Abstract
Background and objectives
Geographic variation in stroke rates is well established in the general population, with higher rates in the South than in other areas of the United States. A similar pattern of geographic variation in ischemic strokes has also recently been reported in patients undergoing long-term dialysis, but whether this is also the case for hemorrhagic stroke is unknown.
Design, setting, participants, & measurements
Medicare claims from 2000 to 2005 were used to ascertain hemorrhagic stroke events in a large cohort of incident dialysis patients. A Poisson generalized linear mixed model was generated to determine factors associated with stroke and to ascertain state-by-state geographic variability in stroke rates by generating observed-to-expected (O/E) adjusted rate ratios (ARRs) for stroke.
Results
A total of 265,685 Medicare-eligible incident dialysis patients were studied. During a median follow-up of 15.5 months, 2397 (0.9%) patients sustained a hemorrhagic stroke. African Americans (ARR, 1.43; 95% confidence interval [CI], 1.30 to 1.57), Hispanics (ARR, 1.78; 95% CI, 1.57 to 2.03), and individuals of other races (ARR, 1.51; 95% CI, 1.26 to 1.80) had a significantly higher risk for hemorrhagic stroke compared with whites. In models adjusted for age and sex, four states had O/E ARRs for hemorrhagic stroke that were significantly greater than 1.0 (California, 1.15; Maryland, 1.25; North Carolina, 1.25; Texas, 1.19), while only 1 had an ARR less than 1.0 (Wisconsin, 0.79). However, after adjustment for race and ethnicity, no states had ARRs that varied significantly from 1.0.
Conclusion
Race and ethnicity, or other factors that covary with these, appear to explain a substantial portion of state-by-state geographic variation in hemorrhagic stroke. This finding suggests that the factors underlying the high rate of hemorrhagic strokes in dialysis patients are likely to be system-wide and that further investigations into regional variations in clinical practices are unlikely to identify large opportunities for preventive interventions for this disorder.
Keywords: cardiovascular disease, chronic dialysis, clinical epidemiology
Introduction
Stroke is substantially more common in patients undergoing long-term dialysis than in the general population (1). Hemorrhagic stroke, in particular, confers a major burden on both affected individuals and society as a whole (2–5). In the general population, older age, male sex, and hypertension emerge as consistent risk factors for hemorrhagic stroke (6–9). Improved understanding of the factors associated with hemorrhagic stroke in the dialysis population, which is at particularly high risk of bleeding by virtue of regular exposure to heparin and consequences of the uremic milieu itself (10), might therefore inform surveillance or education measures designed to improve patient care.
We have recently reported the existence of an ischemic “Stroke Belt” in United States dialysis patients, centered in the southern states (11). That report focused on ischemic stroke, which is approximately 4.5-fold more common than hemorrhagic stroke in United States dialysis patients (12). However, because hemorrhagic stroke differs substantially from ischemic stroke in its pathophysiology (4,5), it is uncertain whether findings associated with the latter would be associated with the former. Although important work has previously been undertaken examining the epidemiology of hemorrhagic stroke in dialysis patients (13–15), no report to date has specifically examined geographic variation in hemorrhagic stroke rates and how race and ethnicity might affect any such potential association. Race and ethnicity, which are characterized not only by differences in genetics and biology but also by variation in environmental, psychosocial, cultural, and behavioral factors, may affect access to and deliverance of care (16,17).
Accordingly, we sought to investigate whether specific demographic, anthropometric, functional status, comorbidity, or geographic factors might be associated with hemorrhagic stroke in dialysis patients. We were also particularly interested in whether state-by-state geographic variation exists for hemorrhagic stroke and, if so, whether race and ethnicity might partially account for such a finding, given that older data suggest that hemorrhagic stroke rates are higher in individuals of African descent than in those of European descent (7). Using data from the US Renal Data System (USRDS), Medicare, and Medicaid, we studied a large cohort of incident dialysis patients. Our underlying hypothesis was that if major regional differences in hemorrhagic stroke persisted after controlling for these patient-specific factors, this might indicate an opportunity to explore regional differences in treatment practices that might lead to opportunities to improve efforts to prevent this catastrophic condition.
Materials and Methods
Study Design and Data Sources for Analysis
We performed a retrospective cohort analysis of incident, Medicare-eligible long-term dialysis patients and a secondary analysis in a cohort of dually eligible (Medicare and Medicaid) patients. The latter patients are a particularly vulnerable group who have lower socioeconomic status, which enables us to minimize the potential confounding effects of this factor. Our approach to data acquisition and cohort creation has been described previously (18,19), and further detail can be found in the Supplemental Appendix. We used the USRDS, a national system that collects data on almost all patients undergoing long-term dialysis in the United States. To make possible the study of dually eligible individuals, the USRDS performed a deterministic match of these Medicaid beneficiaries against the core USRDS files to identify dually eligible individuals receiving long-term dialysis.
Study Cohorts and Rationale for Analytic Approach
The cohorts consisted of individuals >18 years of age who initiated long-term dialysis on or after January 1, 2000, and before October 2, 2005. Participants had survived at least 90 days after the initiation of dialysis, at which time (day 91) the observation window commenced, and they had to be continuously enrolled in Medicare (primary analysis) or both Medicare and Medicaid (secondary analysis) upon initiation. To ensure that all Medicare claims were observable, we studied only individuals who were Medicare-eligible from the time of dialysis initiation and for whom Medicare was the primary payer. Individuals were censored at the time that they lost Medicare eligibility, at the time of kidney transplantation, or at the time of death, if any of these occurred. Otherwise, they were censored on the last day of follow-up (December 31, 2005). For the dually eligible cohort (Medicare plus Medicaid) subgroup analysis, individuals were censored when they lost either coverage.
Covariates and Descriptive Variables
Demographic, comorbidity, and clinical variables were drawn from the Centers for Medicare & Medicare Services (CMS) 2728 dialysis intake form. Details of our approach to comorbid conditions have been described before (18,19) and are outlined in the Supplemental Appendix. Because previous stroke is a strong predictor of future stroke, we examined stroke claims in the first 90-day run-in period before the start of the observation window; an individual was considered to have a preexisting or “prevalent” stroke if a cerebrovascular accident (CVA, either ischemic or hemorrhagic) was declared on the CMS 2728 form as having occurred before dialysis initiation or if a stroke appeared in claims during the first 90 days of dialysis.
Hemorrhagic strokes occurring after this period (i.e., during the observation window) were considered new strokes. Additionally, we supplemented the medical information from the CMS 2728 form with a modified form of the Liu comorbidity index (a summary measure of comorbidity burden), whereby evidence from claims is used to supplement the CMS 2728 comorbid conditions (20).
Stroke Outcomes
Our primary outcome was hemorrhagic stroke, as indicated by International Classification of Diseases, Ninth Revision, codes 430 and 431. These codes have been shown to be highly specific for identifying hemorrhagic strokes (21). A sensitivity analysis, using a much more inclusive definition for hemorrhagic stroke, added codes 432, 852.0, 852.2, 852.4, and 853.
Statistical Analyses
Person-Level Analyses.
We generated descriptive statistics to illustrate how individuals who experienced hemorrhagic strokes differed from those who did not. Bivariate analyses comparing each of the explanatory variables by hemorrhagic stroke versus nonstroke were performed using Pearson chi-squared test or t test, as appropriate. To identify factors associated with hemorrhagic stroke, we generated a multilevel Poisson model using generalized linear mixed modeling (GLMM) (22) with hemorrhagic stroke rate (number of strokes per unit of exposure time) being regressed simultaneously on all a priori selected explanatory variables as fixed effects and with state modeled as a random intercept. Although cause of ESRD was not included among these a priori selected variables, we included, in modified form, the Liu comorbidity index (20), which incorporates cause of ESRD. The Poisson model fit was assessed by ensuring that the ratio of the generalized chi-squared test statistic to its degrees of freedom was close to 1.
State-by-State Differences in Hemorrhagic Stroke.
After developing the Poisson GLMM, we examined the variability in stroke rates by state of residence and compared states. For each state, we determined whether the observed number of incident strokes, called the “observed” (O) value, was above or below the “expected” (E) value given the total exposure time for persons belonging to that state. The random-effect estimates for each state calculated by our model facilitated the O/E rate ratio comparisons. Specifically, we obtained the estimates of the random effects for each state because these parameters modify each state’s log-rates of ischemic stroke from the overall cross-state (fixed) model effects. Exponentiation of these estimates generated state-specific observed versus expected (O/E) adjusted rate ratios (ARRs) adjusted for the effect of other covariates. Using the estimated SEMs of the prediction, we estimated confidence intervals for these state-specific O/E rate ratios. Because of the large sample size of our cohort, statistical significance was inferred only when the P value was <0.01. All statistical analyses were done with SAS software, version 9.2 (SAS Institute, Inc.). We then sequentially adjusted for age; then age and sex; then for age, sex, and race; and, finally, for all factors based on our GLMM, which accounted for individual-level characteristics.
Sensitivity Analyses
To examine the robustness of our results, we performed several sensitivity analyses. First, as stated above, we repeated the analysis with a more sensitive method for identifying hemorrhagic stroke (21). Second, we performed the analysis with the modified Liu comorbidity index (20) as a summary measure of overall illness burden. Third, we performed an analysis in the cohort of dually eligible (Medicare and Medicaid) dialysis patients, which creates a more homogenous cohort based on socioeconomic status (23). Finally, to provide possible insights as to whether it might be racial milieu, rather than individual-level race, that might be responsible for the association of interest, we substituted state-level race distribution for race/ethnicity at the individual level. That is, we computed the racial distribution of the dialysis patients within each state at the midpoint of the cohort and modeled this rather than individual-level race.
Compliance and Protection of Human Research Participants
The institutional review board at the University of Kansas Medical Center approved the study. The work was undertaken in accordance with the principles of the Declarations of Helsinki. Data use agreements between University of Kansas Medical Center and the USRDS and CMS were in place.
Results
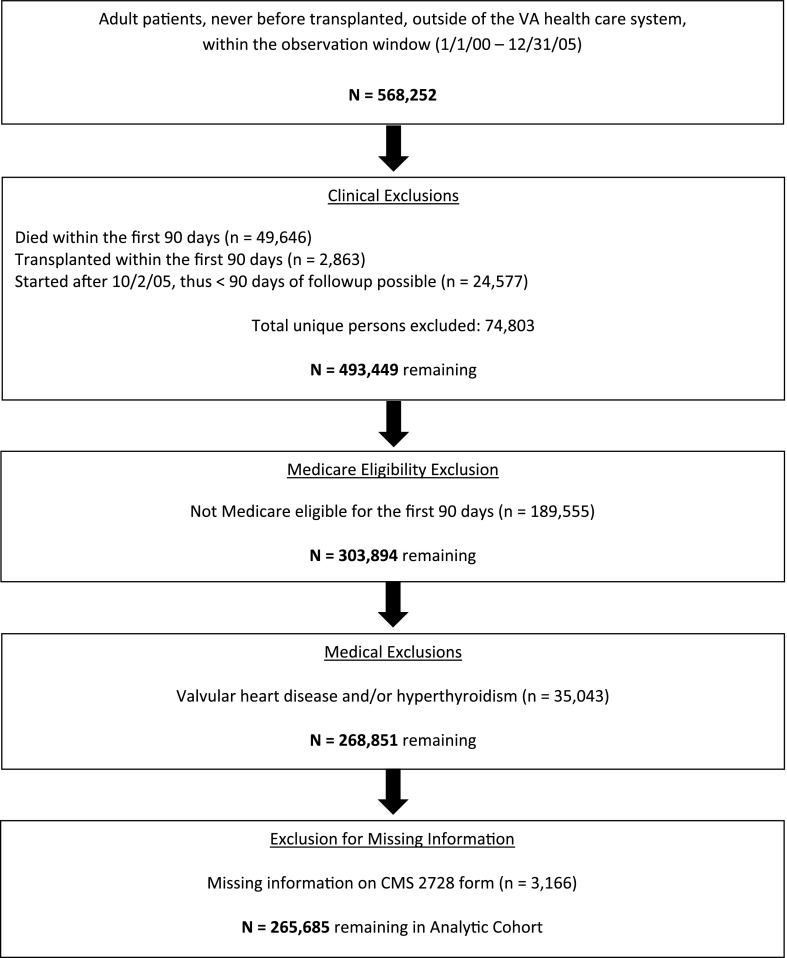
Figure 1 shows the construction of the Medicare-eligible cohort and the numbers excluded at successive stages. A total of 265,685 Medicare-eligible individuals initiated dialysis between January 1, 2000, and October 2, 2005, who survived at least 90 days prior to of the end of the follow-up period (December 31, 2005), and who were not excluded for various causes.
Figure 1.
Exclusion flowchart demonstrates the creation of the study cohort.
The characteristics of the Medicare-eligible cohort are shown in Table 1. Mean age±SD was 64.7±15.1 years, 52.9% of patients were male, and whites made up the largest group at 54.9%, followed by African-Americans at 30.0%. Diabetes, at 47.2%, was the leading cause of ESRD. In terms of comorbid conditions, 84.4% of patients had hypertension, 33.2% had heart failure, and 10.3% had a history of a CVA upon dialysis initiation. Over 93% were undergoing in-center hemodialysis. During a median follow-up period of 15.5 months (25% and 75% percentiles, 6.1 and 30.3 months, respectively), 2397 (0.9%) of the patients sustained a hemorrhagic stroke. Of those who had hemorrhagic strokes, 98.2% had a single event; 43 individuals (1.8%) had two, and only 1 had three; no patient had more than three hemorrhagic strokes. There were a total of 2442 events in 437,817.7 patient-years, for 5.6 hemorrhagic strokes per 1000 patient-years. Bivariate analyses between individuals who did and did not have hemorrhagic strokes during the observation period revealed significant differences (P<0.05) for all covariates examined.
Table 1.
Descriptive characteristics of the Medicare-eligible cohort
| Characteristics | All | Hemorrhagic Stroke | No Hemorrhagic Stroke | P Valuea |
|---|---|---|---|---|
| Patients (n) | 265,685 | 2397 | 263,288 | |
| Age (yr) | 64.7±15.1 | 63.1±14.3 | 64.7±15.2 | <0.001 |
| Men, n (%) | 140,599 (52.9) | 1,202 (50.2) | 139,397 (52.9) | 0.006 |
| Race/ethnicity, n (%) | <0.001 | |||
| African American | 79,693 (30.0) | 896 (37.4) | 78,797 (29.9) | |
| White | 145,768 (54.9) | 987 (41.2) | 144,781 (55.0) | |
| Hispanic | 27,738 (10.4) | 370 (15.4) | 27,368 (10.4) | |
| Other | 12,486 (4.7) | 144 (6.0) | 12,342 (4.7) | |
| Body mass index category, n (%) | <0.001 | |||
| <20 kg/m2 | 25,710 (9.7) | 318 (13.3) | 25,392 (9.6) | |
| 20–24.9 kg/m2 | 84,154 (31.7) | 877 (36.6) | 83,277 (31.6) | |
| 25–29.9 kg/m2 | 76,043 (28.6) | 669 (27.9) | 75,374 (28.6) | |
| ≥30 kg/m2 | 79,778 (30.0) | 533 (22.2) | 79,245 (30.1) | |
| Smoker, n (%) | 14,893 (5.6) | 158 (6.6) | 14,735 (5.6) | 0.04 |
| Substance abuser, n (%) | 5730 (2.2) | 117 (4.9) | 5613 (2.1) | <0.001 |
| Unemployed, n (%) | 252,749 (95.1) | 2318 (96.7) | 250,431 (95.1) | <0.001 |
| Unable to ambulate, n (%) | 11,443 (4.3) | 109 (4.6) | 11,334 (4.3) | 0.54 |
| Unable to transfer, n (%) | 4075 (1.5) | 38 (1.6) | 4037 (1.5) | 0.80 |
| In-center hemodialysis, n (%)b | 247,584 (93.2) | 2292 (95.6) | 245,292 (93.2) | <0.001 |
| Hemoglobin < 11.0 g/dl, n (%) | 176,664 (72.9) | 1706 (77.8) | 174,958 (72.8) | <0.001 |
| Comorbid conditions, n (%) | ||||
| Hypertension | 224,159 (84.4) | 2096 (87.4) | 222,063 (84.3) | <0.001 |
| Diabetes mellitus | 141,067 (53.1) | 1328 (55.4) | 139,739 (53.1) | 0.02 |
| Congestive heart failure | 88,226 (33.2) | 701 (29.2) | 87,525 (33.2) | <0.001 |
| Coronary artery disease | 74,734 (28.1) | 552 (23.0) | 74,182 (28.2) | <0.001 |
| Peripheral vascular disease | 40,662 (15.3) | 305 (12.7) | 40,357 (15.3) | <0.001 |
| Prior cerebrovascular accident | 27,304 (10.3) | 416 (17.4) | 26,888 (10.2) | <0.001 |
| Liu comorbidity score | 5.1±2.8 | 4.9±2.6 | 5.1±2.8 | 0.01 |
| Cause of ESRD, n (%) | <0.001 | |||
| Diabetes | 125,220 (47.2) | 1209 (50.4) | 124,011 (47.1) | |
| Hypertension | 70,117 (26.4) | 690 (28.8) | 69,427 (26.4) | |
| Glomerulonephritis | 22,817 (8.6) | 169 (7.1) | 22,648 (8.6) | |
| Other | 47,531 (17.9) | 329 (13.7) | 47,202 (17.9) |
P value is for the comparison between individuals who did and did not experience a hemorrhagic stroke.
In-center hemodialysis is contrasted to self-care dialysis, which consists of peritoneal dialysis and home
hemodialysis.
Person-Level Factors Associated with Hemorrhagic Stroke
Table 2 shows factors that, after multivariable adjustment, were independently associated with hemorrhagic stroke occurrence. Older age, female sex, lower body mass index, substance abuse, unemployed status, and being initiated on in-center hemodialysis were associated with hemorrhagic stroke. Comorbid conditions of atrial fibrillation, hypertension, and history of a CVA were also significantly associated with hemorrhagic stroke, while the presence of heart failure and coronary artery disease were inversely associated with hemorrhagic stroke risk. In terms of race and ethnicity, compared with whites, hemorrhagic stroke was significantly higher in African Americans (ARR, 1.43; 95% CI, 1.30 to 1.57), Hispanics (ARR, 1.78; 95% CI, 1.57 to 2.03), and members of other races (ARR, 1.51; 95% CI, 1.26 to 1.80), with P<0.001 in each case.
Table 2.
Generalized linear mixed model illustrating patient-level factors independently associated with hemorrhagic stroke in the Medicare-eligible cohort
| Variable | ARR (95% CI) | P Value |
|---|---|---|
| Age, per decade | 1.08 (1.06 to 1.10) | <0.001 |
| Female sex | 1.15 (1.06 to 1.25) | <0.001 |
| Race/ethnicity | ||
| White | Reference | |
| African American | 1.43 (1.30 to 1.57) | <0.001 |
| Hispanic | 1.78 (1.57 to 2.03) | <0.001 |
| Other | 1.51 (1.26 to 1.80) | <0.001 |
| Body mass index category | ||
| <20 kg/m2 | 1.18 (1.04 to 1.34) | 0.01 |
| 20–24.9 kg/m2 | Reference | |
| 25–29.9 kg/m2 | 0.84 (0.76 to 0.93) | <0.001 |
| ≥30 kg/m2 | 0.61 (0.55 to 0.68) | <0.001 |
| Smoker | 1.03 (0.87 to 1.22) | 0.70 |
| Substance abuser | 1.99 (1.63 to 2.42) | <0.001 |
| Unemployed | 1.49 (1.19 to 1.87) | <0.001 |
| Inability to ambulate | 1.01 (0.80 to 1.27) | 0.97 |
| Inability to transfer | 0.95 (0.65 to 1.39) | 0.79 |
| In-center hemodialysisa | 1.50 (1.23 to 1.83) | <0.001 |
| Comorbid conditions | ||
| Atrial fibrillation | 1.13 (1.00 to 1.28) | 0.05 |
| Diabetes mellitus | 1.02 (0.88 to 1.18) | 0.83 |
| Hypertension | 1.19 (1.05 to 1.35) | 0.006 |
| Congestive heart failure | 0.87 (0.79 to 0.96) | 0.005 |
| Coronary artery disease | 0.84 (0.75 to 0.93) | 0.008 |
| Peripheral vascular disease | 0.83 (0.73 to 0.94) | 0.003 |
| Prior cerebrovascular accident | 1.94 (1.74 to 2.17) | <0.001 |
| Cause of ESRD | ||
| Diabetes | 1.14 (1.01 to 1.27) | 0.03 |
| Hypertension | 1.04 (0.95 to 1.14) | 0.39 |
| Glomerulonephritis | 0.88 (0.77 to 1.00) | 0.06 |
| Other | Reference |
ARR, adjusted rate ratio; 95% CI, 95% confidence interval.
In-center hemodialysis is contrasted to self-care dialysis, which consists of peritoneal dialysis and home hemodialysis.
Geographic Factors Associated with Hemorrhagic Stroke
Table 3 demonstrates geographic variation in hemorrhagic stroke under various modeling strategies. An unadjusted model is followed by models adjusted for age; then age and sex; then age, sex, and race/ethnicity; and finally for all factors in Table 2. As can be seen, in each of the first three models, California, Maryland, North Carolina, and Texas all had O/E ratios statistically significantly greater than 1.0; after incorporation of race/ethnicity, no state had an O/E ratio significantly greater than 1.0. Wisconsin and Ohio had O/E ratios statistically significantly lower than 1.0 in the unadjusted model, but only Wisconsin had an O/E ratio significantly less than 1.0 in the subsequent models. After race/ethnicity was incorporated, no state had an O/E ratio statistically significantly different from 1.0. We then incorporated into the model all other factors in Table 2; results were unchanged.
Table 3.
States with observed-to-expected adjusted rate ratios for hemorrhagic stroke, after successive adjustments, in the Medicare-eligible cohort
| State | Unadjusted | Observed-to-Expected ARR (95% CI) | ||
|---|---|---|---|---|
| Age | Age and Sex | Age, Sex, and Race/Ethnicity | ||
| CA | 1.16 (1.02 to 1.33)a | 1.15 (1.01 to 1.30)a | 1.15 (1.01 to 1.31)a | 1.00 (0.90 to 1.12) |
| MD | 1.27 (1.03 to 1.56)a | 1.25 (1.03 to 1.53)a | 1.25 (1.03 to 1.53)a | 1.11 (0.96 to 1.29) |
| NC | 1.29 (1.08 to 1.52)a | 1.26 (1.07 to 1.49)a | 1.25 (1.06 to 1.48)a | 1.13 (0.99 to 1.29) |
| TX | 1.22 (1.07 to 1.40)a | 1.20 (1.05 to 1.36)a | 1.19 (1.05 to 1.35)a | 1.02 (0.91 to 1.14) |
| OH | 0.82 (0.68 to 0.99)a | 0.83 (0.69 to 1.00) | 0.83 (0.69 to 1.00) | 0.92 (0.80 to 1.06) |
| WI | 0.77 (0.60 to 0.98)a | 0.79 (0.62 to 0.99)a | 0.79 (0.62 to 0.99)a | 0.91 (0.78 to 1.07) |
Results not adjusted for the other covariates in Table 2. After adjustment for age, sex, and race/ethnicity, further covariate adjustment did not alter results. ARR, adjusted rate ratio; CI, confidence interval; CA, California; MD, Maryland; NC, North Carolina; TX, Texas; OH, Ohio; WI, Wisconsin.
Statistically significant at P<0.05.
Several sensitivity analyses demonstrated generally similar findings. With use of a more sensitive definition of hemorrhagic stroke, only California, Maryland, and Texas had initial O/E ratios statistically significantly greater than 1.0 (i.e., North Carolina no longer appeared in this group); as before, once race/ethnicity was incorporated, no state had an O/E ratio significantly greater than 1.0. Indiana joined Ohio and Wisconsin as having O/E ratios statistically significantly below 1.0 in the initial models, but, as before, the incorporation of race/ethnicity resulted in no state having an O/E ratio significantly below unity. A second analysis using the dually eligible patient population demonstrated findings that tended to bolster those of the primary analysis. This is shown in Supplemental Table 1. Because the dually eligible study sample was only 26% as large as the Medicare sample, our ability to observe statistically significant differences, if they existed, was reduced, and so the more sensitive hemorrhagic stroke definition was used. Only California had an O/E ratio statistically significantly greater than 1.0 in the initial models; this was no longer the case when race and ethnicity were taken into account. The states with the next-highest (but not statistically significant) O/E ratios were Maryland and Texas, constituting a pattern broadly consistent with that of the Medicare patients. However, of note, the point estimates in the dually eligible sample generally clustered closer to unity than did those in the Medicare sample.
Finally, we substituted race distribution by state in place of individual-level race and found similar effects for race. However, when we modeled both individual-level and state racial distribution simultaneously, the former maintained statistical significance while the latter no longer did, suggesting that individual-level race is the better marker by which to capture the effects of race/ethnicity.
Discussion
In this study, we sought to determine whether there was geographic variability in hemorrhagic stroke rates at the United States state level among patients undergoing long-term dialysis, and, if so, whether any factors, particularly race and ethnicity, might be associated with such variability. Using a large cohort of incident dialysis patients, we found some states to have hemorrhagic stroke rates that differed significantly from unity in the unadjusted analysis. After adjustment for age and then sex, as has been done in previous studies examining geographic variation in stroke rates (24), our findings persisted. However, after race/ethnicity was incorporated in the analyses, no state differed significantly from unity, suggesting that race/ethnicity, or factors that covary with these, appear to explain a substantial portion of state-by-state geographic variation in hemorrhagic stroke in United States dialysis patients. This finding was concordant with our person-level model in which African Americans, Hispanics, and individuals of other races had, approximately, 1.4-, 1.8-, and 1.5-fold increases in hemorrhagic stroke compared with whites, even after adjustment for a wide range of other factors.
We have recently reported the existence of an ischemic “Stroke Belt” in dialysis patients centered on the southern United States (11). This same basic finding was present when we combined ischemic and hemorrhagic strokes in the outcome of “total strokes” (authors’ unpublished data). However, given substantial pathophysiologic differences between ischemic and hemorrhagic strokes, it was unclear whether geographic variability in hemorrhagic strokes, if it existed, would mimic that of ischemic strokes. Although the association of minority race with hemorrhagic stroke is far from universal (8,9), we nevertheless hypothesized that race and ethnicity might at least partially account for any findings that initially appeared attributable to geography.
The present findings must be contextualized in view of the population studied. Our primary analysis examined individuals who had Medicare eligibility upon dialysis initiation. Therefore, this group, whose mean age was slightly under 65 years, is composed primarily of individuals who were >65 years of age when dialysis commenced or were younger and had Medicare by virtue of a substantial preexisting disability. Both types of individuals would probably have more comorbid conditions, on average, than other incident dialysis patients. Our sensitivity analysis examined individuals with Medicaid, which is a program for medically needy, low-income individuals; these individuals are, on average, roughly 10 years younger than individuals with Medicare alone (23). Although Medicaid eligibility varies substantially by state (18), we nevertheless specifically selected this group for analysis in an attempt to create a cohort that was more socioeconomically homogeneous. While this cohort was only about one quarter the size of the Medicare cohort, our findings were broadly similar to those of the larger cohort. The results did not reach statistical significance (probably attributable to lack of power), but inspection of the point estimates suggests that socioeconomic factors could still account for some degree of geographic variation. Because the point estimates of some states in the fully adjusted model using the Medicare cohort were higher than the analogous results obtained using the dually eligible cohort, it appears possible that socioeconomic status (as indicated by eligibility for Medicaid) may be partially responsible for state-by-state variation in hemorrhagic stroke rates. This possibility warrants further scrutiny.
One potential reason for our findings might be differences in medication use by race. For example, it may actually be warfarin use rates that are responsible for our findings, because systemic anticoagulation is a risk factor for hemorrhagic stroke. However, for this to explain our findings, African Americans (and, presumably, other minority groups) would have to be treated with warfarin at higher rates than whites for disorders such as chronic atrial fibrillation, a phenomenon that would not be expected given what has been published on differences in treatment rates, by race, for a wide variety of conditions (25–35). If anything, the potential that warfarin treatment rates are lower in minorities would be expected to strengthen the association we report.
Beyond race/ethnicity, we found many expected associations between various risk factors and hemorrhagic strokes in our analysis, bolstering the study’s face validity. Older age was associated with hemorrhagic stroke, as was history of a CVA. Hypertension, present in most (approximately 85%) individuals in our cohort, was also associated with hemorrhagic stroke risk, as would be expected. The presence of congestive heart failure and its antecedent condition, coronary artery disease, was inversely associated with hemorrhagic stroke risk, probably because individuals with these conditions tend to have lower BP by the time they are receiving dialysis. Notably, lower body mass index was associated with hemorrhagic stroke, a finding that was also in evidence in ischemic strokes (11) and that may be attributable to paradoxical associations (36).
Our findings should be interpreted in the context of the study’s limitations. First, our outcomes were based on claims, rather than on clinical data (such as chart reviews, type of dialysis access, brain imaging, or laboratory results). For example, information on the severity or location (intra-axial versus extra-axial) of stroke is not available. Thus, our claims-based approach, although widely used (21), is imperfect, and there are acknowledged limitations in the CMS 2728 form (37). However, it seems unlikely that this would introduce a bias by state or, if present, that such a bias would be sufficiently large to account for the effects we attribute to race and ethnicity. Second, our unit of geographic variation, namely United States state, was relatively large, meaning that fine resolution of geographic variation, such as at that which might be present at the level of a county or postal code, was not possible using our analytic strategy. The limited number of hemorrhagic strokes meant that a higher degree of resolution might not have been informative. By the same token, estimates generated from states with smaller dialysis populations are vulnerable to instability because a relatively small number of events can exert disproportionate influence in the O/E ratios. Third, our primary analysis used only individuals who were Medicare-eligible from the start of dialysis. As such, caution should be exercised in attempting to extrapolate our results to other populations, such as individuals with private insurance, those with insurance coverage via the Department of Veterans Affairs, and those who acquire Medicare eligibility later in the course of dialysis. Fourth, we are lacking information on exposure to warfarin, which is likely to be an important hemorrhagic stroke risk factor. We have used a claims-based definition of chronic atrial fibrillation (19) as a rough proxy for both that condition itself and potential “treatments” for it, such as warfarin. However, any discussion of the role chronic atrial fibrillation might play in hemorrhagic stroke risk is unsatisfactory unless warfarin use can be fully accounted for. Finally, observational studies invariably suffer from residual confounding and competing risks. These limitations are probably counterbalanced by the large sample size, the richness of the data used, and our use of multiple sensitivity analyses, which bolstered the findings of the primary analysis and improved generalizability.
In conclusion, we initially found apparent geographic variability in hemorrhagic stroke rates by state in patients undergoing long-term dialysis. However, nonwhites in the United States are at a higher risk of hemorrhagic stroke than are whites, and adjustment for race and ethnicity accounted for a substantial portion of this geographic variation. This suggests that race and ethnicity, or factors for which these may be proxies, largely explain observations that initially appear to be geographic in nature. Even so, the possibility that socioeconomic factors are also associated with some level of variation in hemorrhagic stroke risk warrants further investigation. Future studies should explore whether race and ethnicity are markers of other hemorrhagic stroke risk factors and how practices that are common across geographic boundaries influence hemorrhagic stroke in dialysis patients, including issues related to use of anticoagulants.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Disease) grants K23-DK085378-01 (J.B.W.) and R01-DK080111-02 (T.I.S.), by a National Kidney Foundation Young Investigator Award (J.B.W.), and by a Sandra A. Daugherty Foundation Grant (J.B.W.).
The data reported here have been supplied by the USRDS (DUA #2007-10 & 2009-19) and the CMS (DUA #19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06980713/-/DCSupplemental.
References
- 1.United States Renal Data System: USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Tomaszewski A, Kutarski A, Tomaszewski M: [Fibrotic tissue reflecting lead course after percutaneous leads extraction]. Kardiol Pol 69: 619–620, 2011 [PubMed] [Google Scholar]
- 3.Xu PQ, Gong JP: [Effect of all trans retinoid acid (ATRA) on differentiation and apoptosis of HL-60 cell]. Chin J Cancer 23: 118–123, 2004 [PubMed]
- 4.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G, Investigators R, REGARDS Investigators : Association of race and sex with risk of incident acute coronary heart disease events. JAMA 308: 1768–1774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw FE: Long-term mortality after stroke among adults aged 18 to 50 years. JAMA 309: 1136–1144, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA: Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 24: 1166–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero JR, Wolf PA: Epidemiology of Stroke: Legacy of the Framingham Heart Study. Global heart 8: 67-75, 2013 [DOI] [PMC free article] [PubMed]
- 8.Baber U, Gutierrez OM, Levitan EB, Warnock DG, Farkouh ME, Tonelli M, Safford MM, Muntner P: Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am Heart J 166: 373-380 e372, 2013 [DOI] [PMC free article] [PubMed]
- 9.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, Zhou X, Mukhopadhyay P, Shireman TI: Stroke and the “stroke belt” in dialysis: Contribution of patient characteristics to ischemic stroke rate and its geographic variation. J Am Soc Nephrol 24: 2053–2061, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, Spertus JA, Zhou X, Hou Q, Shireman TI: Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 23: 112–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO: Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 64: 603–609, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO: Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol 14: 2623–2631, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Power A, Chan K, Singh SK, Taube D, Duncan N: Appraising stroke risk in maintenance hemodialysis patients: A large single-center cohort study. Am J Kidney Dis 59: 249–257, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Nicholas SB, Kalantar-Zadeh K, Norris KC: Racial disparities in kidney disease outcomes. Semin Nephrol 33: 409–415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler SB, Reeder-Hayes KE, Carey LA: Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist 18: 986–993, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI: Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis 58: 73–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI: The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 81: 469–476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH: A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 100–128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCulloch C, Searle S: Generalized, Linear, and Mixed Models, New York, John Wiley & Sons, Inc., 2001 [Google Scholar]
- 23.Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI: Considering health insurance: How do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant 25: 198–205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard G, Labarthe DR, Hu J, Yoon S, Howard VJ: Regional differences in African Americans’ high risk for stroke: The remarkable burden of stroke for Southern African Americans. Ann Epidemiol 17: 689–696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonel AF, Good CB, Mulgund J, Roe MT, Gibler WB, Smith SC, Jr, Cohen MG, Pollack CV, Jr, Ohman EM, Peterson ED, CRUSADE Investigators : Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: Insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 111: 1225–1232, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Sheats N, Lin Y, Zhao W, Cheek DE, Lackland DT, Egan BM: Prevalence, treatment, and control of hypertension among African Americans and Caucasians at primary care sites for medically under-served patients. Ethn Dis 15: 25–32, 2005 [PubMed] [Google Scholar]
- 27.Riehle JF, Lackland DT, Okonofua EC, Hendrix KH, Egan BM: Ethnic differences in the treatment and control of hypertension in patients with diabetes. J Clin Hypertens (Greenwich) 7: 445–454, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC: Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf 19: 834–842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olomu AB, Grzybowski M, Ramanath VS, Rogers AM, Vautaw BM, Chen B, Roychoudhury C, Jackson EA, Eagle KA, American College of Cardiology Foundation Guidelines Applied in Practice Steering Committee : Evidence of disparity in the application of quality improvement efforts for the treatment of acute myocardial infarction: The American College of Cardiology’s Guidelines Applied in Practice Initiative in Michigan. Am Heart J 159: 377–384, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Vulic D, Lee BT, Dede J, Lopez VA, Wong ND: Extent of control of cardiovascular risk factors and adherence to recommended therapies in US multiethnic adults with coronary heart disease: From a 2005-2006 national survey. Am J Cardiovasc Drugs 10: 109–114, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Mehta JL, Bursac Z, Mehta P, Bansal D, Fink L, Marsh J, Sukhija R, Sachdeva R: Racial disparities in prescriptions for cardioprotective drugs and cardiac outcomes in Veterans Affairs Hospitals. Am J Cardiol 105: 1019–1023, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Poon I, Lal LS, Ford ME, Braun UK: Racial/ethnic disparities in medication use among veterans with hypertension and dementia: A national cohort study. Ann Pharmacother 43: 185–193, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Litaker D, Koroukian S, Frolkis JP, Aron DC: Disparities among the disadvantaged: Variation in lipid management in the Ohio Medicaid program. Prev Med 42: 313–315, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM: Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis 49: 276–283, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopson S, Frankenfield D, Rocco M, McClellan W: Variability in reasons for hemodialysis catheter use by race, sex, and geography: Findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis 52: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K: What is so bad about reverse epidemiology anyway? Semin Dial 20: 593–601, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Eggers PW: CMS 2728: What good is it? Clin J Am Soc Nephrol 5: 1908–1909, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.