Abstract
Background and objectives
Insulin resistance participates in the pathogenesis of multiple metabolic and cardiovascular diseases. CKD patients have impaired insulin sensitivity, but the clinical correlates and outcome associations of impaired insulin sensitivity in this vulnerable population are not well defined.
Design, setting, participants, & measurements
The prospective cohort study was from the third examination cycle of the Uppsala Longitudinal Study of Adult Men, a population-based survey of elderly men ages 70–71 years; insulin sensitivity was assessed by glucose disposal rate as measured with euglycemic clamps. Inclusion criterion was eGFR<60 ml/min per 1.73 m2 (n=543). Exclusion criteria were incomplete data on euglycemic clamp and diabetes (n=97), leaving 446 men with CKD stages 3 and 4 (eGFR median=51.9 ml/min per 1.73 m2; range=20.2–59.5 ml/min per 1.73 m2).
Results
The mean of glucose disposal rate was 5.4±1.9 mg/kg per minute. In multivariable analysis, the independent clinical correlates of glucose disposal rate were eGFR (slope, 0.02; 95% confidence interval, 0.01 to 0.04), hypertension (−0.48; 95% confidence interval, −0.86 to −0.11), hyperlipidemia (−0.51; 95% confidence interval, −0.84 to −0.18), and body mass index (−0.32; 95% confidence interval, −0.37 to −0.27). During follow-up (median=10.0 years; interquartile range=8.7–11.0 years), 149 participants died. In Cox regression models, glucose disposal rate was not associated with all-cause or cardiovascular mortality. Multiplicative interactions (P<0.05) were observed between glucose disposal rate and physical activity or smoking in total mortality association. After subsequent stratification, glucose disposal rate was an independent correlate of all-cause mortality in smokers (adjusted hazard ratio, 0.72; 95% confidence interval, 0.54 to 0.96 per 1 mg/kg per minute glucose disposal rate increase) and physically inactive individuals (hazard ratio, 0.77; 95% confidence interval, 0.61 to 0.97) but not their counterparts.
Conclusion
eGFR, together with various components of the metabolic syndrome, contributed to explain the variance of insulin sensitivity in men with CKD stages 3 and 4. Insulin sensitivity was associated with a lower mortality risk in individuals who smoked and individuals who were physically inactive.
Introduction
Insulin resistance (IR) is defined as reduced sensitivity of target organs to the biologic effects of insulin. IR plays an important role in the pathogenesis of multiple metabolic diseases and cardiovascular diseases (CVDs), and it is associated with increased risk of cardiovascular events and premature mortality in diverse community-based populations (1–3). Multiple mechanisms may explain a potentially causal link in this association, including promotion of endothelial dysfunction, oxidative stress, inflammation, and other cardiovascular risk factors (obesity, hypertension, and dyslipidemia) that are parts of the metabolic syndrome (4).
IR is common in patients with ESRD, and possibly, it is also common in moderate to severe stages of CKD; however, evidence on the latter is scarce (5–7). Prospective data concerning the association between impaired insulin sensitivity (IS) and clinical outcomes in CKD patients are also scarce and conflicting. Some (5,8) but not all (9) studies have documented that IR is an independent predictor of cardiovascular mortality in ESRD. These studies use surrogate indices of IR, like the homeostasis model assessment–IR (HOMA-IR) (10), and its validity in the context of uremic retention is not yet fully established (7). The objectives of this study were to investigate the clinical and biochemical characteristics associated with IR (or its reciprocal IS) in a large cohort of nondiabetic men with CKD stages 3 and 4 as well as establish plausible associations with mortality. We assessed IS by the gold standard—the hyperinsulinemic euglycemic clamp technique (11).
Materials and Methods
Study Population
This analysis includes individuals with reduced kidney function (eGFR<60 ml/min per 1.73 m2) from the Uppsala Longitudinal Study of Adult Men (ULSAM) community-based cohort (described in detail at http://www2.pubcare.uu.se/ULSAM/). This cohort, initiated in the 1970s, invited all 50-year-old men living in the Uppsala region to participate, and subsequent re-examinations were planned. The present analyses are based on the third examination cycle of the ULSAM cohort, when participants were all 70–71 years of age (examinations performed during 1991–1995; n=1221). Inclusion criterion for analysis was eGFR<60 ml/min per 1.73 m2 (n=543). Exclusion criteria were incomplete data on euglycemic clamp (n=23) and diabetes (defined as fasting plasma glucose≥7.0 mmol/L, 2-hour postload glucose level≥11.1 mmol/L, or the use of oral hypoglycemic agents or insulin; n=74). The present study, therefore, comprises 446 nondiabetic elderly men with CKD according to the current Kidney Disease Outcomes Quality Initiative definition (12). All participants gave written consent, and the Ethics Committee of Uppsala University approved the study.
Investigations were performed under standardized conditions. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters. Waist circumference was measured midway between the lowest rib and the iliac crest in the supine position. Smoking status was defined as current smoking versus nonsmoking. Leisure time physical activity was assessed using the following questions. (1) Do you spend most of your time reading, watching television, going to the cinema, or engaging in other mostly sedentary activities? (2) Do you often go walking or cycling for pleasure? (3) Do you engage in any active recreational sports or heavy gardening for at least 3 hours every week? (4) Do you regularly engage in hard physical training or competitive sport? Based on these questions, four physical activity categories were constructed: sedentary (1), moderate (2), regular (3), and athletic (4). The highest positive physical activity response level was used for each participant (13). Previous CVD was defined as history of any CVD as recorded in the Swedish Hospital Discharge Registry (International Classification of Diseases [ICD] -8 codes 390–458 or ICD-9 codes 390–459). BP was measured in duplicate in the right arm with the subject in the supine position after resting for 10 minutes. Hypertension was defined as BP≥140/90 mmHg or the use of antihypertensive medications. Hyperlipidemia was defined as serum cholesterol>6.5 mmol/L and/or serum triglycerides>2.3 mmol/L and/or treatment with lipid-lowering medications.
Laboratory Analyses
Venous blood samples were drawn after an overnight fast and stored at −70°C until analyses. The assays were performed at the Department of Clinical Chemistry, University Hospital, Uppsala, Sweden. Serum cystatin C was measured by latex-enhanced reagent (N Latex Cystatin C; Dade Behring, Deerfield, IL) with a Behring BN ProSpec Analyzer (Dade Behring). The total analytical imprecision of the method was 4.8% at 0.56 mg/L and 3.7% at 2.85 mg/L. eGFR was calculated from serum cystatin C concentrations (milligrams per liter) with the formula eGFR=77.24×cystatin C−1.2623, which has been shown to be closely correlated with iohexol clearance (14). This formula gave an almost identical patient selection compared with the serum cystatin C Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. However, the serum creatinine CKD-EPI equation yielded only 157 individuals with CKD (<60 ml/min per 1.73 m2). Cholesterol and triglyceride concentrations were analyzed in serum and the isolated lipoprotein fractions by enzymatic techniques. HDL particles were separated by precipitation with magnesium chloride/phosphotungstate. Fasting plasma glucose was measured by the glucose dehydrogenase method (Gluc-DH; Merck, Darmstadt, Germany). High-sensitivity C-reactive protein measurements were performed by latex-enhanced reagent (Dade Behring) using a Behring BN ProSpec Analyzer (Dade Behring). IL-6 was analyzed by an ELISA kit (IL-6 HS; R&D Systems, Minneapolis, MN). Urinary albumin excretion rate (UAER) was measured in one overnight urine collection and expressed in micrograms per minute. The assay used a commercially available radioimmunoassay kit (Albumin RIA 100; Pharmacia, Uppsala, Sweden).
Dietary Assessment
Dietary habits were assessed by a 7-day dietary record based on a validated precoded menu book (15) by the Swedish National Food Administration. Because previous literature correlates sodium and fiber intake with IR (16), the intake of these nutrients was explored in the present analysis after correction for total energy intake by the residual method (17).
IS
The euglycemic hyperinsulinemic clamp technique according to the work by DeFronzo et al. (11) was used with a slight modification to suppress hepatic glucose production for estimation of in vivo sensitivity to insulin (18). Insulin (Actrapid Human; Novo, Copenhagen, Denmark) was infused in a primary dose for the first 10 minutes and then, as a continuous infusion (389 pmol/min per meter2 body surface area) for 2 hours to maintain steady state hyperinsulinemia, achieving a level of about 660 pmol/L. The target plasma glucose level was 5.1 mmol/L, which was maintained by measuring plasma glucose every 5 minutes. The glucose infusion rate during the last hour was used as a measure of glucose disposal rate (M; milligrams per kilogram per minute). The coefficient of variation of the M value was 12% on repeated clamp investigations within 30 days in the same individual. The IS index (M/insulin [I] ratio) is a measure of the tissue sensitivity to insulin expressed per unit of insulin, and it was calculated by dividing M by the mean I concentration during the same period of the clamp. Thus, M/I represents the amount of glucose metabolized per unit of plasma insulin, and it is given in milligrams per kilogram per minute per 1 mU/L insulin multiplied by 100.
Follow-Up and Mortality
Mortality was assessed after a median follow-up time of 10.0 years (interquartile range [IQR]=8.7–11.0 years; range=0.1–12.4 years]. We define all-cause mortality as our primary end point to reflect the pleiotropic effects of IR. Cardiovascular mortality was examined as a secondary end point. The Swedish national register recording was used to define total and cardiovascular mortality (defined as death from ischemic heart disease, cerebrovascular disease, or other CVD; ICD-9 codes 410–414 or 430–438 or ICD-10 codes I20–I25 or I60–I69/G45), with no loss to follow-up.
Statistical Analyses
Values were expressed as mean±SD for normally distributed continuous variables, median (IQR) for skewed variables, or percentages of total for categorical variables. Univariable and eGFR-adjusted regressions were calculated to determine correlations between clamp M and its risk factors. Multivariable regression models were fitted to assess the independence of potential clinical correlates of M. Data are expressed as slopes (true value β or estimator standard β) and 95% confidence intervals (95% CIs).
Associations with total and cardiovascular mortality were investigated with Cox proportional hazards analyses (both per unit of M increase as well as per prespecified multicategory [M quartiles] and threshold models [M quartiles 2–4 versus M quartile 1]). Proportional hazards assumptions were confirmed using Schoenfeld’s test. The relations of M and mortality were investigated in four sets of models in a hierarchical fashion: (1) unadjusted model; (2) model adjusted for age and lifestyle parameters: BMI, smoking status, and physical activity; (3) model also adjusted for cardiovascular risk factors: prevalence of CVD and UAER; and (4) model also adjusted for eGFR. Data are presented as hazard ratios (HRs) and 95% CIs.
Based on the preceding evidence regarding effects of lifestyle on IS (19), the values of glucose infusion rate for primary outcome (all-cause mortality) were determined in subgroup proportional hazards Cox analyses. Individuals were dichotomized according to BMI (normal versus overweight/obese), smoking status (nonsmokers versus smokers), or physical activity levels (active versus inactive). Subjects of athletic and regular levels were considered to be physically active, whereas subjects of sedentary and moderate levels were considered to be physically inactive because of the limited numbers of subjects in the two extreme categories. Data are expressed as HRs and 95% CIs for every 1 mg/kg per minute increase in M in each strata as well as P values for interaction. Cardiovascular mortality analysis was not performed in these subgroup analyses because of potentially insufficient statistical power. No other interaction terms other than the above-mentioned terms were tested. All statistical analyses were performed using statistical software STATA version 12 (Stata Corporation, College Station, TX). All tests were two-tailed, and P<0.05 was considered significant.
Results
Baseline Characteristics
Median eGFR in the study population was 51.9 (IQR=45.9–56.6; range=20.2–59.5) ml/min per 1.73 m2. M showed a normal distribution with a mean of 5.4±1.9 mg/kg per minute. The mean of M/I was 5.1±2.2 100×mg/kg per minute per 1 mU/L insulin. As many as 24.0% of the subjects were considered insulin-resistant (M<4.0 mg/kg per minute), 63.5% of the subjects were considered insulin-intolerant (M=4.0–7.5 mg/kg per minute), and only 12.5% of the subjects were considered insulin-sensitive (M>7.5 mg/kg per minute) (20). Clinical and biochemical characteristics of included subjects are shown in Table 1 stratified by quartiles of M distribution. Across increasing M quartiles, patients had higher eGFRs, higher physical activity levels, lower BMIs, and trends to decreased prevalence of CVD. Components of the metabolic syndrome were less prevalent with increasing M: waist circumference, serum triglycerides, systolic and diastolic BP, and fasting glucose were significantly lower, whereas HDL cholesterol was increased. Other biomarkers (C-reactive protein, IL-6, and UAER) decreased across increasing M quartiles. Energy-adjusted dietary sodium and fiber intake did not vary in a statistically significant fashion.
Table 1.
Baseline characteristics according to quartiles of glucose disposal rate by euglycemic clamp in 446 nondiabetic men with CKD
| Parameters | Quartiles of Glucose Disposal Rate (range; milligrams per kilogram per minute) | P for Trend | |||
|---|---|---|---|---|---|
| Quartile 1 (0.9, 4.1) | Quartile 2 (4.1, 5.3) | Quartile 3 (5.3, 6.7) | Quartile 4 (6.7, 11.3) | ||
| n | 111 | 111 | 113 | 111 | |
| Age, yr | 71.0±0.7 | 71.0±0.5 | 71.0±0.5 | 71.1±0.6 | 0.02 |
| eGFR, ml/min per 1.73 m2 | 50.1 (44.8–54.4) | 52.9 (43.4–56.0) | 52.9 (46.9–57.1) | 53.4 (48.7–57.1) | 0.002 |
| Body mass index, kg/m2 | 29.0±3.0 | 26.6±2.8 | 25.3±2.5 | 23.6±2.3 | <0.001 |
| Smokers, n (%) | 20 (18) | 28 (25) | 28 (25) | 28 (25) | 0.20 |
| Physical activity, n (%) | 0.002 | ||||
| Sedentary | 7 (7) | 7 (7) | 5 (5) | 1 (1) | |
| Moderate | 43 (43) | 34 (35) | 33 (32) | 33 (32) | |
| Regular | 49 (49) | 54 (56) | 58 (56) | 63 (60) | |
| Athletic | 2 (1) | 2 (2) | 8 (7) | 7 (7) | |
| Cardiovascular disease, n (%) | 45 (41) | 36 (32) | 41 (36) | 30 (27) | 0.07 |
| Hypertension, n (%) | 99 (89) | 85 (76) | 87 (77) | 74 (67) | <0.001 |
| Hyperlipidemia, n (%) | 50 (45) | 37 (33) | 41 (36) | 30 (27) | 0.01 |
| Dietary factors (g/kcal per day) | |||||
| Sodium intake | 2.6±0.7 | 2.5±0.3 | 2.4±0.3 | 2.4±0.3 | 0.10 |
| Fiber intake | 16.4±3.7 | 16.0±3.9 | 16.5±3.4 | 16.9±4.4 | 0.52 |
| Metabolic syndrome | |||||
| Waist circumference, cm | 103±9 | 96±9 | 92±6 | 87±8 | <0.001 |
| Triglycerides, mmol/L | 1.5 (1.1–2.2) | 1.5 (1.0–1.7) | 1.3 (0.9–1.6) | 1.0 (0.8–1.4) | <0.001 |
| HDL, mmol/L | 1.1 (0.9–1.2) | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) | 1.4 (1.2–1.7) | <0.001 |
| Systolic BP, mmHg | 151±18 | 149±20 | 147±17 | 144±20 | 0.001 |
| Diastolic BP, mmHg | 86±9 | 85±9 | 84±9 | 82±10 | 0.02 |
| Fasting glucose, mmol/L | 5.6±0.7 | 5.3±0.5 | 5.3±0.5 | 5.1±0.5 | <0.001 |
| Other biomarkers | |||||
| C-reactive protein, mg/L | 2.9 (1.7–5.7) | 2.6 (1.1–4.6) | 2.0 (1.0–5.3) | 1.6 (0.9–3.6) | <0.001 |
| IL-6, ng/L | 4.9 (2.6–8.1) | 4.5 (2.2–9.7) | 3.3 (2.1–6.3) | 3.4 (2.1–5.3) | 0.001 |
| UAER, μg/min | 6.2 (3.8–13.2) | 5.8 (3.7–13.1) | 4.9 (3.2–13.5) | 3.7 (2.9–6.6) | <0.001 |
Data are expressed as mean±SD, median (interquartile range), or number (percentage) as appropriate. eGFR, estimated GFR; UAER, urinary albumin excretion rate.
Correlates of M
Univariable and eGFR-adjusted regressions are shown in Table 2. The metabolic syndrome components, as well as IL-6, were significantly associated with M. UAER was not associated with M in crude or eGFR-adjusted regression models. A multivariable regression analysis was fitted to study correlates of M (Table 3), and eGFR, hypertension, hyperlipidemia, and BMI were considered independent contributors to the variance of IS.
Table 2.
Clinical correlates of glucose disposal rate (milligrams per kilogram per minute) by euglycemic clamp in 446 nondiabetic men with CKD
| Variables | Crude | eGFR Adjusted | ||
|---|---|---|---|---|
| Slope (95% CI) | P Value | Slope (95% CI) | P Value | |
| Metabolic syndrome components | ||||
| Waist circumference, cm | −0.12 (−0.13 to −0.10) | <0.001 | −0.11 (−0.13 to −0.10) | <0.001 |
| Triglycerides, mmol/L | −0.94 (−1.17 to −0.71) | <0.001 | −0.92 (−1.15 to −0.69) | <0.001 |
| HDL, mmol/L | 1.87 (1.41 to 2.34) | <0.001 | 1.79 (1.33 to 2.27) | <0.001 |
| Systolic BP, 10 mmHg | −0.12 (−0.21 to −0.03) | 0.009 | −0.11 (−0.20 to −0.02) | 0.01 |
| Diastolic BP, 10 mmHg | −0.26 (−0.45 to −0.08) | 0.005 | −0.28 (−0.46 to −0.10) | 0.003 |
| Fasting glucose, mmol/L | −1.0 (−1.28 to −0.71) | <0.001 | −0.98 (−1.26 to −0.69) | <0.001 |
| Other biomarkers | ||||
| C-reactive protein, mg/L | −0.03 (−0.07 to 0.01) | 0.055 | −0.03 (−0.06 to 0.01) | 0.15 |
| IL-6, ng/L | −0.03 (−0.05 to −0.01) | 0.002 | −0.03 (−0.05 to −0.01) | 0.006 |
| UAER, 200 μg/min | −0.04 (−0.34 to 0.27) | 0.81 | 0.08 (−0.24 to 0.40) | 0.63 |
95% CI, 95% confidence interval.
Table 3.
Multivariable regression model with glucose disposal rate (milligrams per kilogram per minute) in 446 nondiabetic men with CKD
| Independent Variables (Adjusted R2=0.42) | Slope (95% CI) | P Value |
|---|---|---|
| eGFR, ml/min per 1.73 m2 | 0.02 (0.01 to 0.04) | 0.02 |
| Age, 10 yr | 1.99 (−0.68 to 4.67) | 0.14 |
| Body mass index, kg/m2 | −0.32 (−0.37 to −0.27) | <0.001 |
| Smokers | 0.11 (−0.24 to 0.46) | 0.53 |
| Physical activity (reference: sedentary)a | ||
| Moderate | 0.46 (−0.27 to 1.19) | 0.21 |
| Regular | 0.65 (−0.07 to 1.37) | 0.08 |
| Athletic | 1.64 (0.66 to 2.62) | 0.001 |
| Cardiovascular disease | −0.08 (−0.40 to 0.24) | 0.63 |
| Hypertension | −0.48 (−0.86 to −0.11) | 0.01 |
| Hyperlipidemia | −0.51 (−0.84 to −0.18) | 0.003 |
| IL-6, ng/L | −0.02 (−0.03 to 0.01) | 0.10 |
| UAER, 200 μg/min | 0.12 (−0.17 to 0.41) | 0.40 |
P for trend=0.005.
Cox Regression Models
During follow-up, 149 participants died from any cause (incidence rate=4.45/100 person-years at risk), of which 73 deaths were caused by cardiovascular causes (incidence rate=1.99/100 person-years at risk). Specific cardiovascular causes of death are presented in Supplemental Table 1, with no linear trends observed across increasing M quartiles. As shown in Table 4, M did not associate with all-cause (HR, 0.96; 95% CI, 0.87 to 1.06 for 1 mg/kg per minute increase of M) or cardiovascular mortality (HR, 0.96; 95% CI, 0.83 to 1.10 for 1 mg/kg per minute increase of M) after adjustment for age, lifestyle, and cardiovascular factors and eGFR. The multicategory and threshold models largely confirmed these findings. We observed similar results when using M/I as the exposure (Supplemental Tables 2 and 3).
Table 4.
Cox regression analysis between all-cause and cardiovascular mortality and glucose disposal rate in 446 nondiabetic men with CKD
| Cox Regression Models | M (milligrams per kilogram per minute) | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Crude Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | ||
| All-cause mortality | |||||
| Continuous model | 1-mg/kg per minute increment | 0.96 (0.89 to 1.03) | 0.94 (0.85 to 1.04) | 0.95 (0.86 to 1.05) | 0.96 (0.87 to 1.06) |
| Multicategory model | |||||
| Quartile 1 | ≤4.1 | Reference | Reference | Reference | Reference |
| Quartile 2 | 4.1–5.3 | 0.73 (0.47 to 1.15) | 0.74 (0.44 to 1.23) | 0.72 (0.42 to 1.22) | 0.76 (0.45 to 1.29) |
| Quartile 3 | 5.3–6.7 | 0.79 (0.51 to 1.24) | 0.75 (0.43 to 1.28) | 0.82 (0.47 to 1.44) | 0.88 (0.50 to 1.53) |
| Quartile 4 | >6.7 | 0.77 (0.49 to 1.21) | 0.68 (0.38 to 1.23) | 0.71 (0.38 to 1.30) | 0.78 (0.42 to 1.44) |
| P for trend | 0.53 | 0.58 | 0.60 | 0.75 | |
| Threshold model | |||||
| Quartile 1 | ≤4.1 | Reference | Reference | Reference | Reference |
| Quartile 2–4 | >4.1 | 0.76 (0.53 to 1.10) | 0.73 (0.46 to 1.15) | 0.77 (0.48 to 1.23) | 0.80 (0.50 to 1.28) |
| Cardiovascular mortality | |||||
| Continuous model | 1-mg/kg per minute increment | 0.90 (0.81 to 1.01) | 0.90 (0.78 to 1.04) | 0.95 (0.83 to 1.09) | 0.96 (0.83 to 1.10) |
| Multicategory model | |||||
| Quartile 1 | ≤4.1 | Reference | Reference | Reference | Reference |
| Quartile 2 | 4.1–5.3 | 0.63 (0.34 to 1.14) | 0.67 (0.34 to 1.31) | 0.73 (0.37 to 1.46) | 0.74 (0.37 to 1.48) |
| Quartile 3 | 5.3–6.7 | 0.49 (0.25 to 0.94) | 0.45 (0.20 to 0.99) | 0.60 (0.27 to 1.34) | 0.61 (0.27 to 1.36) |
| Quartile 4 | >6.7 | 0.53 (0.28 to 1.01) | 0.47 (0.20 to 1.08) | 0.64 (0.27 to 1.50) | 0.66 (0.28 to 1.55) |
| P for trend | 0.10 | 0.20 | 0.62 | 0.65 | |
| Threshold model | |||||
| Quartile 1 | ≤4.1 | Reference | Reference | Reference | Reference |
| Quartile 2–4 | >4.1 | 0.55 (0.34 to 0.89) | 0.56 (0.30 to 1.04) | 0.67 (0.36 to 1.26) | 0.68 (0.36 to 1.29) |
Covariance in adjusted model 1 includes age, body mass index, smoking status, and physical activity. Covariance in adjusted model 2 includes age, body mass index, smoking status, physical activity, cardiovascular disease, and UAER. Covariance in adjusted model 3 includes age, body mass index, smoking status, physical activity, cardiovascular disease, UAER, and eGFR. M, glucose disposal rate.
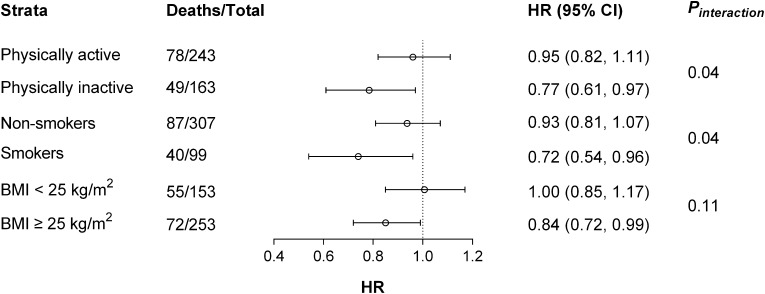
A significant interaction product term in mortality association was observed between M and physical activity or smoking (both P=0.04) together with a nonsignificant interaction term (P=0.11) containing M and BMI>25 kg/m2. In separated strata, M is an independent correlate of mortality among individuals with unhealthy lifestyles: smokers (HR, 0.72; 95% CI, 0.54 to 0.96), physically inactive individuals [HR, 0.77; 95% CI, 0.61 to 0.97), and individuals with BMI≥25 kg/m2 (HR, 0.84; 95% CI, 0.72 to 0.99). In nonsmokers, physically active individuals, and individuals with BMI<25 kg/m2, M was not associated with mortality (Figure 1).
Figure 1.
Association between insulin sensitivity and mortality in subgroup populations. All-cause mortality in relation to 1 mg/kg per minute increment of glucose disposal rate (M) in the study population as stratified by their physical activity, smoking status, and body mass index (BMI) categories. Represented are hazard ratios (HRs; circles) and 95% confidence intervals (95% CIs; error bars) of multivariable Cox regression analyses adjusted for age, the prevalence of cardiovascular disease, urinary albumin excretion rate, and eGFR. In addition, BMI as a continuous variable, smoking status, and physical activity were included in the corresponding models not stratified by those parameters.
Discussion
Our study has two main findings. First, reduced eGFR and various features of the metabolic syndrome were independently associated with impaired IS in nondiabetic elderly men with CKD stages 3 and 4. Second, although IS per se did not associate with total and cardiovascular mortality in this population, IS was a predictor of mortality in CKD individual strata with unhealthy but modifiable lifestyles.
Two important characteristics should be brought to the front when interpreting our findings. First, our cohort is a cohort of relatively healthy individuals, which is attributed in part to the lower prevalence of cardiovascular risk factors in Nordic countries and the nature of this screening program, where individuals more concerned about their health and lifestyle may have been prone to participate. This finding may explain, among others, the relatively normal range of UAER, despite reduced GFR and old age. Second, our cohort is a cohort of 70-year-old men, an age category that is becoming increasingly common among incident dialysis patients, but different from previous literature in the field. This homogeneity translated into an overall narrow eGFR range of individuals with CKD stages 3 and 4 in our study. Nonetheless, even in the narrow range, eGFR was independently associated with IS. This finding accords with the previous study by Kobayashi et al. (21) of 29 CKD individuals with varying degrees of renal function and widespread age as well as the work by Landau et al. (22) of 2418 individuals ages 70–79 years using HOMA-IR. Of interest, recent prospective cohort studies found impaired IS to be associated with a more rapid decline of renal function (23,24). In the present study, other risk factors, including BMI, physical activity, hypertension, and hyperlipidemia, emerged as independent contributors to the variance of IS, which is in line with the recommendation that obesity therapies (e.g., exercise) effectively improve IS (25). The impact of selected foods (e.g., fiber, sodium, and magnesium) on the modulation of IS has been proposed (16). In our study, however, energy-adjusted dietary fiber and sodium did not associate with IS. Additionally, aging and genetic factors are important causes of IR in the general population (7), and both inflammation (26,27) and albuminuria (28) are known risk factors for IR in nonrenal populations. However, despite decreased IL-6 and UAER levels across increasing M quartiles, they did not arise as independent predictors of IS in multivariable models. Again, this lack of association could be attributed to the homogeneity of the included population as per study design. However, it may also be possible that increased IL-6 and UAER levels in this setting represent the consequence of other risk factors, such as reduced renal function, obesity, and hypertension, which may be more strongly linked with IS.
We report that IS did not associate with mortality in elderly men with CKD stages 3 and 4. Likewise, a recent study also reported a lack of mortality association in community individuals with reduced renal function using the IS index derived from oral glucose tolerance tests (9). We are aware of only two previous studies that investigated a linear relationship between the level of IR and outcomes in CKD patients (5,8). First, the study by Shinohara et al. (5), based on HOMA-IR, reported a significant association of IS with cardiovascular mortality (22 events) in hemodialysis patients, but this association was seen only when threshold models were applied (top versus lower two tertiles). Second, Becker et al. (8) showed in logistic regressions that patients who had experienced cardiovascular events had significantly higher HOMA-IR at study inclusion. The study subjects of the above-mentioned studies were younger than those subjects included in our analysis. Our data, therefore, expand this knowledge in CKD stages 3 and 4, representing the largest sample size study of this kind so far. We speculate that coexistent well established risk factors (e.g., BMI, hypertension, and hyperlipidemia) might attenuate mortality association of M. Moreover, the fact that only 24% of the population was truly insulin-resistant (M<4 mg/kg per minute) may also prevent a positive mortality association.
The potential mechanisms linking IR and clinical outcomes are incompletely understood. Other than impaired insulin removal, risk factors leading to IR, such as obesity, sedentary lifestyle, and an unhealthy diet (29), are also highly prevalent among CKD patients. Thus, an important finding in our study is the observation that multiplicative interactions between IS and some of these modifiable risk factors (namely physical inactivity and smoking) may influence mortality. At a general population level, some studies have shown that components of the metabolic syndrome attenuate the relationship between HOMA-IR and outcomes as well (19,30), and it has, therefore, been suggested that other metabolic pathways may indirectly explain this link. Assuming that these associations are causal, the clinical applicability of our study is the identification of modifiable risk factors that can be targeted to improve IS and thereby, potentially attenuate the outcome implications of impaired IS in the CKD population. Supporting this concept, a recent community study showed that chronic cigarette smokers had a lower IS than nonsmokers and that IS improves, although it does not normalize, after 1–2 weeks of smoking cessation (31). Nevertheless, when interpreting these findings, it should be noted that, despite the overall relatively large sample size of the current study, it may not be powered enough to draw firm conclusions in substrata analysis, and additional studies are warranted to confirm this hypothesis.
The observational nature of our study does not allow us to establish causality in the associations observed. Other than the high prevalence of risk factors for IR in our population, CKD-specific causes of IR have also been reported, including post-translational defects, specific uremic toxins, metabolic acidosis, and vitamin D deficiency (32,33). Using the common belief (7), we assume in this study that CKD leads to the development of IR. However, the reverse is also possible (that is, that impaired IS promotes kidney disease through activation of the sympathetic nervous system, sodium retention, or decreased Na+/K+-ATPase activity) (34,35). Indeed, endoplasmic reticulum stress seems to be the factor linking inflammation and IR with proteinuria-induced podocyte damage, glomerulosclerosis, and tubulointerstitial damage (36). Thus, the hypothesis that impaired renal function may represent a sensitive marker of the adverse biologic pathways activated by IR (indirectly supported by our results of additional adjustment for renal function) should also be contemplated.
Because in normal conditions, 30%–80% of insulin in the circulation is removed by the kidney (37), the use of the gold standard euglycemic–hyperinsulinemic clamps in our study is a strength, because the assessment of IS was not subjected to bias by GFR (6). Using serum levels of cystatin C to estimate GFR in our cohort should also represent an advantage over creatinine assessments. Because of our inclusion criteria, our results are unbiased by important confounders of the associations studied, but they may not be representative of the general CKD stages 3 and 4 population. Future studies should confirm our findings in populations with broader ranges of age and GFR as well as both sexes.
To conclude, various components of the metabolic syndrome together with eGFR contributed to explain the variance of IS in elderly men with CKD stages 3 and 4. IS per se did not associate with mortality in this population, but it became an independent correlate of mortality in patient strata with unhealthy lifestyles. On the basis of these results, we speculate that increased physical activity and smoking cessation can be preventive strategies to improve IS in the CKD population, potentially attenuating the negative impact of reduced IS on clinical outcomes. Interventional studies addressing this hypothesis would be very attractive.
Disclosure
B.L. is affiliated with Baxter Healthcare. None of the other authors declares a conflict of interest.
Supplementary Material
Acknowledgments
We acknowledge research grants from the Swedish Research Council, the Strategic Research Programme in Diabetes at Karolinska Institutet, the Swedish Heart-Lung Foundation, the Center for Gender Medicine, and the Westman’s Foundation. Baxter Novum is the result of a grant from Baxter Healthcare Corporation to Karolinska Institutet.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05230513/-/DCSupplemental.
See related editorial, “Insulin Resistance in CKD,” on pages 638–640.
References
- 1.Zethelius B, Lithell H, Hales CN, Berne C: Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 48: 862–867, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr., Wilson PW: Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 54: 3252–3257, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE: Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116: 151–157, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F, American Heart Association. National Heart, Lung, and Blood Institute : Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, Miki T, Tabata T, Nishizawa Y: Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 13: 1894–1900, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Pham H, Robinson-Cohen C, Biggs ML, Ix JH, Mukamal KJ, Fried LF, Kestenbaum B, Siscovick DS, de Boer IH: Chronic kidney disease, insulin resistance, and incident diabetes in older adults. Clin J Am Soc Nephrol 7: 588–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham H, Utzschneider KM, de Boer IH: Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertens 20: 640–646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D, MMKD Study Group : Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The mild and moderate kidney disease study. J Am Soc Nephrol 16: 1091–1098, 2005 [DOI] [PubMed] [Google Scholar]
- 9.de Boer IH, Katz R, Chonchol MB, Fried LF, Ix JH, Kestenbaum B, Mukamal KJ, Peralta CA, Siscovick DS: Insulin resistance, cystatin C, and mortality among older adults. Diabetes Care 35: 1355–1360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[2 Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 13.Byberg L, Zethelius B, McKeigue PM, Lithell HO: Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 44: 2134–2139, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Larsson A, Malm J, Grubb A, Hansson LO: Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64: 25–30, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Becker W: Food Habits and Intake in Sweden 1989, Uppsala, Sweden: The Swedish National Food Administration, 1994 [Google Scholar]
- 16.Weickert MO: What dietary modification best improves insulin sensitivity and why? Clin Endocrinol (Oxf) 77: 508–512, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Howe GR, Kushi LH: Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65[4 Suppl]: 1220S–1228S, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Pollare T, Vessby B, Lithell H: Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler Thromb 11: 1192–1203, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Meigs JB, Rutter MK, Sullivan LM, Fox CS, D’Agostino RB, Sr., Wilson PW: Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care 30: 1219–1225, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hung AM, Sundell MB, Egbert P, Siew ED, Shintani A, Ellis CD, Bian A, Ikizler TA: A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol 6: 767–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T: Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Landau M, Kurella-Tamura M, Shlipak MG, Kanaya A, Strotmeyer E, Koster A, Satterfield S, Simsonick EM, Goodpaster B, Newman AB, Fried LF, Health, Aging and Body Composition Study : Correlates of insulin resistance in older individuals with and without kidney disease. Nephrol Dial Transplant 26: 2814–2819, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng HT, Huang JW, Chiang CK, Yen CJ, Hung KY, Wu KD: Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J Clin Endocrinol Metab 97: 1268–1276, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Nerpin E, Risérus U, Ingelsson E, Sundström J, Jobs M, Larsson A, Basu S, Arnlöv J: Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31: 1550–1555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH, American Heart Association Council on Nutrition, Physical Activity, and Metabolism : Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation 110: 2952–2967, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK: Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54: 2939–2945, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Zethelius B, Helmersson J, Berne C, Larsson A, Arnlöv J: Cytokine-mediated inflammation is independently associated with insulin sensitivity measured by the euglycemic insulin clamp in a community-based cohort of elderly men. Int J Clin Exp Med 4: 164–168, 2011 [PMC free article] [PubMed] [Google Scholar]
- 28.De Cosmo S, Menzaghi C, Prudente S, Trischitta V: Role of insulin resistance in kidney dysfunction: Insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 28: 29–36, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Trirogoff ML, Shintani A, Himmelfarb J, Ikizler TA: Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3-4 chronic kidney disease patients. Am J Clin Nutr 86: 1642–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Chien KL, Hsu HC, Su TC, Chen MF, Lee YT, Hu FB: Fasting and postchallenge hyperglycemia and risk of cardiovascular disease in Chinese: The Chin-Shan Community Cardiovascular Cohort study. Am Heart J 156: 996–1002, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Bergman BC, Perreault L, Hunerdosse D, Kerege A, Playdon M, Samek AM, Eckel RH: Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61: 3156–3166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegra V, Mengozzi G, Martimbianco L, Vasile A: Glucose-induced insulin secretion in uremia: Effects of aminophylline infusion and glucose loads. Kidney Int 38: 1146–1150, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Mak RH: Insulin resistance in uremia: Effect of dialysis modality. Pediatr Res 40: 304–308, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC: Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol 2012: 691369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Ottosson-Seeberger A, Alvestrand A: Renal hemodynamics and sodium handling in moderate renal insufficiency: The role of insulin resistance and dyslipidemia. J Am Soc Nephrol 5: 1751–1760, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Cybulsky AV: Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int 77: 187–193, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Duckworth WC, Bennett RG, Hamel FG: Insulin degradation: Progress and potential. Endocr Rev 19: 608–624, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.