples for gene-expression analyses. The normalized data available on the public databases were tested as reported previously.
To integrate data in biologic networks, Cytoscape software (which allows visualization of data in the context of networks) was applied (46). Cytoscape is widely used open-source software for the analyses of bimolecular interaction networks. MiMI integrates data on 119,880 molecules, 330,153 interactions, and 579 complexes from multiple, well known protein-interaction databases. An MiMI plugin, version 3.1.1, installed within Cytoscape 2.8.3, was used to determine the genetic interactions in positional/functional networks. Direct query of genes and their nearest neighbors from all data resources was done, and no further modifications were made.
Results
Analyses of SLE Risk Alleles in IgAN Show Suggestive IgAN Protective Alleles
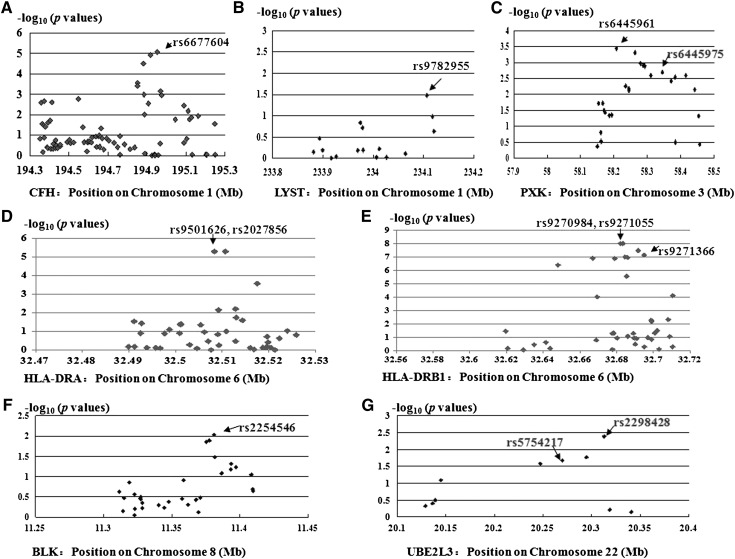
Among the selected 96 SNPs, 10 SNPs of the lupus risk alleles in the region of HLA, CFH (suggested to be a tagging SNP for CFHR1, 3Δ), PXK, BLK, UBE2L3, and LYST showed evidence for association at an allele-type level (P<0.05) (Table 1). Of note, the associations between alleles in the HLA region, CFH, and IgAN were the top signals in our previous reports on GWAS. Interestingly, in comparing odds ratio (OR) values for these alleles in SLE and IgAN, all the directions of association were opposite those observed in the SLE studies, except for UBE2L3. Control allele frequencies were similar to those reported in SLE GWAS data. Thus, these findings suggested that the SLE risk alleles may be protective for susceptibility to IgAN. However, only SNPs in CFH and HLA regions could retain statistically significant evidence for association (Table 1). Although nonsignificant after applying a Bonferroni correction, PXK, BLK, UBE2L3, and LYST remained interesting candidates for further investigation.
Table 1.
Association results for systemic lupus erythematosus risk variants in IgA nephropathy
| Chr | Base Pair | Locus | SNP | Major/Minor Allele | MAF Case/Control (%) | Trend Test P Values | Allele OR (95% CI) by SLE Risk Allelea | Dominant P Values | Recessive P Values | Genotype P Values | SLE Risk Allele OR (Reference) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 194953541 | CFH | rs6677604 | G/A | 4.10/7.26 | 8.41×10−6 | 0.55 (0.42 to 0.72) | 3.37×10−5b | 1.80×10−2 | 5.47×10−5 | 1.19 (41) |
| 1 | 234106500 | LYST | rs9782955 | C/T | 12.87/10.71 | 3.31×10−2 | 0.81 (0.67 to 0.98) | 6.31×10−3 | 0.18 | 3.15×10−3 | 1.18 (35) |
| 3 | 58345217 | PXK | rs6445975 | T/G | 23.79/19.79 | 2.01×10−3 | 0.79 (0.68 to 0.92) | 3.78×10−3 | 0.07 | 9.00×10−3 | 1.20 (31) |
| 6 | 32508322 | HLA-DRA | rs9501626 | C/A | 11.39/16.26 | 4.91×10−6 | 0.66 (0.55 to 0.79) | 4.68×10−6b | 0.12 | 2.60×10−5 | 1.86 (32) |
| 6 | 32694832 | HLA-DRB1 | rs9271366 | A/G | 12.60/18.65 | 6.96×10−8 | 0.63 (0.53 to 0.75) | 4.37×10−10b | 0.81 | 3.40×10−10 | 1.26 (36) |
| 8 | 11377591 | BLK | rs7812879 | C/T | 26.59/23.23 | 1.23×10−2 | 0.83 (0.72 to 0.96) | 3.28×10−2 | 0.05 | 0.04 | 1.45 (34) |
| 8 | 11381089 | BLK | rs2254546 | G/A | 26.63/23.12 | 9.32×10−3 | 0.83 (0.72 to 0.95) | 2.35×10−2 | 4.88×10−2 | 2.96×10−2 | 1.42 (32) |
| 8 | 11381382 | BLK | rs2736340 | T/C | 29.94/26.94 | 3.33×10−2 | 0.86 (0.75 to 0.99) | 7.78×10−2 | 0.07 | 0.08 | 1.35 (36) |
| 22 | 20247190 | UBE2L3 | rs131654 | T/G | 46.48/49.94 | 2.63×10−2 | 1.15 (1.02 to 1.30) | 0.17 | 2.38×10−2 | 0.06 | 1.28 (34) |
| 22 | 20269675 | UBE2L3 | rs5754217 | G/T | 47.32/43.74 | 2.11×10−2 | 1.16 (1.02 to 1.31) | 2.36×10−2 | 0.15 | 0.06 | 1.20 (36) |
The reported SLE risk alleles are set in boldface. Chr, chromosome; SNP, single-nucleotide polymorphism; MAF, minor allele frequency; OR, odds ratio; 95% CI, 95% confidence interval; SLE, systemic lupus erythematosus.
ORs were calculated on the basis of SLE risk alleles for comparison. Reported ORs were derived from the references listed.
Only SNPs in CFH and HLA regions could retain statistical significance after multiple correction.
Analyses of Neighboring SNPs Support Disease Effects
To reduce the chance of false-positive findings using a single marker by chance, all the positive associations were checked further by analyzing the neighboring SNPs. Multiple significant association signals were observed (Figure 1). In CFH, HLA-DRA, and BLK, the top signals were from the SNPs selected. In HLA-DRA, rs2027856 (protective allele T; P=4.91×10−6; OR, 0.66; 95% CI, 0.55 to 0.79) also showed the same significance compared with rs9501626 in association with IgAN (r2 value between rs2027856 and rs9501626 is 1.00). In HLA-DRB1, rs9270984 (protective allele G; OR, 0.63; 95% CI, 0.53 to 0.74) and rs9271055 (protective allele T; OR, 0.63; 95% CI, 0.53 to 0.74) showed the same most significance with P=9.46×10−9 (r2=1.00 between rs9270984 and rs9271055; r2=0.84 between rs9270984 and rs9271366). HLA-DRB1 rs9270984 and rs9271055 showed no better fit (P=3.1×10−9 for both) in association at the genotype level than that of rs9271366 (P=3.40×10−10) as well as their incomplete information in eQTL analyses; rs9271366 was still selected as tag SNPs in further analyses. In PXK, rs6445961 (r2=0.92 between rs6445961 and rs6445975) showed the most significant association, with P=3.62×10−4 (protective allele A; OR, 0.77; 95% CI, 0.66 to 0.89). In UBE2L3, rs2298428 (r2=0.95 between rs2298428 and rs5754217) showed the most significant association, with P=4.07×10−3 (risk allele T; OR, 1.21; 95% CI, 1.06 to 1.37). These findings suggested that associations with PXK, BLK, and UBE2L3 may be true associations because all the associations reported herein were corroborated by associations at neighboring SNPs. Nevertheless, the significances were too weak to meet the threshold in multiple testing. However, the associations between SNPs within LYST and IgAN were not convincing.
Figure 1.
Regional plots of identified loci in Chinese patients with IgA nephropathy. Genotyped SNPs are plotted with their P values (as–log10[P values]) as a function of genomic position (Human Genome Build 18) within a region surrounding the reported systemic lupus erythematosus risk alleles. SNP, single-nucleotide polymorphism.
eQTL Analyses Provides Functional Clues
We investigated whether the most associated SNPs were expression SNPs because they affected the abundance of a protein or gene product by altering transcription. In lymphocyte cell lines from HapMap individuals, rs2298428 and rs9271366 were correlated consistently with UBE2L3 expression (P=0.01–5.0×10−5) and HLA-DRB1 expression (P=4.7×10−13–3.9×10−19), respectively, without population restrictions (Table 2). This conclusion was confirmed by data from lymphoblastoid cell lines from 405 siblings in the United Kingdom (45). These findings suggested that the associations between rs6677604 and CFH, rs2254546, and BLK were more pronounced in Asian populations (although similar trends between genotypes and gene expressions could be observed among different populations). For rs6445961 and PXK, the correlation was marginally significant in white patients living in Utah and Han Chinese from Beijing, China. However, different association patterns appeared to exist between white and Chinese individuals.
Table 2.
Correlation between genotypes of identified IgA nephropathy–associated single-nucleotide polymorphisms with gene expression in Epstein–Barr virus–transformed lymphoblastoid cell lines from an open database
| SNP | Gene | HapMap 3 Unrelated Individuals (P Value) | Children Siblings of British Descent (n=405) | |||
|---|---|---|---|---|---|---|
| CEU (n=165) | CHB (n=137) | JPT (n=113) | YRI (n=203) | |||
| rs6445961-A | PXK | 0.27 (4.10×10−3)a | −0.20 | −0.18 | 0.02 | ND |
| 0.07 | 0.10 | 0.83 | ||||
| rs2298428-C | UBE2L3 | −0.28 (3.30×10−3)a | −0.28 (0.01)a | −0.43 (5.00×10−5)a | – | −0.390 (8.50×10−5)a |
| rs6677604-A | CFH | 0.12 (0.22) | 0.02 (0.84) | 0.26 (0.03)a | 0.11 (0.26) | – |
| rs9501626-A | HLA-DRA | – | – | – | – | – |
| rs9270984-G | HLA-DRB1 | 0.59 (1.00×10−11)a | 0.72 (1.30×10−13)a | 0.68 (1.40×10−12)a | 0.68 (4.90×10−16)a | – |
| rs9271366-G | HLA-DRB1 | 0.63 (4.70×10−13)a | 0.74 (3.80×10−15)a | 0.75 (3.10×10−16)a | 0.73 (3.90×10−19)a | 0.878 (4.00×10−17)a |
| rs2254546-G | BLK | 0.02 (0.82) | −0.43 (8.20×10−5)a | −0.51 (1.10×10−6)a | −0.06 (0.57) | ND |
With the function of each increase of the risk allele, the table depicts the correlation between genotypes and gene expressions. Protective alleles were regarded as reference alleles in the correlation. Pearson correlation coefficients are presented with P values in brackets. CEU, Caucasians living in Utah who were of northern and western European ancestries; CHB, Han Chinese from Beijing, China; JPT, Japanese in Tokyo, Japan; YRI, Yoruba in Ibadan, Nigeria; ND, no data could be derived from the database.
P<0.05.
Differential Gene-Expression Analyses Suggest Gene Involvement in IgAN
We ascertained whether the associated genes described above were expressed differently in patients with IgAN and healthy controls. Except for PXK (for which data were not available), all of the genes were differentially expressed from IgAN than those of controls, with elevated expressions of CFH, HLA-DRA, and HLA-DRB1 in renal biopsy specimens as well as BLK and UBE2L3 in whole-blood samples (Table 3). Only HLA genes were significantly differentially expressed when subjected to multiple testing, but only in renal biopsy specimens rather than in blood samples.
Table 3.
Differential candidate gene expressions in patients with IgA nephropathy compared with healthy controls from an open database
| Candidate Gene | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Renal Biopsies | Whole Blood: Experiment E-GEOD-14795 | ||||||||
| Experiment E-GEOD-37460 | Experiment E-GEOD-35489 | ||||||||
| IgAN (n=27) | Controls (n=27) | P Value | IgAN (n=25) | Controls (n=6) | P Value | IgAN (n=12) | Controls (n=8) | P Value | |
| CFH | 9.41±0.94 | 8.95±0.64 | 4.09×10−2a | 5.72±0.32 | 5.51±0.14 | 0.14 | 96.90±56.10 | 88.11±61.04 | 0.74 |
| HLA-DRA | 11.59±0.33 | 10.89±0.54 | 6.56×10−7a,b | 9.42±0.76 | 8.62±0.27 | 2.56×10−4a,b | 8576.43±2251.01 | 8638.24±2355.87 | 0.95 |
| HLA-DRB1 | 13.10±0.26 | 12.52±0.51 | 4.22×10−6a,b | 11.31±0.65 | 10.43±0.28 | 5.58×10−5a,b | 16661.58±5086.23 | 15779.10±3730.21 | 0.68 |
| PXK | – | – | – | – | – | – | – | – | – |
| BLK | 4.91±0.25 | 4.82±0.17 | 0.14 | 4.48±0.13 | 4.44±0.13 | 0.53 | 372.31±148.09 | 245.60±104.07 | 3.75×10−2a |
| UBE2L3 | 9.58±0.18 | 9.66±0.29 | 0.21 | 7.94±0.13 | 7.75±0.16 | 3.24×10−3a | 492.78±94.12 | 362.57±132.65 | 1.90×10−2a |
Data are the means±SD. IgAN, IgA nephropathy.
P<0.05.
P values remained significant after multiple correction using Benjamini and Hochberg false-discovery rate methods.
Additive and Multiplicative Interaction Analyses Suggest Gene–Gene Interactions
Fifteen tests involving different combinations of six of the most significantly associated SNPs (n×[n−1])/2) within their respective loci (PXK rs6445961, UBE2L3 rs2298428, CFH rs6677604, HLA-DRB1 rs9271366, HLA-DRA rs9271366, and BLK rs2254546) were conducted in the Chinese population. Supplemental Table 2 shows the results of analyses for additive and multiplicative interactions between identified SNPs categorized by whether they had or did not have protective alleles. There was a modest additive (but not multiplicative) gene–gene interaction between PXK rs6445961 and HLA-DRA rs9501626, with the proportion of risk due to an additive interaction of 2.86 (0.55–5.16), interaction P=1.51×10−2 for IgAN. Significant multiplicative interactions were observed between FH rs6677604 and HLA-DRB1 rs9271366 (P=1.77×10−2), as well as for HLA-DRA rs9501626 and HLA-DRB1 rs9271366 (P=3.23×10−2).
Analyses of Joint Effects Suggest Cumulative Effects on the Risk of Disease
To determine the cumulative effect of six SNPs, disease risk was assessed according to the number of protective alleles they had. Individuals with more protective alleles seemed to be less prone to IgAN (whole model P=5.96×10−13) (Table 4). With each increase in the number of protective alleles, the disease risk decreased by approximately 7% (r2=–0.97; P=1.38×10−3). The disease risk decreased up to seven-fold in individuals with eight or more protective alleles compared with those with fewer than 2.
Table 4.
Joint effects of newly identified loci stratified by the number of protective alleles
| Protective Alleles (n) | Frequency in Cases/Controls (%/%) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| ≤2 | 5.4/1.9 | 1.00 (Reference) | |
| 3 | 13.5/10.3 | 0.46 (0.25 to 0.83) | 9.11×10−3 |
| 4 | 25.7/19.5 | 0.46 (0.26 to 0.82) | 6.68×10−3 |
| 5 | 26.3/25.4 | 0.36 (0.21 to 0.64) | 2.73×10−4 |
| 6 | 19.0/21.4 | 0.31 (0.18 to 0.55) | 3.06×10−5 |
| 7 | 6.4/13.3 | 0.17 (0.09 to 0.31) | 1.44×10−9 |
| ≥8 | 3.7/8.0 | 0.16 (0.08 to 0.31) | 8.77×10−9 |
Integrating Identifies Molecules in Cytoscape-Supported Network Involvement
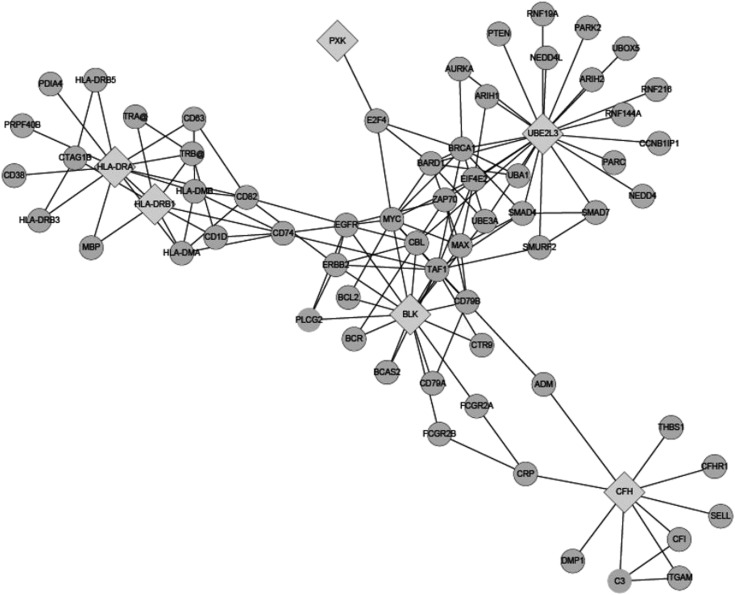
The six identified molecules showed physical interactions between genes or through their products/neighbors (Figure 2). The network was divided mainly into four modules, representative of pathways: MHC class II antigen presentation, complement regulation, signaling by the B-cell receptor (BCR), and ubiquitin/proteasome-dependent degradation. Several cellular interrelated genes have been suggested to participate in the pathogenesis of IgAN as well as SLE: HLA, ITGAM, C3, CFI, FCGR, and PTEN (26,47–50). We also checked the differential expression of those interrelated genes: great enrichment of differences in gene expression between IgAN and healthy controls was observed (Supplemental Table 3). Whole genome-wide expression data were just from tens of samples, but C3 (it was linked with CFH), ITGAM (CFH), CD74 (HLA-DRB1), HLA-DMA (HLA-DRB1), HLA-DMB (HLA-DRA), EGFR (BLK), SMAD7 (UBE2L3), and PTEN (UBE2L3) still produced significant associations in the context of multiple testing.
Figure 2.
A network plot of connections between identified loci in Chinese patients with IgA nephropathy.
Discussion
In recent years, three GWASs in IgAN have been conducted. They uncovered several susceptibility loci and greatly broadened our understanding of the genetic architecture of the susceptibility to IgAN (5,7–9). Among these three GWAS, we took part in two of them (7,8). As reported, all the identified associations within the regions of MHC, 1q32, 8p23, 17p13, and 22q12 could be confirmed in our cohort (7,8), which proved to be the cornerstone of credibility of the present study. The findings of the present study added to the loci showing associations with IgAN, as well as overlap between IgAN and SLE: CFH, HLA-DRA, HLA-DRB1, PXK, BLK and UBE2L3. Although some of the associations did not remain significant after the Bonferroni correction was applied, all the associations reported herein were corroborated by associations at neighboring SNPs, suggesting that they are true associations.
It is widely accepted that initial GWAS can detect just the greatest effects rather than all the susceptibility variants. The ORs of all the novel variants were much weaker (0.8 or 1.2) than the ORs from variants within CFH and HLA (0.6), both of which were previously identified signals in GWAS. The observation that the associated allele was the reverse of that reported previously for SLE was in accordance with a report stating that the protective alleles within MHC, 1q32 and 22q12 regions for IgAN had been implicated as risk factors for other autoimmune disorders (8). Most of these associated loci showed the same tendency for disease susceptibility, so the result is not likely to be a coincidence. The different association directions of the same alleles nevertheless supported the notion of pleiotropy (effect of a single gene on multiple phenotypes), quantitative genetics (combination of the influences of multiple genes together with environmental variation resulting in continuous distributions of phenotypes), and the human “diseasome” (the synthesis of all human genetic disorders [“disease phenome”]) and all human disease genes [“disease genome”]). Ideally, GWAS testing for identifying the common or shared genetic influences on SLE and IgAN in the same population should be carried out and is underway.
GWAS have been used to identify multiple SNPs associated with disease risk, and attention has turned to explaining the underlying molecular mechanisms of action (5). One hypothesis is that a proportion of the causal variants tagged by these disease-associated markers may affect the abundance of a protein (or the relative abundance of its different isoforms) by altering transcription. Efficient identification of additional susceptibility loci with more modest effects might benefit from the integration of statistical evidence with some assessment of functional candidacy. Here, we investigated the positive correlations between identified SNPs and their corresponding gene expression, especially for HLA-DRB1, UBE2L3, and BLK. The data further supported the candidacy of those genes as causal factors in IgAN. Data from Epstein–Barr virus B cell–transformed lymphoblastoid cell lines should be more illustrative than data based on other cell lines in IgAN, because gene expression and eQTLs can be tissue-specific and because IgAN is a disease characterized by production of the nephritogesnic IgA1 molecule from B cells. Confirmation from a different gene-expression database strongly supported the probability of reliability (45). In addition, immortalized lymphoblasts that were clonal could more readily be studied without the environmental influences or transcriptome diversity found in mixed lymphocyte populations in vivo (51). Also, when differential gene expressions in IgAN patients were checked, the expression of all of those genes was upregulated in IgAN patients. However, the associations seemed to have tissue specific-characteristics because elevated expressions of CFH, HLA-DRA and HLA-DRB1 seemed to be restricted to renal biopsies and BLK and UBE2L3 to whole-blood samples. More widespread gene-expression analyses will be warranted, especially in specific cell clones. It seemed that HLA-DRA and HLA-DRB1 protective alleles corresponded to lower gene expressions, whereas PXK, BLK, and UBE2L3 protective alleles corresponded to higher gene expressions, which may indicate an abnormal balance between antigen presentation and lymphocyte signaling. Nevertheless, future studies linking alleles and differential gene expressions in specific tissues will be needed. In addition, rare variants, which may have a greater effect in conferring disease risk and may contribute to a substantial fraction of heritability, will need further evaluations in future genetic studies in IgAN.
Furthermore, to determine whether the identified genes cause effects in a joint manner or epistatic fashion, we conducted gene–gene interaction analyses as well as cumulative gene effect analysis. Investigating genetic interactions has proved difficult, and an optimal statistical approach is not available, so combining several analytical methods may be best for detecting epistatic interactions. Gene–gene interactions can be assessed with additive or multiplicative mathematical models. We demonstrated significant additive and multiplicative interactions among the identified SNPs: that is, additive interactions between PXK rs6445961and HLA-DRA rs9501626, as well as multiplicative interactions between CFH rs6677604 and HLA-DRB1 rs9271366, and between HLA-DRA rs9501626 and HLA-DRB1 rs9271366. However, because of the moderate effects of these alleles and a low incidence of IgAN (estimated incidence in the general population, 25–50 cases per 100,000 individuals), our study remained underpowered to detect epitasis with our sample size (calculated power for epistasis was approximately 0.1–0.2). In joint analyses, we observed that the disease risk decreased by about 7% with each increase in the alleles, and it decreased up to 7-fold in individuals with eight or more protective alleles compared with those who have fewer than two. These results repeatedly supported the notion that the identified genes were the susceptibility genes for IgAN.
One of the most compelling reasons for identifying the genetic underpinnings of common diseases is to generate new hypotheses about the mechanisms and pathogenesis of disease (5). Hence, we checked further the newly identified genes in a pathway-based manner. A molecular network using a correlation structure was produced in which all the identified genes were connected to each other by intermediary genes, and four modules were highlighted. Great enrichment of differences in gene expression between IgAN and healthy controls was observed even though the whole genome-wide expression data were just from tens of samples. The pathways were MHC class II antigen presentation, complement regulation, signaling by the BCR, and ubiquitin/proteasome-dependent degradation. The role of MHC and complement in IgAN has been supported strongly by several observational studies. BLK encodes a tyrosine kinase that is involved in the regulation of B-cell activation. B-cell signaling may have a key role in the pathogenesis of IgAN through elevation of IgA levels in serum, production of autoantibodies, antigen presentation to T cells, and cytokine production (52). Also, B-cell depletion has proved successful in the treatment of GN. UBE2L3 encodes a ubiquitin-conjugating enzyme involved in ubiquitin/proteasome-dependent degradation, which is important in the cell cycle, cell differentiation, apoptosis, sodium-channel function, and modulation of inflammatory responses. The ubiquitin/proteasome pathway has been suggested to be implicated in the development of multiple kidney diseases (53), and proteasome inhibitors have been efficacious in some forms of renal disorders, such as lupus nephritis (54), renal ischemia-reperfusion injury (55), and ANCA-induced GN (56). PXK encodes a multimodular protein composed of a phox homology domain, a protein kinase–like domain, and a Wiskott-Aldrich syndrome protein homology 2 domain. The gene product of PXK regulates the activity of Na-ATPase and K-ATPase ion transport, and is expressed in the kidney (57,58). Recent data suggest that PXK has a critical role in trafficking of the EGF receptor through modulation of ligand-induced ubiquitination of the receptor. Thus, the present study provided important clues for better elucidation of IgAN pathophysiology in the future and possible therapy optimization. Nevertheless, one must be cautious because most of the findings from the initial GWA studies were association signals rather than direct information about susceptibility genes.
The degree of shared genes between IgAN and SLE is substantial, but is likely to still be an underestimate. First, we analyzed only associated loci at the P<1×10−5 level, and the SNPs or genes meeting this criterion are increasing with enrollment of larger sample sizes. Second, because of different linkage disequilibrium between variants in cases and controls, a different variant in the same locus may be responsible for disease risk in a second phenotype. Third, GWASs directly conducted in patients with lupus nephritis are still underway. For these reasons, the gene overlap between the two diseases may be higher than that identified in the present study.
In conclusion, we identified CFH, HLA-DRA, HLA-DRB1, PXK, BLK, and UBE2L3 as shared loci between IgAN and SLE. Many of the alleles that are risk alleles for SLE are protective alleles for IgAN. Genotypes were correlated with the corresponding gene expression, suggesting a cis-eQTL effect. Positive gene–gene interactions were observed, and disease risk decreased with accumulation of protective alleles. Four pathways (MHC class II antigen presentation, complement regulation, signaling by the BCR, and ubiquitin/proteasome-dependent degradation) were highlighted. From the “systems genetics” perspective, our data represent important clues for future studies on pleiotropy in IgA nephropathy and lupus nephritis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank our collaborators, Ali G. Gharavi and Krzyszt of Kiryluk (Department of Medicine, Columbia University College of Physicians and Surgeons, New York, NY), for kindly providing GWAS data and giving advice for the manuscript. We thank Sai-Nan Zhu (Department of Biostatistics, Peking University First Hospital) for assistance with statistical analysis. We thank ELIXIGEN for their editing assistance. We are grateful to the patients and their families for their participation in this study.
This work was supported by grants from the Major State Basic Research Development Program of China (973 program, No. 2012CB517700), the National Natural Science Foundation of China (No. 81200524), the Research Fund of Beijing Municipal Science and Technology for the Outstanding Program (20121000110), the Foundation of Ministry of Education of China (20120001120008), and the Natural Science Fund of China to the Innovation Research Group (81021004).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01860213/-/DCSupplemental.
References
- 1.Floege J: The pathogenesis of IgA nephropathy: What is new and how does it change therapeutic approaches? Am J Kidney Dis 58: 992–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lai KN: Pathogenesis of IgA nephropathy. Nat Rev Nephrol 85: 275–283, 2012 [DOI] [PubMed]
- 3.Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J: An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 81: 833–843, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiryluk K, Novak J, Gharavi AG: Pathogenesis of immunoglobulin A nephropathy: Recent insight from genetic studies. Annu Rev Med 64: 339–356, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, Novak L, Matousovic K, Novak J: IgA nephropathy: Molecular mechanisms of the disease. Annu Rev Pathol 8: 217–240, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ: HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21: 1791–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhernakova A, van Diemen CC, Wijmenga C: Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet 10: 43–55, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Li GS, Zhu L, Zhang H, Lv JC, Ding JX, Zhao MH, Shen Y, Wang HY: Variants of the ST6GALNAC2 promoter influence transcriptional activity and contribute to genetic susceptibility to IgA nephropathy. Hum Mutat 28: 950–957, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Ding J, Zhu L, Shi S, Jiang L, Zhao M, Zhang H: Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant 24: 3372–3375, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Tang W, Li G, Lv J, Ding J, Yu L, Zhao M, Li Y, Zhang X, Shen Y, Zhang H, Wang H: Interaction between variants of two glycosyltransferase genes in IgA nephropathy. Kidney Int 76: 190–198, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lv J, Xu D, Perkovic V, Ma X, Johnson DW, Woodward M, Levin A, Zhang H, Wang H, TESTING Study Group : Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol 23: 1108–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H: The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82: 790–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang DY, Luo H, Zhou XJ, Chen M, Zhao MH: Association of HLA genes with clinical outcomes of ANCA-associated vasculitis. Clin J Am Soc Nephrol 7: 1293–1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo H, Chen M, Cui Z, Yang R, Xu PC, Zhou XJ, Zhao MH: The association of HLA-DQB1, -DQA1 and -DPB1 alleles with anti- glomerular basement membrane (GBM) disease in Chinese patients. BMC Nephrol 12: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou XJ, Cheng FJ, Lv JC, Luo H, Yu F, Chen M, Zhao MH, Zhang H: Higher DEFB4 genomic copy number in SLE and ANCA-associated small vasculitis. Rheumatology (Oxford) 51: 992–995, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, Su Y, Li ZG, Zhang H: Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis 70: 1330–1337, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Zhou XJ, Lu XL, Nath SK, Lv JC, Zhu SN, Yang HZ, Qin LX, Zhao MH, Su Y, Shen N, Li ZG, Zhang H, International Consortium on the Genetics of Systemic Lupus Erythematosus : Gene-gene interaction of BLK, TNFSF4, TRAF1, TNFAIP3, and REL in systemic lupus erythematosus. Arthritis Rheum 64: 222–231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou XJ, Lv JC, Bu DF, Yu L, Yang YR, Zhao J, Cui Z, Yang R, Zhao MH, Zhang H: Copy number variation of FCGR3A rather than FCGR3B and FCGR2B is associated with susceptibility to anti-GBM disease. Int Immunol 22: 45–51, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Zhou XJ, Lv JC, Yu L, Cui Z, Zhao J, Yang R, Han J, Hou P, Zhao MH, Zhang H: FCGR2B gene polymorphism rather than FCGR2A, FCGR3A and FCGR3B is associated with anti-GBM disease in Chinese. Nephrol Dial Transplant 25: 97–101, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Zhou XJ, Lv JC, Zhao MH, Zhang H: Advances in the genetics of anti-glomerular basement membrane disease. Am J Nephrol 32: 482–490, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Zhou XJ, Zhang H: Autophagy in immunity: Implications in etiology of autoimmune/autoinflammatory diseases. Autophagy 8: 1286–1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Guo J, Zhou X, Li R, Liu X, Zhao Y, Zhu B, Liu X, Xu J, Zhu P, Wu X, He J, Liu X, Zhang H, Li Z: Deletion of LCE3C_LCE3B is associated with rheumatoid arthritis and systemic lupus erythematosus in the Chinese Han population. Ann Rheum Dis 70: 1648–1651, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Vernon KA, Cook HT: Complement in glomerular disease. Adv Chronic Kidney Dis 19: 84–92, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Corrado A, Quarta L, Di Palma AM, Gesualdo L, Cantatore FP: IgA nephropathy in systemic lupus erythematosus. Clin Exp Rheumatol 25: 467–469, 2007 [PubMed] [Google Scholar]
- 28.Horino T, Takao T, Terada Y: IgA nephropathy in a patient with systemic lupus erythematosus. Lupus 19: 650–654, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Basile C, Semeraro A, Montanaro A, Giordano R, De Padova F, Marangi AL, Ligorio VA, Santese D: IgA nephropathy in a patient with systemic lupus erythematosus. Nephrol Dial Transplant 13: 1891–1892, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lessard CJ, Adrianto I, Ice JA, Wiley GB, Kelly JA, Glenn SB, Adler AJ, Li H, Rasmussen A, Williams AH, Ziegler J, Comeau ME, Marion M, Wakeland BE, Liang C, Ramos PS, Grundahl KM, Gallant CJ, Alarcón-Riquelme ME, Alarcón GS, Anaya JM, Bae SC, Boackle SA, Brown EE, Chang DM, Cho SK, Criswell LA, Edberg JC, Freedman BI, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Kim JH, Martin J, Merrill JT, Niewold TB, Park SY, Petri MA, Pons-Estel BA, Ramsey-Goldman R, Reveille JD, Scofield RH, Song YW, Stevens AM, Tsao BP, Vila LM, Vyse TJ, Yu CY, Guthridge JM, Kaufman KM, Harley JB, Wakeland EK, Langefeld CD, Gaffney PM, Montgomery CG, Moser KL, BIOLUPUS Network. GENLES Network : Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet 90: 648–660, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, Kaufman KM, Jacob CO, Vyse TJ, Tsao BP, Kimberly RP, Gaffney PM, Alarcón-Riquelme ME, Harley JB, Langefeld CD, International Consortium on the Genetics of Systemic Erythematosus : A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet 7: e1002406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada Y, Shimane K, Kochi Y, Tahira T, Suzuki A, Higasa K, Takahashi A, Horita T, Atsumi T, Ishii T, Okamoto A, Fujio K, Hirakata M, Amano H, Kondo Y, Ito S, Takada K, Mimori A, Saito K, Kamachi M, Kawaguchi Y, Ikari K, Mohammed OW, Matsuda K, Terao C, Ohmura K, Myouzen K, Hosono N, Tsunoda T, Nishimoto N, Mimori T, Matsuda F, Tanaka Y, Sumida T, Yamanaka H, Takasaki Y, Koike T, Horiuchi T, Hayashi K, Kubo M, Kamatani N, Yamada R, Nakamura Y, Yamamoto K: A genome-wide association study identified AFF1 as a susceptibility locus for systemic lupus eyrthematosus in Japanese. PLoS Genet 8: e1002455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W, Shen N, Ye DQ, Liu Q, Zhang Y, Qian XX, Hirankarn N, Ying D, Pan HF, Mok CC, Chan TM, Wong RW, Lee KW, Mok MY, Wong SN, Leung AM, Li XP, Avihingsanon Y, Wong CM, Lee TL, Ho MH, Lee PP, Chang YK, Li PH, Li RJ, Zhang L, Wong WH, Ng IO, Lau CS, Sham PC, Lau YL, Asian Lupus Genetics Consortium : Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet 6: e1000841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, Zhang XJ: Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41: 1234–1237, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvänen AC, Rönnblom L, Behrens TW, Graham RR, Vyse TJ: Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet 7: e1002341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jönsen A, Bengtsson AA, Rantapää-Dahlqvist S, Baechler EC, Brown EE, Alarcón GS, Edberg JC, Ramsey-Goldman R, McGwin G, Jr, Reveille JD, Vilá LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Rönnblom L, Criswell LA, Syvänen AC, Behrens TW, Graham RR: A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41: 1228–1233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapää-Dahlqvist S, Petri M, Manzi S, Seldin MF, Rönnblom L, Syvänen AC, Criswell LA, Gregersen PK, Behrens TW: Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med 358: 900–909, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA, International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) : Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 40: 204–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob CO, Zhu J, Armstrong DL, Yan M, Han J, Zhou XJ, Thomas JA, Reiff A, Myones BL, Ojwang JO, Kaufman KM, Klein-Gitelman M, McCurdy D, Wagner-Weiner L, Silverman E, Ziegler J, Kelly JA, Merrill JT, Harley JB, Ramsey-Goldman R, Vila LM, Bae SC, Vyse TJ, Gilkeson GS, Gaffney PM, Moser KL, Langefeld CD, Zidovetzki R, Mohan C: Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A 106: 6256–6261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, Wu YL, Yu CY, Tang Y, Chen JY, Yang W, Wong M, Kawasaki A, Tsuchiya N, Sumida T, Kawaguchi Y, Howe HS, Mok MY, Bang SY, Liu FL, Chang DM, Takasaki Y, Hashimoto H, Harley JB, Guthridge JM, Grossman JM, Cantor RM, Song YW, Bae SC, Chen S, Hahn BH, Lau YL, Tsao BP: Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A 107: 15838–15843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, Kaufman KM, Langefeld CD, Williams AH, Comeau ME, Ziegler JT, Marion MC, Adler A, Glenn SB, Alarcón-Riquelme ME, Pons-Estel BA, Harley JB, Bae SC, Bang SY, Cho SK, Jacob CO, Vyse TJ, Niewold TB, Gaffney PM, Moser KL, Kimberly RP, Edberg JC, Brown EE, Alarcon GS, Petri MA, Ramsey-Goldman R, Vilá LM, Reveille JD, James JA, Gilkeson GS, Kamen DL, Freedman BI, Anaya JM, Merrill JT, Criswell LA, Scofield RH, Stevens AM, Guthridge JM, Chang DM, Song YW, Park JA, Lee EY, Boackle SA, Grossman JM, Hahn BH, Goodship TH, Cantor RM, Yu CY, Shen N, Tsao BP, BIOLUPUS Network. GENLES Network : Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7: e1002079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham RR, Hom G, Ortmann W, Behrens TW: Review of recent genome-wide association scans in lupus. J Intern Med 265: 680–688, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, Costenbader KH: Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis 69: 54–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosmer DW, Lemeshow S: Confidence interval estimation of interaction. Epidemiology 3: 452–456, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC, Taylor J, Burnett E, Gut I, Farrall M, Lathrop GM, Abecasis GR, Cookson WO: A genome-wide association study of global gene expression. Nat Genet 39: 1202–1207, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD: Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2: 2366–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox SN, Sallustio F, Serino G, Pontrelli P, Verrienti R, Pesce F, Torres DD, Ancona N, Stifanelli P, Zaza G, Schena FP: Altered modulation of WNT-beta-catenin and PI3K/Akt pathways in IgA nephropathy. Kidney Int 78: 396–407, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Suzuki Y, Tsuge T, Kanamaru Y, Horikoshi S, Monteiro RC, Tomino Y: FcgammaRIIa-131R allele and FcgammaRIIIa-176V/V genotype are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 20: 2439–2445, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Zhou XJ, Lv JC, Qin LX, Yang HZ, Yu F, Zhao MH, Zhang H: Is FCGR2A a susceptibility gene to systemic lupus erythematosus in Chinese? Lupus 20: 1198–1202, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Zhou XJ, Cheng FJ, Qi YY, Zhao YF, Hou P, Zhu L, Lv JC, Zhang H: FCGR2B and FCRLB gene polymorphisms associated with IgA nephropathy. PLoS ONE 8: e61208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grundberg E, Adoue V, Kwan T, Ge B, Duan QL, Lam KC, Koka V, Kindmark A, Weiss ST, Tantisira K, Mallmin H, Raby BA, Nilsson O, Pastinen T: Global analysis of the impact of environmental perturbation on cis-regulation of gene expression. PLoS Genet 7: e1001279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL: Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukasawa H: The role of the ubiquitin-proteasome system in kidney diseases. Clin Exp Nephrol 16: 507–517, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE: The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 14: 748–755, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Huber JM, Tagwerker A, Heininger D, Mayer G, Rosenkranz AR, Eller K: The proteasome inhibitor bortezomib aggravates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 297: F451–F460, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R: Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol 22: 336–348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao H, Ferguson TS, Cibulsky SM, Holmqvist M, Ding C, Fei H, Levitan IB: MONaKA, a novel modulator of the plasma membrane Na,K-ATPase. J Neurosci 25: 7934–7943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou X, Qiu G, Chen C, Wu M, Hu Y, Zheng H, Li X, Gu S, Ji C, Mao Y: Expression pattern and subcellular localization of five splice isoforms of human PXK. Int J Mol Med 16: 701–707, 2005 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.