Abstract
Background and objectives
The benefit of the initiation of dialysis for AKI may differ depending on patient factors, but, because of a lack of robust evidence, the decision to initiate dialysis for AKI remains subjective in many cases. Prior studies examining dialysis initiation for AKI have examined outcomes of dialyzed patients compared with other dialyzed patients with different characteristics. Without an adequate nondialyzed control group, these studies cannot provide information on the benefit of dialysis initiation. To determine which patients would benefit from initiation of dialysis for AKI, a propensity-matched cohort study was performed among a large population of patients with severe AKI.
Design, setting, participants, & measurements
Adults admitted to one of three acute care hospitals within the University of Pennsylvania Health System from January 1, 2004, to August 31, 2010, who subsequently developed severe AKI were included (n=6119). Of these, 602 received dialysis. Demographic, clinical, and laboratory variables were used to generate a time-varying propensity score representing the daily probability of initiation of dialysis for AKI. Not-yet-dialyzed patients were matched to each dialyzed patient according to day of AKI and propensity score. Proportional hazards analysis was used to compare time to all-cause mortality among dialyzed versus nondialyzed patients across a spectrum of prespecified variables.
Results
After propensity score matching, covariates were well balanced between the groups, and the overall hazard ratio for death in dialyzed versus nondialyzed patients was 1.01 (95% confidence interval, 0.85 to 1.21; P=0.89). Serum creatinine concentration modified the association between dialysis and survival, with a 20% (95% confidence interval, 9% to 30%) greater survival benefit from dialysis for each 1-mg/dl increase in serum creatinine concentration (P=0.001). This finding persisted after adjustment for markers of disease severity. Dialysis initiation was associated with more benefit than harm at a creatinine concentration≥3.8 mg/dl.
Conclusions
Dialysis was associated with increased survival when initiated in patients with AKI who have a more elevated creatinine level but was associated with increased mortality when initiated in patients with lower creatinine concentrations.
Introduction
The decision to initiate dialysis for AKI is affected by multiple factors (1), is difficult for physicians and families, and is costly to society (2,3). While certain emergency indications for dialysis exist, clinicians may initiate dialysis for metabolic derangements that could resolve in the absence of therapy (4). Initiating dialysis early in the course of AKI may have potential benefits, including increased control of volume status and metabolic derangements, and may allow for more robust nutritional supplementation (5). But early initiation carries the risk of greater exposure to the dialysis membrane and dialysis catheter and may provoke hypotension, leading to delays in recovery of renal function (6,7). Pursuing the hypothesis that dialysis does not have a mortality benefit among all individuals with AKI, we created a propensity-matched cohort of dialyzed and nondialyzed patients to identify modifiers of the effects of dialysis on mortality within a population experiencing AKI.
Materials and Methods
Patients
Patients were drawn from the University of Pennsylvania Health System AKI cohort (8). All inpatient admissions to any of three University of Pennsylvania Health System hospitals from January 1, 2004, to August 31, 2010 (n=518,089), were examined. Adults were included if their baseline creatinine (defined as the lowest serum creatinine concentration within 48 hours of hospital admission) was ≤1.4 mg/dl for men and ≤1.2 mg/dl for women, and subsequently doubled during the hospital admission. This threshold was chosen to identify a population who sustained a significant decrement in renal function. Patients receiving dialysis within 24 hours of admission or those receiving a renal transplant during hospitalization were excluded. If a patient met inclusion criteria in more than one hospitalization in the dataset, only the first hospitalization was used. The Institutional Review Board of the University of Pennsylvania approved this study. A waiver of informed consent was granted.
Definition of an Episode of AKI
AKI onset was defined by the Acute Kidney Injury Network stage 1 creatinine criteria (a 50% increase in serum creatinine or absolute increase of 0.3 mg/dl within 48 hours [9]) during the first episode of AKI in which the creatinine doubled. An episode of AKI was considered terminated once the serum creatinine decreased to within 10% of the established baseline value.
Covariate Ascertainment
Race was determined by self-report. Comorbidity information was assembled using International Classification of Disease, Ninth Revision (ICD-9) codes, as documented on admission by a billing database and by using the enhanced ICD-9 scheme developed and validated by Quan et al. (10) All data from the comprehensive electronic medical record from each hospitalization were available for analysis. This included laboratory data obtained during routine clinical care; medication orders, including dates of initiation and discontinuation; and procedural data, including transfusion, intubation, extubation, and dialysis initiation. Daily sequential organ failure assessment (SOFA) scores were calculated but excluded the neurologic and renal components (11). Estimated GFR was calculated using the CKD-Epidemiology Collaboration equation (12).
Dialysis therapy was considered initiated on the first day that an order for hemodialysis, continuous venovenous hemodialysis, or continuous venovenous hemofiltration was recorded. Isolated ultrafiltration and slow continuous ultrafiltration are not included in the definition. Slow low-efficiency dialysis and peritoneal dialysis are not used for AKI at the study institutions.
Propensity-Score Matching
Patients were assigned a propensity score to initiate dialysis on each day from the onset of AKI until 14 days later or until the day of initiation of dialysis therapy. Propensity to initiate dialysis was estimated using a logistic regression model. Model selection was performed using stepwise regression retaining covariates with P≤0.20.
On the day of initiation of dialysis, dialyzed patients were matched to not-yet-dialyzed patients with the same duration of AKI (in days) in a greedy-nearest-neighbor fashion using a 0.1-SD caliper to the propensity score. We ensured that matched patients had AKI for the same duration of time before matching to eliminate survivorship bias. To recapitulate the design of a randomized trial, patients who would receive dialysis later in their hospital course were still eligible to be matched to dialyzed patients if they had not yet initiated dialysis.
Statistical Analyses
Continuous covariates are described in terms of means±SDs or medians and interquartile ranges depending on skewness. Unadjusted associations between categorical outcomes and continuous variables were assessed using the t test or the Wilcoxon rank-sum test as appropriate, and unadjusted associations between categorical variables were assessed using the chi-squared test in the full cohort. In the matched cohort, unadjusted comparisons were performed via paired t tests, sign-rank tests, and McNemar tests as appropriate.
In the matched cohort, Cox proportional hazards regression, stratified by matched pairs, was used to assess associations between covariates and mortality rate. The proportional hazards assumption was evaluated using Schoenfeld residuals and log-log survival plots. Logistic regression was used to assess associations between covariates and 90-day mortality. To explore factors associated with serum creatinine concentration, equal-sized tertiles were created. All statistical tests were performed using Stata software, version 12.1 (Stata Corp., College Station, TX).
Primary Outcome
The primary outcome was time to all-cause mortality. Time zero was defined as the date of initiation of dialysis or matching. Date of death was determined from hospital discharge records for patients who died during their hospitalization and, with the Social Security Death Master File, was matched by last name and Social Security number, for those who survived to hospital discharge. Modifiers of the dialysis-mortality relationship were assessed by including interaction terms in the Cox model. The threshold creatinine level at which the hazard ratio (HR) for dialysis versus no dialysis fell below 1 was calculated on the basis of a linear combination of coefficients from the interaction model. Our specific a priori hypotheses were that dialysis therapy would confer particular benefit in patients with higher BUN, higher serum creatinine, younger age, shorter AKI duration, and greater propensity to receive dialysis.
Results
A total of 6119 patients with in-hospital AKI met entry criteria for the full cohort, and 602 (9.8%) of these went on to receive dialysis within 14 days of meeting AKI criteria. Of the 602, 401 began receiving continuous venovenous hemodialysis and 201 began receiving hemodialysis. Median follow-up was 263 days (range, 1–2429 days). In-hospital, 90-day, and 1-year mortality rates were 27%, 34%, and 41%, respectively, in the overall cohort and 64%, 67%, and 79% among patients who received dialysis.
Baseline characteristics of patients, stratified by receipt of dialysis therapy, are presented in Table 1. The mean age±SD of the cohort was 60.9±16.3 years. Fifty-three percent of patients were men. Table 2 presents the results of our propensity model and reports multivariable-adjusted odds ratios for factors associated with the initiation of dialysis on any given day according to daily covariates. The final model contains the 32 variables listed. As might be expected, the initiation of dialysis was strongly associated with higher concentrations of serum creatinine, potassium, and BUN. Male sex and black race were negatively associated with initiation of dialysis, as was the presence of a do-not-resuscitate order. Sunday (versus other days of the week) was also negatively associated with initiation of dialysis. The area under the receiver-operator characteristic curve was 0.97 (95% confidence interval [95% CI], 0.97 to 0.98), indicating that the model had outstanding predictive ability.
Table 1.
Baseline (hospital admission) characteristics and unadjusted analyses of dialyzed versus nondialyzed patients in the University of Pennsylvania Health System AKI Cohort
| Characteristic | Nondialyzed (n=5517) | Dialyzed (n=602) | Total (n=6119) | P Value (Dialyzed versus Nondialyzed) |
|---|---|---|---|---|
| Men | 52.2 | 63.1 | 53.3 | <0.001 |
| Black (versus other races) | 28.9 | 16.8 | 27.7 | <0.001 |
| Cardiac surgical service | 16.9 | 29.2 | 18.1 | <0.001 |
| Other surgical service | 28.4 | 29.1 | 28.4 | 0.72 |
| HIV/AIDS | 2.4 | 1.3 | 2.3 | 0.08 |
| Malignancy | 11.5 | 8.6 | 11.2 | 0.03 |
| Congestive heart failure | 35.3 | 44.7 | 36.2 | <0.001 |
| Cardiovascular disease | 9.8 | 10.6 | 9.9 | 0.50 |
| Dementia | 1.1 | 0.2 | 1.0 | 0.03 |
| Diabetes mellitus | 27.2 | 25.6 | 27.0 | 0.41 |
| Hemiplegia | 2.5 | 2.8 | 2.5 | 0.59 |
| Metastatic solid tumor | 9.5 | 4.8 | 9.0 | 0.001 |
| Myocardial infarction | 13.7 | 17.9 | 14.2 | 0.005 |
| Mild liver disease | 14.1 | 38.7 | 16.5 | <0.001 |
| Moderate-severe liver disease | 5.3 | 15.4 | 6.3 | <0.001 |
| Pulmonary disease | 15.3 | 11.5 | 14.9 | 0.01 |
| Peripheral vascular disease | 13.4 | 19.1 | 14.0 | 0.001 |
| Rheumatic disease | 3.3 | 2.7 | 3.2 | 0.42 |
| Peptic ulcer disease | 2.3 | 5.0 | 2.6 | 0.001 |
| Age (yr) | 61.1±16.4 | 59.3±15.7 | 60.9±16.3 | 0.009 |
| Baseline creatinine (mg/dl) | 0.79±0.26 | 0.95±0.25 | 0.81±0.26 | <0.001 |
| Baseline eGFR (ml/min per 1.73 m2) | 96±28 | 84±24 | 95±28 | <0.001 |
| Hospital stay prior to AKI (IQR) (d) | 3 (2–8) | 3 (1–7) | 3 (2–8) | 0.15 |
| Onset of AKI to doubling of creatinine (IQR) (d) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.63 |
| Time from AKI to RRT (IQR) (d) | NA | 3 (1–6) | NA | NA |
Unless otherwise noted, values are percentages of patients. Values expressed with a plus/minus sign are the mean±SD. eGFR, estimated GFR; IQR, interquartile range; RRT, renal replacement therapy.
Table 2.
Multivariable-adjusted odds ratio for initiation of dialysis based upon daily covariates
| Covariate | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Demographics | ||
| Age (per 10 yr) | 0.92 (0.84 to 1) | 0.06 |
| Male sex | 0.56 (0.45 to 0.7) | <0.001 |
| Black (versus other races) | 0.52 (0.37 to 0.72) | <0.001 |
| Hospital location | ||
| Intensive care unit (versus other hospital location) | 1.78 (1.23 to 2.57) | 0.002 |
| Comorbid conditions (present at admission) | ||
| Liver disease | 1.24 (0.98 to 1.57) | 0.07 |
| Malignancy | 0.76 (0.52 to 1.12) | 0.17 |
| Metastatic solid tumor | 0.63 (0.35 to 1.14) | 0.13 |
| Paraplegia | 0.64 (0.35 to 1.19) | 0.16 |
| Laboratory values | ||
| Anion gap (per mEq/L) | 1.03 (1.01 to 1.05) | 0.004 |
| Baseline eGFR (per 10 ml/min per 1.73 m2) | 0.93 (0.88 to 0.99) | 0.03 |
| Bicarbonate (per mEq/L) | 0.95 (0.93 to 0.98) | <0.001 |
| BUN (per 10 mg/dl) | 1.13 (1.09 to 1.17) | <0.001 |
| Change in creatinine over 24 hr (per mg/dl) | 1.19 (1.02 to 1.38) | 0.03 |
| Creatinine (per mg/dl) | 2.08 (1.86 to 2.32) | <0.001 |
| Fraction of inspired oxygen (per 10% increase) | 1.06 (1.02 to 1.11) | 0.004 |
| Platelet count (per 50,000/µl) | 0.9 (0.85 to 0.95) | <0.001 |
| Potassium (per mEq/L) | 1.59 (1.42 to 1.78) | <0.001 |
| Sodium (per mEq/L) | 0.95 (0.94 to 0.97) | <0.001 |
| White blood cell count (per 1000/µl) | 1.01 (1 to 1.01) | 0.05 |
| Medications | ||
| Number of sedatives (per each additional) | 1.17 (1.03 to 1.31) | 0.01 |
| Number of antibiotics (per each additional) | 1.1 (1.02 to 1.19) | 0.01 |
| Number of pressors (per each additional) | 1.55 (1.4 to 1.73) | <0.001 |
| Paralytic exposure | 1.86 (1.37 to 2.53) | <0.001 |
| Potassium-sparing diuretic | 1.85 (1.01 to 3.4) | 0.04 |
| Prior aminoglycoside exposure | 1.39 (1.12 to 1.72) | 0.003 |
| Thiazide diuretic | 2.25 (1.73 to 2.93) | <0.001 |
| Other orders | ||
| Blood culture ordered | 1.26 (0.91 to 1.76) | 0.17 |
| Do-not-resuscitate order | 0.33 (0.21 to 0.53) | <0.001 |
| Fresh frozen plasma transfusion | 1.84 (1.39 to 2.44) | <0.001 |
| Packed red blood cell transfusion | 1.53 (1.22 to 1.93) | <0.001 |
| Mechanical ventilation | 1.21 (0.93 to 1.58) | 0.16 |
| Day of week | ||
| Sunday (versus other days of the week) | 0.54 (0.39 to 0.74) | <0.001 |
Factors excluded from the model by stepwise regression included admission year, surgical admission, cardiovascular disease, congestive heart failure, dementia, diabetes mellitus, HIV disease, peptic ulcer disease, peripheral vascular disease, pulmonary disease, rheumatic disease, serum hemoglobin, cryoglobulin transfusion, platelet transfusion, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use and loop diuretic use. eGFR, estimated GFR.
Of the 602 patients who initiated dialysis, 545 were matched to 545 patients who had not initiated dialysis on the same day of AKI. The remaining 57 could not be matched because of an inadequate number of nondialyzed patients with sufficiently high propensity to receive dialysis. The mean propensity score among unmatched dialyzed patients was 69% (range, 35%–98%). Match quality was excellent, with mean difference in paired propensity scores of 0.7%±2.0% (standardized difference, −0.04). The distribution of propensity scores in the full and propensity-matched cohorts is illustrated in Supplemental Figure 1. Table 3 illustrates match-day covariates within the matched cohort and demonstrates balance across all measured covariates after propensity-score matching.
Table 3.
Distribution of covariates within the full cohort and matched cohort
| Variable | Matched, Nondialyzed Patients | Dialyzed Patients | P Value |
|---|---|---|---|
| Patients (n) | 545 | 545 | |
| Covariate | |||
| Age (yr) | 58±17 | 60±16 | 0.24 |
| Men (%) | 62 | 62 | 0.90 |
| Race (%) | |||
| Black | 16 | 17 | 0.87 |
| White | 60 | 64 | 0.20 |
| Other | 23 | 19 | 0.08 |
| Hospital location | |||
| Intensive care unit (%) | 91 | 89 | 0.54 |
| Comorbid conditions (present at admission) (%) | |||
| Liver disease | 42 | 41 | 0.65 |
| Malignancy | 10 | 9 | 0.84 |
| Metastatic solid tumor | 5 | 5 | 0.78 |
| Paraplegia | 3 | 3 | 0.86 |
| Laboratory values | |||
| Anion gap (mEq/L) | 14±7 | 14±6 | 0.58 |
| Baseline eGFR (ml/min) | 84±22 | 84±24 | 0.84 |
| Bicarbonate (mEq/L) | 17±6 | 17±5 | 0.93 |
| BUN (mg/dl) | 69±36 | 71±40 | 0.34 |
| Change in creatinine over 24 hr (mg/dl) | 0.69±1.00 | 0.65±0.72 | 0.34 |
| Creatinine (mg/dl) | 3.88±2.09 | 3.88±1.66 | 0.99 |
| Fraction of inspired oxygen (IQR) (%) | 70 (40–100) | 75 (40–100) | 0.45 |
| Platelet count (×1000/μl) | 112±98 | 118±109 | 0.33 |
| Potassium (mEq/L) | 5.2±1.1 | 5.1±1.0 | 0.59 |
| Sodium (mEq/L) | 138±7 | 138±6 | 0.50 |
| White blood cell count (×1000/μl) | 18.4±15.4 | 18.0±13.5 | 0.64 |
| Medications | |||
| Number of sedatives (IQR) | 2 (0–2) | 2 (1–2) | 0.98 |
| Number of antibiotics (IQR) | 2 (1–3) | 2 (1–3) | 0.16 |
| Number of pressors (IQR) | 2 (0–3) | 2 (0–3) | 0.30 |
| Paralytic exposure (%) | 19 | 17 | 0.47 |
| Potassium-sparing diuretic (%) | 3 | 2 | 0.84 |
| Prior aminoglycoside exposure (%) | 34 | 35 | 0.62 |
| Thiazide diuretic (%) | 23 | 23 | 0.88 |
| Other orders | |||
| Blood culture ordered (%) | 10 | 12 | 0.54 |
| Do-not-resuscitate order (%) | 8 | 8 | 0.66 |
| Fresh frozen plasma transfusion (%) | 21 | 20 | 0.81 |
| Packed red blood cell transfusion (%) | 52 | 52 | 0.95 |
| Ventilated, % | 64 | 67 | 0.31 |
| Day of week | |||
| Sunday (%) | 9 | 9 | 0.99 |
| Severity of illness | |||
| SOFA score | 10.4±3.1 | 10.7±3.2 | 0.12 |
Data are presented as mean±SD unless otherwise noted. P values reflect univariable paired analyses between days of dialysis initiation and the matched nondialysis days. eGFR, estimated GFR; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
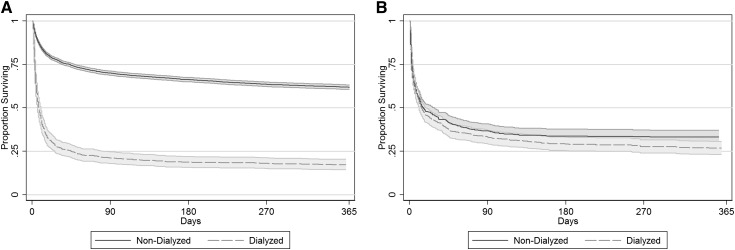
In the full cohort, initiation of dialysis therapy was strongly associated with mortality: unadjusted HR, 3.42 (95% CI, 3.09 to 3.78; P<0.001). After propensity score matching, the risk associated with dialysis was ablated: HR, 1.01 (95% CI, 0.85 to 1.21; P=0.89), indicating that patient factors accounts for the observed harm associated with dialysis in the full cohort. Similarly, 90-day mortality was no different (67% among dialyzed patients and 63% among matched nondialyzed patients; P=0.26). Kaplan–Meier survival plots of the full and matched cohorts are illustrated in Figure 1.
Figure 1.
Kaplan–Meier survival curves of dialyzed and nondialyzed patients in the (A) full and (B) matched cohorts. Time 0 is the onset of AKI in the full cohort and the day of initiation of dialysis (or match day) in the matched cohort.
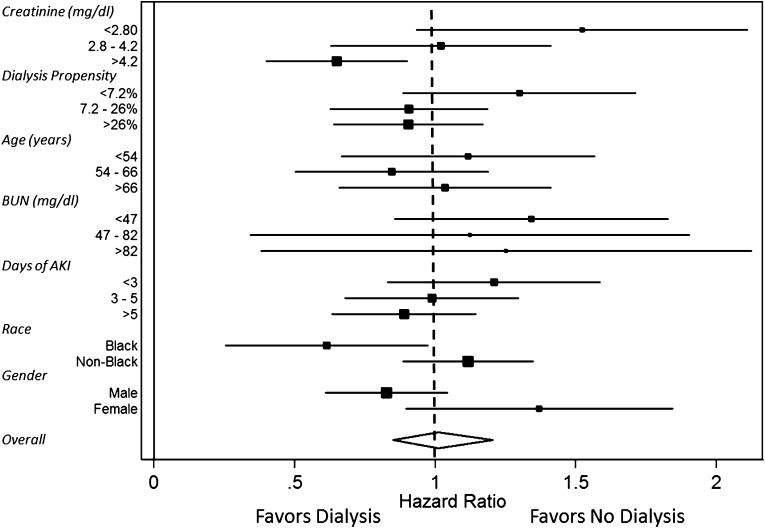
We analyzed key covariates that were selected a priori as possible effect modifiers of the relationship between dialysis and all-cause mortality in the propensity-matched cohort. As serum creatinine increased, the HR for death of dialysis initiation decreased with a reduction in the association between dialysis and death of 20% (95% CI, 9% to 30%) for each mg/dl higher creatinine level (P for interaction=0.001). The HR (for dialysis versus no dialysis) became <1, favoring dialysis, at creatinine concentrations≥3.8 mg/dl. This effect modification was also evident in logistic regression models examining death at 90 days (P=0.01). Multivariable modeling of the interaction of creatinine on dialysis mortality risk, adjusting for age, sex, race, medical versus surgical status, intensive care unit (ICU) status, congestive heart failure, liver disease, BUN, potassium, bicarbonate, fraction of inspired oxygen, modified SOFA score, and day of AKI did not substantially modify the association of higher creatinine concentration and improved outcomes among dialyzed versus nondialyzed patients. No modification of the relationship between dialysis and death was seen across the range of propensity score (P=0.36), patient age (P=0.97), BUN concentration (P=0.43), duration of AKI (P=0.86), or race (P=0.08). A weak interaction was detected according to sex. The HR for dialysis versus no dialysis was 1.37 (95% CI, 0.96 to 1.94; P=0.07) in women and 0.83 (95% CI, 0.64 to 1.07; P=0.15) in men, a 40% reduction (P for interaction=0.04). This finding remained after adjustment for creatinine concentration. These analyses are depicted in Figure 2.
Figure 2.
Forest plot demonstrating the net benefit (or risk) of dialysis across specified subgroups. The difference in the hazard ratio of dialysis versus nondialysis between the highest creatinine tertile and the lowest is significant (P=0.003), as is the difference in hazard ratio seen in women versus men (P=0.04).
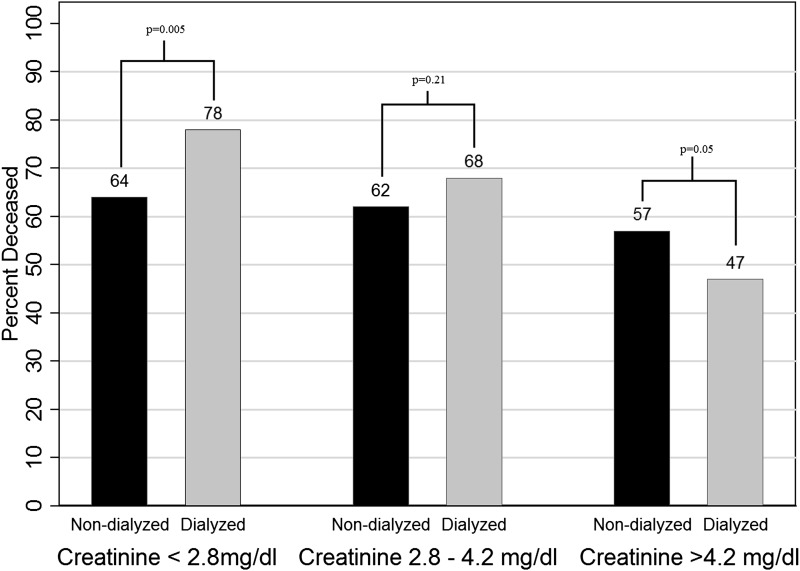
To explore characteristics of patients dialyzed or matched at various creatinine levels, we divided the matched cohort into tertiles by serum creatinine level. The in-hospital mortality rate for patients in the highest tertile (>4.2 mg/dl) was 47% for dialyzed patients versus 57% for nondialyzed patients (P=0.05). For those in the lowest tertile (<2.8 mg/dl), in-hospital mortality was 78% among dialyzed patients and 64% among nondialyzed patients (P=0.005) (Figure 3). Patients in the highest tertile were younger: mean age, 58±16 versus 60±17 years in the lowest tertile (P=0.03). In addition, the highest tertile included more male (76% versus 43%; P<0.001) and black (23% versus 8%; P<0.001) patients, and fewer patients in the ICU (82% versus 95%; P<0.001). There was no significant association between creatinine category and modified SOFA score, with mean modified SOFA score of 10.5±2.9 in the low creatinine group and 10.1±3.5 in the high creatinine group (P=0.12). Time from AKI to RRT was greater as creatinine tertile increased: Two days (interquartile range [IQR], 1–6 days) in the lowest tertile, 3 days (IQR, 1–7 days) in the middle tertile, and 5 days (IQR, 3–9 days) in the highest tertile (P<0.001). Matched nondialyzed patients in the low or medium creatinine groups were less likely to go on to receive dialysis later in their hospital stay than those in the high creatinine group (24% and 26% versus 40%, respectively; P=0.001). Among the nondialyzed patients in the highest creatinine tertile who went on to receive dialysis (n=80), the inpatient mortality was 44% compared with 68% in the 103 high tertile patients who never received dialysis (P=0.001).
Figure 3.
Proportion of patients who died during the hospitalization in the matched cohort, stratified by creatinine tertile at the time of matching.
There was no association between parenteral or enteral nutritional supplementation and creatinine concentration in the matched cohort (P=0.19). In the subset of patients with an available serum albumin (n=727), there was no association between creatinine and albumin levels (ρ=0.02; P=0.67). Mean albumin levels were similar in the matched cohort despite not being used in the propensity score: 2.9±1.0 among nondialyzed patients and 2.8±1.0 among dialyzed patients (P=0.28).
Effect of Urine Output
Accurate urine output and fluid balance data were not available for many patients in this cohort (primarily due to absence of urinary catheters or less intensive level of care). We assessed urine output among a subsample of matched pairs (wherein both members were in an ICU at the time of matching) via direct chart review (n=52 pairs). Urine output was significantly higher in the nondialyzed patients versus their matched, dialyzed counterparts on the match day and the day before match day. The median difference in urine output between nondialyzed and dialyzed patients on the day before matching was 223 (IQR, −187.5 to 1476.5) ml (P=0.01). Urine output was not associated with serum creatinine concentration in the dialyzed or matched nondialyzed group (P=0.38 and P=0.10, respectively) (Supplemental Figure 2). Urine output was not associated with all-cause mortality (HR per 500 ml increase, 1.05; 95% CI, 0.98 to 1.12; P=0.21), nor did it modify the association between dialysis and mortality (P=0.64).
Additional Analyses
It is conceivable that some patients were not dialyzed because of a decision to withhold futile care. When patient-days after the placement of a do-not-resuscitate order were excluded from analysis, the HR for dialysis versus withholding dialysis was 1.03 (95% CI, 0.85 to 1.24; P=0.78). Creatinine remained a significant modifier of this relationship, with a 16% reduction in dialysis mortality risk for each 1-mg/dl increase in serum creatinine (P=0.01).
As indications for dialytic initiation may differ in and out of the ICU, we restricted our analysis to the 443 matched pairs of patients who were both in an ICU at the time of matching. Again, dialysis was more beneficial at higher creatinine concentrations, with a 23% (95% CI, 10% to 34%) reduction in mortality risk in dialyzed versus nondialyzed patients for each 1-mg/dl increase in serum creatinine (P=0.001).
Discussion
Nephrologists are frequently asked to comment on the benefit of dialysis in particular patients with AKI. In a cohort of well matched dialyzed and nondialyzed patients, we found a strong association between dialysis and survival at higher creatinine levels and between dialysis and mortality at lower creatinine levels. No other patient factors were strongly associated with dialytic benefit.
Prior studies examining the initiation of dialysis for AKI have not generated consensus (13–22). Significantly heterogenous, these studies fail to include an adequate nondialyzed control group. Without this, no conclusion regarding the effectiveness of dialysis versus nondialysis can be made. In general, studies examining patients dialyzed at a high creatinine versus those dialyzed at a low creatinine have demonstrated that outcomes are better when dialysis is initiated at a higher creatinine concentration (20,22–25). Our results confirm and expand upon those seen in prior studies. By including a well matched nondialyzed group, we can conclude that patients with a higher creatinine at initiation of dialysis fare better not only than those who initiate dialysis at a lower creatinine but also those who do not initiate dialysis at a high creatinine. Conversely, patients who initiate dialysis at a low creatinine do worse than matched patients who do not initiate dialysis at a low creatinine.
Unmeasured Confounding
Unmeasured confounding cannot be fully dismissed in any observational study. One interpretation of our results could be that, at a high creatinine, healthy patients are dialyzed and sick patients are not, whereas at a low creatinine, sick patients are dialyzed and healthy patients are not. That said, SOFA scores were well matched between the groups, urine output did not differ by creatinine status, and serum albumin (a factor not built into the propensity score) was well matched among pairs. Indeed, the in-hospital mortality among nondialyzed patients in the lowest creatinine tertile was 64% compared with 57% in the highest tertile, demonstrating that the matched nondialyzed patients were severely ill, even at low creatinine levels. The fact that there was no indication of harm from dialysis at low propensity scores or benefit from dialysis at high propensity scores (no propensity score by treatment interaction) also suggests that match quality was excellent. The lack of such an interaction suggests that although unmeasured factors may be associated with treatment status, they are not associated simultaneously with outcome; thus, they are not true confounders (26,27). To use urine output as an example, we demonstrated that urine output was associated with the exposure (dialysis) but not with the outcome (death) or the effect modifier (serum creatinine concentration). This finding runs counter to previously published studies wherein low urine output was associated with higher mortality (28–30). We suspect that the lack of a relationship between urine output and mortality in the matched cohort is due to the fact that low urine output leads to mortality through pathways that appear in our propensity score (such as hyperkalemia, acidosis, and fraction of inspired oxygen). Taken together, these analyses may suggest an independent effect whereby dialysis at a higher creatinine concentration is associated with improved outcomes in AKI.
Creatinine Kinetics and Dialysis in AKI
The modification of the benefit of dialysis by creatinine concentration should be interpreted in the context of creatinine kinetics. Because dialyzed patients with higher creatinine levels had lower mortality than dialyzed patients with lower creatinine levels, we do not believe that creatinine is only representing GFR in this population. Higher creatinine levels could be due to longer duration of AKI, but the benefit of dialysis did not increase as AKI duration increased. We suspect that the benefit demonstrated herein is due to increased creatinine generation rate. At higher creatinine generation rate, the rate of creatinine rise in AKI is greater. Creatinine generation rate is a proxy for muscle mass, and higher levels may identify a healthier subset of patients with AKI (31). It has been suggested that creatinine generation rate may be increased by catabolic states, although this has not been measured in AKI populations to our knowledge (32). An association between higher creatinine and lower mortality is consistent with findings in long-term dialysis populations, in which a similar association has been observed (23,33,34). The lack of relationship between serum albumin, enteral or parenteral nutrition, and creatinine concentrations argues against nutritional status contributing significantly to the above effect.
Limitations
Limitations of this study include the fact that it was performed within a single health system, although it did encompass a tertiary care center and two community hospitals with a diverse patient population. This cohort enrolled only patients with relatively normal baseline renal function in order to accurately assess the onset of AKI (a key matching covariate); these results may not apply to patients with preexisting CKD in whom the outcome of AKI may be more favorable (35). Approximately 9% of dialyzed patients could not be matched in our primary analysis. This may reflect a clinical scenario in which there is no doubt about the necessity of dialysis. As such, our primary analysis should be interpreted as providing guidance in clinical scenarios when dialysis can reasonably be initiated or deferred. Administrative (ICD-9) coding of comorbidity data may not accurately represent comorbid conditions (10). We did not have a direct indicator of fluid overload, such as daily weight or fluid intake (although we did have data on oxygen requirement); fluid-overloaded patients may be expected to have lower creatinine concentrations, potentially confounding the creatinine-dialysis-mortality relationship. Finally, the requirement of a doubling of serum creatinine may have excluded patients initiated on dialysis extremely early in the course of AKI. These patients are usually dialyzed for urgent indications, however, and thus do not inform the clinical question of when to initiate dialysis for AKI in nonemergent situations.
In conclusion, in the setting of AKI, higher serum creatinine concentrations, perhaps as a marker of muscle mass, are associated with benefit from dialysis. Conversely, low creatinine concentrations, perhaps a marker of frailty, are associated with harm from dialysis. Our findings suggest that there is equipoise for conducting randomized trials to determine which patients benefit from dialysis for AKI and which are in fact harmed by this therapy.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank the Penn Data Store for their assistance in assembling the laboratory and medication databases used in this study. We would like to thank Paul Palevsky for his insight on study design and presentation.
This research was conducted under National Institute of Diabetes and Digestive and Kidney Disease grant 1F32DK093223 awarded to F.P.W.
This research was presented in abstract form at the American Society of Nephrology Meeting, San Diego, CA, in October 30–November 4, 2012.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07630713/-/DCSupplemental.
See related editorial, “Which Patients Benefit from Initiation of Dialysis for AKI?,” on pages 635–637.
References
- 1.Basso F, Ricci Z, Cruz D, Ronco C: International survey on the management of acute kidney injury in critically ill patients: Year 2007. Blood Purif 30: 214–220, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA: Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23: 1970–1974, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kellum JA, Mehta RL, Levin A, Molitoris BA, Warnock DG, Shah SV, Joannidis M, Ronco C, Acute Kidney Injury Network (AKIN) : Development of a clinical research agenda for acute kidney injury using an international, interdisciplinary, three-step modified Delphi process. Clin J Am Soc Nephrol 3: 887–894, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL: Indications for dialysis in the ICU: Renal replacement vs. renal support. Blood Purif 19: 227–232, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hakim RM, Wingard RL, Parker RA: Effect of the dialysis membrane in the treatment of patients with acute renal failure. N Engl J Med 331: 1338–1342, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E: Renal replacement therapy and the kidney: Minimizing the impact of renal replacement therapy on recovery of acute renal failure. Curr Opin Crit Care 11: 548–554, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wilson FP, Yang W, Feldman HI: Predictors of death and dialysis in severe AKI: The UPHS-AKI cohort. Clin J Am Soc Nephrol 8: 527–537, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiao CC, Ko WJ, Wu VC, Huang TM, Lai CF, Lin YF, Chao CT, Chu TS, Tsai HB, Wu PC, Young GH, Kao TW, Huang JW, Chen YM, Lin SL, Wu MS, Tsai PR, Wu KD, Wang MJ, National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) : U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS ONE 7: e42952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccinni P, Dan M, Barbacini S, Carraro R, Lieta E, Marafon S, Zamperetti N, Brendolan A, D’Intini V, Tetta C, Bellomo R, Ronco C: Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med 32: 80–86, 2006 [DOI] [PubMed] [Google Scholar]
- 15.García-Fernández N, Pérez-Valdivieso JR, Bes-Rastrollo M, Vives M, Lavilla J, Herreros J, Monedero P, GEDRCC : Timing of renal replacement therapy after cardiac surgery: A retrospective multicenter Spanish cohort study. Blood Purif 32: 104–111, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Clark E, Wald R, Levin A, Bouchard J, Adhikari NK, Hladunewich M, Richardson RM, James MT, Walsh MW, House AA, Moist L, Stollery DE, Burns KE, Friedrich JO, Barton J, Lafrance JP, Pannu N, Bagshaw SM, Canadian Acute Kidney Injury (CANAKI) Investigators : Timing the initiation of renal replacement therapy for acute kidney injury in Canadian intensive care units: A multicentre observational study. Can J Anaesth 59: 861–870, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM: Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 1: 915–919, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kresse S, Schlee H, Deuber HJ, Koall W, Osten B: Influence of renal replacement therapy on outcome of patients with acute renal failure. Kidney Int Suppl S75–S78, 1999 [PubMed] [Google Scholar]
- 19.De Corte W, Vanholder R, Dhondt AW, De Waele JJ, Decruyenaere J, Danneels C, Claus S, Hoste EA: Serum urea concentration is probably not related to outcome in ICU patients with AKI and renal replacement therapy. Nephrol Dial Transplant 26: 3211–3218, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care 24: 129–140, 2009. 19272549 [Google Scholar]
- 21.Splendiani G, Mazzarella V, Cipriani S, Pollicita S, Rodio F, Casciani CU: Dialytic treatment of rhabdomyolysis-induced acute renal failure: Our experience. Ren Fail 23: 183–191, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Chou Y-H, Huang T-M, Wu V-C, Wang C-Y, Shiao C-C, Lai C-F, Tsai H-B, Chao C-T, Young G-H, Wang W-J, Kao T-W, Lin S-L, Han Y-Y, Chou A, Lin T-H, Yang Y-W, Chen Y-M, Tsai P-R, Lin Y-F, Huang J-W, Chiang W-C, Chou N-K, Ko W-J, Wu K-D, Tsai T-J, Grp NS, NSARF Study Group : Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care 15: R134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganini EP, Halstenberg WK, Goormastic M: Risk modeling in acute renal failure requiring dialysis: The introduction of a new model. Clin Nephrol 46: 206–211, 1996 [PubMed] [Google Scholar]
- 24.Soubrier S, Leroy O, Devos P, Nseir S, Georges H, d’Escrivan T, Guery B: Epidemiology and prognostic factors of critically ill patients treated with hemodiafiltration. J Crit Care 21: 66–72, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Lunt M, Solomon D, Rothman K, Glynn R, Hyrich K, Symmons DP, Stürmer T, British Society for Rheumatology Biologics Register. British Society for Rheumatology Biologics Register Control Centre Consortium : Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol 169: 909–917, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glynn RJ, Schneeweiss S, Stürmer T: Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98: 253–259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz DN, Bolgan I, Perazella MA, Bonello M, de Cal M, Corradi V, Polanco N, Ocampo C, Nalesso F, Piccinni P, Ronco C, North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI) Investigators : North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol 2: 418–425, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PGH: Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 35: 1692–1702, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL: Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 80: 760–767, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Wilson FP, Sheehan JM, Mariani LH, Berns JS: Creatinine generation is reduced in patients requiring continuous venovenous hemodialysis and independently predicts mortality. Nephrol Dial Transplant 27: 4088–4094, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens LA, Levey AS: Measurement of kidney function. Med Clin North Am 89: 457–473, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Fink JC, Burdick RA, Kurth SJ, Blahut SA, Armistead NC, Turner MS, Shickle LM, Light PD: Significance of serum creatinine values in new end-stage renal disease patients. Am J Kidney Dis 34: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM: Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 168: 987–995, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.