Abstract
Background and objective
In the general population, metabolic syndrome (MES) is associated with cardiovascular risk. However, the definition of MES and its prognostic implication among patients undergoing peritoneal dialysis (PD) remain controversial.
Design, setting, participants, & measurements
We studied 329 prevalent PD patients from April 2008 to April 2011 and compared four sets of diagnostic criteria: the original World Health Organization (WHO) criteria, the International Diabetes Federation (IDF) criteria, the original National Cholesterol Education Program (NCEP) criteria, and the modified NCEP criteria. Nutritional status, body composition, and arterial pulse-wave velocity were measured. Patients were followed for 31.7±15.5 months.
Results
Among the 329 patients, 175 (53.2%) fulfilled the WHO criteria, 177 (53.8%) the IDF criteria, 199 (60.5%) the original NCEP criteria, and 218 (66.3%) the modified NCEP criteria. The agreement among the four sets of criteria was fair to moderate (Cohen κ=0.35–0.58). Patients with MES defined by all four criteria had higher adipose tissue mass than the others, although the difference in adipose tissue mass was most pronounced with the IDF criteria (MES versus no MES, 18.2±7.9 versus 10.7±5.9 kg; P<0.001). Patients with MES, as defined by the IDF criteria, were hospitalized longer than those without MES (3.82 [interquartile range, 0.00–12.61] versus 1.07 [interquartile range, 0.00–6.43]) days per year of follow-up; P=0.01). Overall survival, cardiovascular survival, or technique survival did not differ between patients with and without MES, irrespective of the diagnostic criteria after adjustment for diabetic status.
Conclusion
In patients undergoing PD, overall survival, cardiovascular survival, and technique survival did not differ between patients with and without MES, irrespective of diabetic status and diagnostic criteria. Further studies are needed to establish a new definition or clinical scoring system for risk stratification of PD patients.
Keywords: obesity, metabolism, cardiovascular disease
Introduction
Metabolic syndrome (MES) is a medical condition with clustering of major risk factors for cardiovascular diseases and type 2 diabetes (1). In the general population, MES is strongly associated with cardiovascular risk, mortality, diabetes mellitus, stroke, nonalcoholic fatty liver disease, and gout (2,3). More recently, the association of the MES with CKD is also emerging (3). Previous studies show that patients with MES have a higher risk of developing CKD (4–6). Among patients undergoing hemodialysis, MES has been reported to be associated with systemic inflammation (7) and predict hospitalization (8). More recently, the prevalence and prognostic implication of MES in patients undergoing peritoneal dialysis (PD) have attracted attention because, partly as a result of the glucose-containing dialysis solution, PD represents a special challenge to body metabolism. For example, Jiang et al. (9) noted that whereas 22% of patients with ESRD met the diagnostic criteria of MES before dialysis, nearly 70% exhibited MES after commencement of PD. In another study, Johnson et al. (10) reported that MES occurs in 30% of patients with stage 4 and 5 CKD and is associated with age, PD, increased oxidative stress, and an increased risk of future cardiovascular events.
Not only is MES prevalent in PD patients, but the presence of MES per se probably plays an important role in both macrovascular complications and endothelial dysfunction in these patients (11). In addition, the presence of MES is associated with changes in peritoneal solute clearance and solute transport rate (11), as well as arterial pulse-wave velocity (PWV) (12). In a study of 106 nondiabetic PD patients, Park et al. (13) note that nearly 50% had MES, and it was a significant independent predictor of mortality. In another study of 280 nondiabetic PD patients followed for an average of 48 months, Liao et al. (14) found that MES was independently associated with increased risk for cardiovascular death as well as fatal or nonfatal cardiovascular events. Similarly, Rasić et al. (15) noted that the overall prevalence of the MES was nearly 90% in PD patients and that MES represents an important risk factor for the high cardiovascular morbidity rate in these patients.
Unfortunately, the definition of MES is still debated, and no standardized definition has been established for MES as a cluster of risk factors for diabetes or cardiovascular disease in PD patients (16). Traditional criteria for MES may not apply to the PD population because these patients tend to have a larger waist circumference, their metabolic measures may be affected by glucose in the dialysis solution, and there is a confounding factor of excessive body fluid. In 1998, Alberti and Zimmet proposed, for the first time, a definition for MES for the World Health Organization (WHO) (17). In 2001, the National Cholesterol Education Program (NCEP) Expert Panel (Adult Treatment Panel [ATP] III) proposed a simple diagnostic criterion for clinical identification of MES in their third report (18). A modified NCEP III guideline for the diagnosis of MES in PD patients has also been proposed to circumvent the practical difficulty of measuring waist circumference in PD patients (19).
Materials and Methods
Patient Selection
We studied 329 prevalent PD patients from April 2008 to April 2011 in the dialysis unit of a single university hospital in Hong Kong, which is the only dialysis service provider within the region, with a population of 1.2 million. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, and all study procedures adhered to the Declaration of Helsinki. All patients provided inform consent to participate in the study. We excluded patients who had a failed kidney allograft, planned to have elective living donor transplant, or planned transfer to other renal center within 6 months. Clinical data were recorded by chart review. The modified Charlson comorbidity index, which has been validated in PD patients (20), was used to calculate a comorbidity score. Nutritional status was represented by serum albumin level, subjective global assessment (21), comprehensive malnutrition-inflammation score (MIS) (22), and normalized protein nitrogen appearance (23). The calculation of MIS has been described previously (22). Briefly, MIS consists of four main parts: patient’s related medical history, physical examination, body mass index, and laboratory measures, with 10 components in total. These components are scored from 0 (normal) to 3 (very severe); thus, the total score ranges from 0 to 30.
Definition of Metabolic Syndrome
We compared four sets of diagnostic criteria for MES: the original WHO criteria (17), the International Diabetes Federation (IDF) criteria (1), the original NCEP ATP III criteria (18,24), and the modified NCEP criteria (25). The details of these diagnostic criteria are summarized in Table 1. Waist circumference and waist-to-hip ratio were determined by conventional methods. The average of three BP measurements, each obtained at least 1 month apart, was used. Body weight was measured with a dry abdomen or with PD dialysate in abdomen minus the volume of PD dialysate infused, in liters (25). Fasting plasma glucose was measured after an overnight fast but with continuation of PD therapy with 1.5% dextrose dialysate (25).
Table 1.
Diagnostic criteria of metabolic syndrome
| Category | WHO, 1998 (15) | IDF, 2005 (1) | Original NCEP, 2001 (16,22) | Modified NCEP (23) |
|---|---|---|---|---|
| Insulin resistance | Known diabetes/ IFG/IGT or insulin resistance plus ≥2 criteria from the categories below | – | – | – |
| ≥3 of the criteria from the categories below | ≥3 criteria from the categories below | |||
| Obesity | BMI≥30 kg/m2 or waist-to-hip ratio >0.9 for men and >0.85 for women | Waist circumference ≥94 cm for men and ≥80 cm for women (a prerequisite) plus ≥2 of the criteria from the categories below | Waist circumference >102 cm for men and >88 cm for women | BMI>30 kg/m2 for white persons; >25 kg/m2 for Asian persons |
| Dyslipidemia | TG≥151 mg/dl or HDL-C<34.8 mg/dl for men or <38.7 mg/dl for women | TG ≥151 mg/dl | TG ≥151 mg/dl | TG ≥151 mg/dl |
| Low HDL-C<38.7 mg/dl in men or <50.3 mg/dl in women | Low HDL-C<38.7 mg/dl in men or <50.3 mg/dl in women | Low HDL-C<38.7 mg/dl in men or <50.3 mg/dl in women | ||
| Hypertension | Receiving treatment or BP≥140/90 mmHg | Receiving treatment or BP≥130/85 mmHg | Receiving treatment or BP≥130/85 mmHg | Receiving treatment or BP≥130/85 mmHg |
| Microalbuminuria | Urinary AER≥20 μg/min or ACR≥20 mg/g | |||
| Dysglycemia | FPG≥101 mg/dl | FPG≥101 mg/dl | FPG≥101 mg/dl or receiving treatment for diabetesa |
WHO, World Health Organization; IDF, International Diabetes Federation; NCEP, National Cholesterol Education Program; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; AER, albumin excretion rate; ACR, albumin-to-creatinine ratio; FPG, fasting plasma glucose.
Insulin or any oral hypoglycemic agent.
Measurement of Body Fat Content and Body Composition
We used bioimpedance spectroscopy (Body Composition Monitor, Fresenius Medical Care, Germany) to measure the body fluid and fat content. The method was described in our previous study (26). Briefly, electrodes were attached to one hand and one foot with the patient in a supine position. After the patient cable was connected, the measurement would complete automatically in 2 minutes. By measuring the electrical resistance to a wide range of frequencies, the volumes of total body water and the extracellular water could be determined. Adipose tissue mass and volume of overhydration could then be computed as secondary measures.
Arterial PWV
PWV, an index of aortic stiffness, was measured using an automatic computerized recorder, and the results were analyzed using the Complior SP program (Artech Medical, France). The method of PWV measurement was described previously (27). All measurements were performed by the same observer.
Clinical Follow-Up
All patients were followed till December 2012. Clinical management was decided by individual clinician and was not affected by the study. Although all clinicians have access to the biochemical and hospitalization data of each individual patient, the clinical record did not list the diagnosis of MES and the label “MES” was not routinely used in patient care. Outcome measures include patient survival, technique survival, number of hospital admissions, and total duration of hospitalization. Censoring events for patient survival include transfer to long-term hemodialysis, kidney transplant, recovery of renal function, loss to follow-up, and transfer to other dialysis centers. Death within 30 days of switching to hemodialysis was considered death on PD. Death, kidney transplantation, and permanent cessation of PD was defined as technique failure. Kidney transplantation was considered as an event for technique failure because some patients might have received priority organ allocation if they had impending technique failure.
Statistical Analyses
Statistical analyses were performed by SPSS for Windows software, version 15.0 (SPSS, Inc., Chicago, IL). Data are expressed as mean±SD or median (interquartile range [IQR]). Data were compared by t test, chi-squared test, or Pearson correlation coefficient as appropriate. Because the data on hospital admission and duration of hospitalization are highly skewed, they were compared between groups by the Mann–Whitney U test. Survival data are presented in the Kaplan–Meier plot and compared between groups by log-rank test. P<0.05 was considered to represent a statistically significant difference. All probabilities were two tailed.
Results
We studied total of 329 PD patients. The baseline clinical characteristics are summarized in Table 2 and baseline biochemical variables in Table 3. Among the 329 patients, 305 (92.7%) were receiving antihypertensive agents; 122 (37.1%) were treated with statins.
Table 2.
Baseline characteristics of the patients
| Characteristic | All Patients | Diabetic Patients | Nondiabetic Patients |
|---|---|---|---|
| Patients (n) | 329 | 133 | 196 |
| Men:women (n:n) | 165:164 | 74:59 | 91:105 |
| Age (yr)a | 59.8±12.3 | 64.6±9.8 | 56.5±12.7 |
| Body build | |||
| Height (cm) | 160.2±8.0 | 160.5±8.0 | 160.0±8.1 |
| Weight (kg)a | 62.1±12.4 | 66.0±12.5 | 59.4±11.6 |
| Body mass index (kg/m2)a | 24.1±3.9 | 25.5±3.5 | 23.1±3.9 |
| Waist circumference (cm)a | 90.3±10.8 | 95.3±9.7 | 86.9±10.1 |
| Hip circumference (cm)a | 96.4±8.2 | 99.3±8.1 | 94.5±7.7 |
| Waist-to-hip ratioa | 0.94±0.08 | 0.96±0.08 | 0.92±0.07 |
| Duration of dialysis (mo)a,b | 26.6 (13.3–57.0) | 21.9 (11.6–39.7) | 30.0 (14.6–66.2) |
| BP (mmHg) | |||
| Systolic | 141.0±22.0 | 143.1±23.5 | 139.5±20.8 |
| Diastolica | 75.3±12.0 | 71.2±11.2 | 78.1±11.7 |
| Diagnosis, n (%)a | |||
| GN | 104 (31.6) | 10 (7.5) | 94 (48.0) |
| Diabetes | 103 (31.3) | 103 (77.4) | 0 |
| Hypertension | 26 (7.9) | 6 (4.5) | 20 (10.2) |
| Polycystic | 15 (4.6) | 1 (0.8) | 14 (7.1) |
| Obstruction | 17 (5.2) | 2 (1.5) | 15 (7.7) |
| Others/unknown | 64 (19.5) | 11 (8.3) | 53 (27.1) |
| Major comorbidity | |||
| Diabetes, n (%)a | 133 (40.4) | 133 (100) | 0 |
| Coronary heart disease, n (%)a | 61 (18.5) | 39 (29.3) | 22 (11.2) |
| Cerebrovascular disease, n (%)a | 70 (21.3) | 41 (30.8) | 29 (14.8) |
| Charlson comorbidity index score | 5.6±2.6 | 7.6±2.1 | 4.2±1.9 |
Values expressed with a plus/minus sign are the mean±SD.
Significant difference between diabetic and nondiabetic patients.
Data presented as median (interquartile range).
Table 3.
Baseline biochemical profile of the patients
| Variable | All Patients | Diabetic Patients | Nondiabetic Patients |
|---|---|---|---|
| Patients (n) | 329 | 133 | 196 |
| Malnutrition inflammation scorea | 7.0±3.6 | 7.4±3.5 | 6.7±3.6 |
| Subjective global assessment | 5.4±0.9 | 5.4±0.9 | 5.5±0.9 |
| Hemoglobin (g/dl) | 9.20±1.67 | 9.25±1.48 | 9.17±1.79 |
| Serum albumin (g/L)a | 35.4±5.0 | 34.5±4.4 | 36.2±5.3 |
| Fasting plasma glucose (mg/dl)a | 112±42 | 129±58 | 101±18 |
| Lipid profile (mg/dl) | |||
| Total cholesterol | 200±50 | 195±60 | 203±42 |
| Triglyceridea | 168±105 | 189±115 | 154±94 |
| LDL cholesterola | 116±41 | 110±45 | 120±38 |
| HDL cholesterola | 50.3±19.3 | 45.3±20.0 | 53.8±17.8 |
| Total Kt/V | 2.01±0.72 | 2.03±1.00 | 1.99±0.47 |
| Residual GFR (ml/min per 1.73 m2)b | 1.12 (0.00–2.83) | 1.48 (0.00–3.50) | 0.83 (0.00–2.43) |
| NPNA (g/kg per day) | 1.12±0.23 | 1.15±0.26 | 1.10±0.21 |
| Peritoneal transport | |||
| D/P creatinine | 0.59±0.13 | 0.59±0.13 | 0.60±0.13 |
| MTAC creatinine (ml/min per 1.73 m2) | 8.26±4.13 | 7.96±3.76 | 8.45±4.36 |
| Body composition | |||
| Adipose tissue mass (kg)a | 14.5±7.9 | 16.2±6.4 | 13.4±8.8 |
| Overhydration (L)a | 2.7±2.9 | 4.3±3.3 | 1.6±2.0 |
| Carotid-femoral PWV (m/sec)a | 10.4±2.7 | 11.5±2.8 | 9.5±2.3 |
Values expressed with a plus/minus sign are the mean±SD. NPNA, normalized protein nitrogen appearance; D/P, dialysate-to-plasma concentration ratio of creatinine; MTAC, mass transfer area coefficient; PWV, pulse-wave velocity.
Significant difference between diabetic and nondiabetic patients.
Data presented as median (interquartile range).
Prevalence of MES
The prevalence of MES as defined by four sets of criteria was explored. Among the 329 patients, 175 (53.2%) fulfilled the WHO criteria, 177 (53.8%) the IDF criteria, 199 (60.5%) the original NCEP criteria, and 218 (66.3%) the modified NCEP criteria. With the original NCEP criteria, 2, 53, 75, 85, 71, and 43 patients fulfilled zero, one, two, three, four, and five criteria, respectively. With the modified NCEP criteria, the corresponding numbers of patients were 2, 38, 71, 89, 82, and 47.
In general, the agreement among the four sets of criteria was fair to moderate (Cohen κ=0.35–0.58, details not shown), except the original and modified NCEP criteria had good agreement (Cohen κ=0.80).
Relation with Body Fat Content and PWV
The body composition (adipose tissue mass and volume of overhydration as assessed by bioimpedance spectroscopy) and arterial PWV between patients with and without MES, as defined by the four sets of criteria, are compared and summarized in Table 4. In short, patients with MES as defined by any of the four criteria had higher adipose tissue mass than patients without MES, although the difference was most pronounced with the IDF criteria (18.2±7.9 versus 10.7±5.9 kg, P<0.001). Furthermore, the IDF criteria were least affected by the degree of overhydration, as assessed by bioimpedance spectroscopy. The result remained similar when only nondiabetic patients were analyzed, although the interference of overhydration was minimal in nondiabetic patients, irrespective of the criteria.
Table 4.
Comparison of body composition, peritoneal transport, and arterial pulse wave velocity between patients with and without metabolic syndrome
| Variable | All Patients | Nondiabetic Patients | ||||
|---|---|---|---|---|---|---|
| MES | No MES | P Value | MES | No MES | P Value | |
| Adipose tissue mass (kg) | ||||||
| WHO criteria | 15.6±7.9 | 13.1±7.8 | 0.07 | 14.2±11.3 | 13.1±7.8 | 0.6 |
| IDF criteria | 18.2±7.9 | 10.7±5.9 | <0.001 | 18.6±10.4 | 10.3±6.0 | <0.001 |
| Original NCEP criteria | 16.3±7.9 | 11.3±7.0 | 0.001 | 16.7±10.1 | 10.5±6.3 | 0.002 |
| Modified NCEP criteria | 15.9±7.9 | 11.3±7.0 | 0.002 | 16.0±9.9 | 10.5±6.3 | 0.01 |
| Overhydration (L) | ||||||
| WHO criteria | 3.7±3.2 | 1.5±2.0 | <0.001 | 1.8±2.1 | 1.5±2.0 | 0.7 |
| IDF criteria | 3.0±3.2 | 2.5±2.7 | 0.3 | 1.8±2.3 | 1.5±1.9 | 0.5 |
| Original NCEP criteria | 3.1±3.1 | 2.1±2.6 | 0.05 | 1.3±1.7 | 1.7±2.2 | 0.4 |
| Modified NCEP criteria | 3.3±3.1 | 1.4±1.9 | <0.001 | 1.8±2.1 | 1.4±1.9 | 0.5 |
| D/P at 4 hr | ||||||
| WHO criteria | 0.59±0.13 | 0.60±0.13 | 0.9 | 0.60±0.14 | 0.59±0.13 | 0.6 |
| IDF criteria | 0.58±0.12 | 0.61±0.14 | 0.02 | 0.58±0.12 | 0.61±0.13 | 0.13 |
| Original NCEP criteria | 0.58±0.12 | 0.61±0.14 | 0.06 | 0.58±0.12 | 0.61±0.14 | 0.13 |
| Modified NCEP criteria | 0.59±0.13 | 0.60±0.14 | 0.4 | 0.59±0.13 | 0.60±0.13 | 0.6 |
| MTAC creatinine (ml/min per 1.73 m2) | ||||||
| WHO criteria | 8.14±4.15 | 8.39±4.12 | 0.6 | 8.92±5.31 | 8.32±4.06 | 0.4 |
| IDF criteria | 7.66±3.75 | 8.95±4.44 | 0.01 | 7.78±4.06 | 8.91±4.51 | 0.08 |
| original NCEP criteria | 7.87±3.86 | 8.85±4.47 | 0.04 | 8.03±4.09 | 8.78±4.54 | 0.2 |
| modified NCEP criteria | 8.00±3.95 | 8.77±4.44 | 0.12 | 8.28±4.41 | 8.63±4.33 | 0.6 |
| Carotid-femoral PWV (m/sec) | ||||||
| WHO criteria | 11.0±2.9 | 9.6±2.3 | 0.001 | 9.1±2.4 | 9.6 ±- 2.3 | 0.3 |
| IDF criteria | 10.8±3.0 | 10.0±2.4 | 0.03 | 9.6±2.5 | 9.5±2.2 | 0.9 |
| Original NCEP criteria | 10.8±3.0 | 9.6±2.1 | 0.002 | 9.4±2.6 | 9.6±2.1 | 0.7 |
| Modified NCEP criteria | 10.7±2.9 | 9.7±2.4 | 0.02 | 9.4±2.5 | 9.7±2.1 | 0.5 |
Values expressed with a plus/minus sign are the mean±SD. MES, metabolic syndrome; WHO, World Health Organization; IDF, International Diabetes Federation; NCEP, National Cholesterol Education Program; D/P, dialysate-to-plasma creatinine ratio; MTAC, mass transfer area coefficient; PWV, pulse-wave velocity.
Patients with MES as defined by any of the four criteria had higher carotid-femoral PWV than patients without MES, indicating a higher degree of arterial stiffness (see Table 4). However, the difference becomes insignificant when only nondiabetic patients were analyzed.
Patient and Technique Survival
The median duration of follow-up was 32.4 months (IQR, 21.3–45.7 months). During this period, 97 patients died. The causes of death were coronary heart disease (21 cases), sudden cardiac arrest (13 cases), stroke (14 cases), peritonitis (21 cases), nonperitonitis infection (12 cases), termination of dialysis (11 cases), cancer (3 cases), and other specific causes (2 cases). During this period, another 28 patients were converted to hemodialysis, 22 had kidney transplantation, 5 were transferred to other dialysis centers, and 1 had recovery of kidney function.
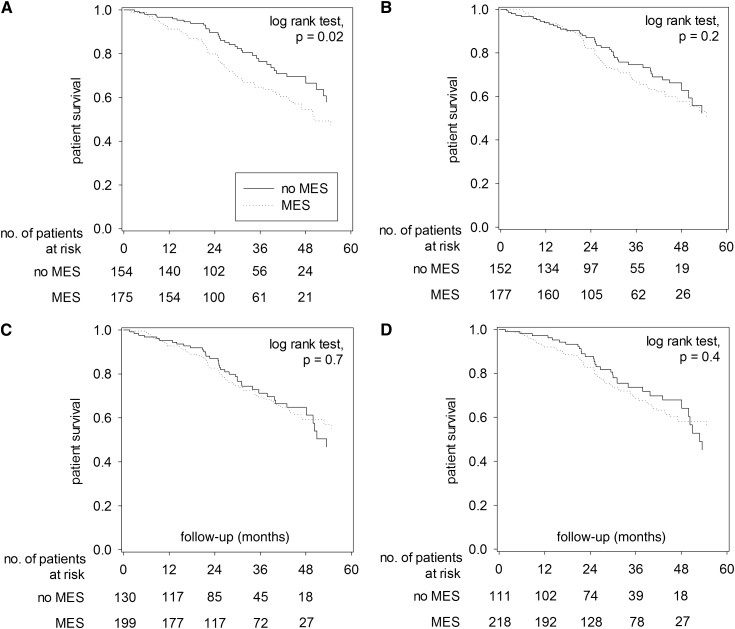
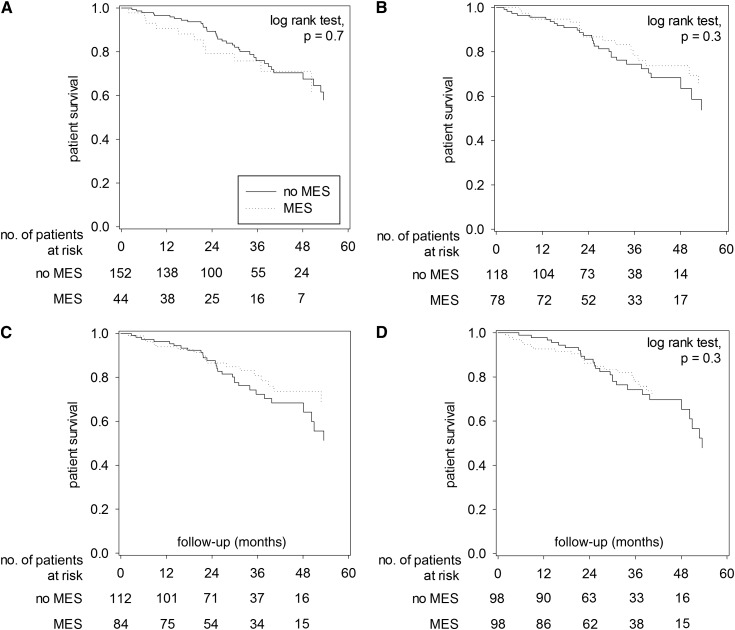
The Kaplan–Meier plots of overall survival, comparing patients with and without MES as defined by the four sets of criteria, are shown in Figure 1. When defined by the WHO criteria, patients with MES had lower overall survival rate than those without MES (4-year survival rate, 54.4% versus 66.6%; log-rank test, P=0.02) (Figure 1A). In contrast, patients with and without MES had similar overall survival when MES was defined by the IDF, original NCEP, or modified NCEP criteria. Furthermore, the effect of MES, as defined by the WHO criteria, on patient survival was due to the effect of diabetes. When only nondiabetic patients were analyzed, patients with and without MES had similar overall survival, irrespective of the diagnostic criteria used for defining MES (Figure 2). Similarly, patients with and without MES, irrespective of the diagnostic criteria, had similar cardiovascular survival and technique survival (details not shown). The result of analysis on technique survival remained similar when only deaths and cessation of PD were considered as technique failure (details not shown).
Figure 1.
Kaplan–Meier plots of patient survival for patients with and without metabolic syndrome (MES). Plots are defined by (A) World Health Organization criteria, (B) International Diabetes Federation criteria, (C) original National Cholesterol Education Program criteria, and (D) modified National Cholesterol Education Program criteria. Data are compared by log-rank test. When both diabetic and nondiabetic patients are analyzed together, there is no significant difference in patient survival between patients with and without MES, except when MES is defined by the World Health Organization criteria.
Figure 2.
Kaplan–Meier plots of patient survival for nondiabetic patients with and without metabolic syndrome (MES). Plots are defined by (A) World Health Organization criteria, (B) International Diabetes Federation criteria, (C) original National Cholesterol Education Program criteria, and (D) modified National Cholesterol Education Program criteria. Data are compared by log-rank test. When only nondiabetic patients are analyzed, there is no significant difference in patient survival between patients with and without MES, irrespective to the defining diagnostic criteria.
Hospitalization
During the follow-up period, there were a total of 1567 hospital admissions. The total duration of hospitalization was 9261 days. We compare the number of hospital admission and duration of hospitalization between patients with and without MES, and the result is summarized in Table 5. In short, patients with MES, as defined by any of the four sets of criteria, had significantly longer hospitalization than those without MES. Similarly, patients with MES had more hospital admission than those without MES, although the difference was not statistically significant when MES was defined by the IDF criteria. However, when only nondiabetic patients were analyzed, only patients with MES defined by the IDF and original NCEP criteria had longer hospitalization than those without MES. Specifically, patients with and without MES, as defined by the IDF criteria, were hospitalized for 3.82 (IQR, 0.00–12.61) and 1.07 (IQR, 0.00–6.43) days per year of follow-up, respectively (P=0.01).
Table 5.
Comparison of number of hospital admissions and duration of hospitalization between patients with and without metabolic syndrome
| Variable | All Patients | Nondiabetic Patients | ||||
|---|---|---|---|---|---|---|
| MES | No MES | P Value | MES | No MES | P Value | |
| Hospital admissions (n/yr follow-up) | ||||||
| WHO criteria | 1.52 (0.00–3.15) | 0.54 (0.00–1.79) | <0.001 | 0.73 (0.00–2.62) | 0.53 (0.00–1.79) | 0.3 |
| IDF criteria | 1.24 (0.00–2.63) | 0.61 (0.00–2.45) | 0.12 | 1.06 (0.00–2.30) | 0.38 (0.00–1.38) | 0.06 |
| Original NCEP criteria | 1.24 (0.00–2.81) | 0.56 (0.00–2.15) | 0.01 | 0.73 (0.00–2.16) | 0.53 (0.00–1.80) | 0.12 |
| Modified NCEP criteria | 1.24 (0.00–2.81) | 0.53 (0.00–2.15) | 0.01 | 0.64 (0.00–2.16) | 0.53 (0.00–1.73) | 0.16 |
| Duration of hospitalization (d/yr follow-up) | ||||||
| WHO criteria | 6.02 (0.00–22.20) | 1.58 (0.00–8.35) | <0.001 | 2.51 (0.00–14.93) | 1.51 (0.00–8.45) | 0.2 |
| IDF criteria | 5.06 (0.00–16.26) | 2.38 (0.00–12.37) | 0.04 | 3.82 (0.00–12.61) | 1.07 (0.00–6.43) | 0.01 |
| Original NCEP criteria | 5.26 (0.00–19.18) | 2.27 (0.00–8.61) | 0.01 | 2.25 (0.00–13.16) | 1.28 (0.00–6.52) | 0.05 |
| Modified NCEP criteria | 4.92 (0.00–17.77) | 2.25 (0.00–8.93) | 0.01 | 1.84 (0.00–12.36) | 1.64 (0.00–8.35) | 0.18 |
Data are presented as median (interquartile range) and compared by the Mann–Whitney U test. MES, metabolic syndrome; WHO, World Health Organization; IDF, International Diabetes Federation; NCEP, National Cholesterol Education Program.
Discussion
In the present study, we found that all four sets of diagnostic criteria for MES could identify PD patients with excessive adipose tissue mass, although the higher body weight of patients with MES was often attributable to excess body water. Although MES is associated with a higher arterial PWV, it appears to be largely the effect of diabetes. We did not find that MES affected patient survival, cardiovascular survival, or technique survival of Chinese PD patients. However, patients with MES, especially when defined by the IDF or original NCEP criteria, had a longer duration of hospitalization compared with patients without MES.
The definition of MES in PD patients has been difficult and controversial (16,25). In the original WHO criteria (17), diabetes or insulin resistance is a prerequisite, which favors a selection bias toward high-risk cases irrespective of the metabolic problem. The IDF criteria put central obesity, as defined by the waist circumference, as the prerequisite (1), which may be more akin to the original idea of Reaven's syndrome X (28). In contrast, the original NCEP criteria (18,24) aim to be generic and use a scoring system rather than having any specific prerequisite condition. In theory, this approach would allow a more flexible definition of MES. To complicate the issue, waist circumference, which is used for the definition of obesity in both the IDF and original NCEP criteria, could not be measured conveniently in PD patients as a result of the intraperitoneal infusion of dialysis solution; thus, it has been proposed that body mass index should be used instead (25). Nonetheless, no study has determined the internal consistency or compared the prognostic value of different sets of criteria. Our results indicate that the agreement among these criteria is modest and that the IDF criteria are probably the preferred choice because they are less affected by overhydration and have the best association with hospitalization during follow-up. Although the modified NCEP criteria avoid the difficulty of measuring waist circumference by using body mass index for the definition of obesity, our result indicates that the IDF and original NCEP criteria are more clinically relevant, partly because measuring waist circumference is less likely to mistake fluid overload as obesity.
The incidence of MES among our cohort of PD patients is similar to that noted in previous reports (9,10,13,14). Unlike previous studies (11,12), we found that the correlation between MES and peritoneal transport or arterial PWV was at best modest and was not statistically significant when only nondiabetic patients were analyzed.
Contrary to the reports of Liao et al. (14) and Rasić et al. (15), we did not find a difference in all-cause mortality or cardiovascular mortality between patients with and without MES. Nonetheless, our result is consistent with the body of literature, all of which show that a higher body mass index is associated with lower mortality in the dialysis population (29–31). Furthermore, several recent studies showed that in nonrenal populations, MES does not predict the development of cardiovascular events with greater power than some of its individual components (32,33). In patients with CKD, MES as a syndrome may be even less relevant because CKD is inherently linked to so many of the MES components (34,35).
Although we did not find a higher mortality in PD patients with MES, we did observe a longer duration of hospitalization during follow-up in this group of patients (see Table 5), which is in line with the previous report by Rasić et al. (15). Unfortunately, we did not determine the causes of hospitalization in this study, and it remains uncertain whether the excessive hospitalization in patients with MES was the result of a higher incidence of cardiovascular disease. Of note, however, the difference in number of hospital admission was actually less pronounced compared with the difference in duration of hospitalization, indicating that PD patients with MES need to stay in the hospital longer for each admission. Further studies are needed to test whether PD patients with MES have more nosocomial complications, which may account for the excessive hospital stay.
Our study had several limitations. First, the sample size may not have been sufficient to compare one set of criteria to another, and the duration of follow-up may not have been long enough to determine the effect of MES on survival. As mentioned above, we did not determine the causes of hospitalization in this study. Our previous study suggests that nearly half of the hospital admissions of our PD patients are due to cardiovascular diseases (36). Unfortunately, the cause of hospital admission could be multiple in many patients, and additional hospital stay is often the result of unrelated reasons (e.g., nosocomial complications). It is therefore difficult to analyze the data on hospitalization for cardiovascular problems.
Because of limitations in the original study design, we did not explore the interaction between MES and other traditional or nontraditional cardiovascular risk factors, such as inflammation, protein energy wasting, and hyperhomocysteinemia. Since >90% of our patients were hypertensive, it was impossible to determine the effect of hypertension on patient outcome. It would be interesting to explore whether the associations between MES and clinical outcomes indeed differ from the associations between individual MES components, and further study is needed to answer this question. Although we measured arterial PWV as a surrogate marker of vascular stiffness, we have no data on the actual degree of endothelial dysfunction or atherosclerosis. In this study, we used bioimpedance spectroscopy to measure the adipose tissue mass. However, the accuracy of adipose tissue mass as measured by bioimpedance spectroscopy remains uncertain. More important, our study shows that MES, irrespective of the diagnostic criteria, may not provide much prognostic information. Further studies are urgently needed to establish a new definition or clinical scoring system for risk stratification of PD patients.
Disclosures
C.C.S. reported receiving research grant and consultancy from Baxter Healthcare.
Acknowledgments
This study was supported in part by the Chinese University of Hong Kong research account 6901031.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group : The metabolic syndrome—a new worldwide definition. Lancet 366: 1059–1062, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Kari JA: Metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens 22: 198–203, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 6: 2364–2373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K: Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int 69: 369–374, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Shahrokh S, Heydarian P, Ahmadi F, Saddadi F, Razeghi E: Association of inflammatory biomarkers with metabolic syndrome in hemodialysis patients. Ren Fail 34: 1109–1113, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Yang SY, Chiang CK, Hsu SP, Peng YS, Pai MF, Ho TI, Hung KY, Wu KD: Metabolic syndrome predicts hospitalization in hemodialysis patients: A prospective Asian cohort study. Blood Purif 25: 252–259, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Jiang N, Qian J, Lin A, Lindholm B, Axelsson J, Yao Q: Initiation of glucose-based peritoneal dialysis is associated with increased prevalence of metabolic syndrome in non-diabetic patients with end-stage renal disease. Blood Purif 26: 423–428, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, Prins JB, Isbel NM: Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 12: 391–398, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chen HY, Kao TW, Huang JW, Chu TS, Wu KD: Correlation of metabolic syndrome with residual renal function, solute transport rate and peritoneal solute clearance in chronic peritoneal dialysis patients. Blood Purif 26: 138–144, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Zhe XW, Zeng J, Tian XK, Chen W, Gu Y, Cheng LT, Chen HM, Axelsson J, Lindholm B, Wang T: Pulse wave velocity is associated with metabolic syndrome components in CAPD patients. Am J Nephrol 28: 641–646, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Park JT, Chang TI, Kim DK, Lee JE, Choi HY, Kim HW, Chang JH, Park SY, Kim E, Yoo TH, Han DS, Kang SW: Metabolic syndrome predicts mortality in non-diabetic patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 25: 599–604, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Liao CT, Kao TW, Chou YH, Wu MS, Chen YM, Chuang HF, Hung KY, Chu TS, Wu KD, Tsai TJ: Associations of metabolic syndrome and its components with cardiovascular outcomes among non-diabetic patients undergoing maintenance peritoneal dialysis. Nephrol Dial Transplant 26: 4047–4054, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Rasić S, Hadzović-Dzuvo A, Rebić D, Uncanin S, Hadzić A, Mujaković A, Kulenović I: The metabolic syndrome in patients on peritoneal dialysis: Prevalence and influence on cardiovascular morbidity. Bosn J Basic Med Sci 10[Suppl 1]: S3–S7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SH, Lindholm B: Definition of metabolic syndrome in peritoneal dialysis. Perit Dial Int 29[Suppl 2]: S137–S144, 2009 [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Li PK, Kwan BC, Ko GT, Chow KM, Leung CB, Szeto CC: Treatment of metabolic syndrome in peritoneal dialysis patients. Perit Dial Int 29[Suppl 2]: S149–S152, 2009 [PubMed] [Google Scholar]
- 20.Beddhu S, Zeidel ML, Saul M, Seddon P, Samore MH, Stoddard GJ, Bruns FJ: The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am J Med 112: 696–701, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Enia G, Sicuso C, Alati G, Zoccali C: Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant 8: 1094–1098, 1993 [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Bergström J, Heimbürger O, Lindholm B: Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int 18: 467–473, 1998 [PubMed] [Google Scholar]
- 24.Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association. European Association for the Study of Diabetes : The metabolic syndrome: time for a critical appraisal: Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28: 2289–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Li PK, Kwan BC, Szeto CC, Ko GT: Metabolic syndrome in peritoneal dialysis patients. Clin Kidney J 4: 206–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwan BC, Szeto CC, Chow KM, Law MC, Cheng MS, Leung CB, Pang WF, Kwong VW, Li PK: Bioimpedance spectroscopy for the detection of fluid verload in Chinese peritoneal dialysis patients. Perit Dial Int, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao N, Kwan BC, Chow KM, Chung KY, Pang WF, Leung CB, Li PK, Szeto CC: Arterial pulse wave velocity and peritoneal transport characteristics independently predict hospitalization in Chinese peritoneal dialysis patients. Perit Dial Int 30: 80–85, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hansen BC: The metabolic syndrome X. Ann N Y Acad Sci 892: 1–24, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Beddhu S, Pappas LM, Ramkumar N, Samore M: Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14: 2366–2372, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA: Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 13: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL: Body mass index, dialysis modality, and survival: Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65: 597–605, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW, Lennon L, Macfarlane PW, Murphy MB, Packard CJ, Stott DJ, Westendorp RG, Whincup PH, Shepherd J, Wannamethee SG: Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet 371: 1927–1935, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM: Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 27: 2676–2681, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Raimundo M, Lopes JA. Metabolic syndrome, chronic kidney disease, and cardiovascular disease: A dynamic and life-threatening triad. Cardiol Res Pract 2011; 2011: 747861. [DOI] [PMC free article] [PubMed]
- 35.Yu M, Ryu DR, Kim SJ, Choi KB, Kang DH: Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: Results from the Korean National Health and Nutrition Examination Survey. Nephrol Dial Transplant 25: 469–477, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Szeto CC, Wong TY, Chow KM, Leung CB, Li PK: Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: A randomized placebo-control trial. J Am Soc Nephrol 14: 2119–2126, 2003 [DOI] [PubMed] [Google Scholar]