Abstract
Background and objectives
ESRD is accompanied by endothelial dysfunction. Because the endothelial glycocalyx (endothelial surface layer) governs interactions between flowing blood and the vessel wall, perturbation could influence disease progression. This study used a novel noninvasive sidestream–darkfield imaging method, which measures the accessibility of red blood cells to the endothelial surface layer in the microcirculation (perfused boundary region), to investigate whether renal function is associated with endothelial surface layer dimensions.
Design, setting, participants, & measurements
Perfused boundary region was measured in control participants (n=10), patients with ESRD (n=23), participants with normal kidney function after successful living donor kidney transplantation (n=12), and patients who developed interstitial fibrosis/tubular atrophy after kidney transplantation (n=10). In addition, the endothelial activation marker angiopoietin-2 and shed endothelial surface layer components syndecan-1 and soluble thrombomodulin were measured using ELISA.
Results
Compared with healthy controls (1.82±0.16 µm), ESRD patients had a larger perfused boundary region (+0.23; 95% confidence interval, 0.46 to <0.01; P<0.05), which signifies loss of endothelial surface layer dimensions. This large perfused boundary region was accompanied by higher circulating levels of syndecan-1 (+57.71; 95% confidence interval, 17.38 to 98.04; P<0.01) and soluble thrombomodulin (+12.88; 95% confidence interval, 0.29 to 25.46; P<0.001). After successful transplantation, the perfused boundary region was indistinguishable from healthy controls (without elevated levels of soluble thrombomodulin or syndecan-1). In contrast, however, patients who developed interstitial fibrosis and tubular atrophy showed a large perfused boundary region (+0.36; 95% confidence interval, 0.09 to 0.63; P<0.01) and higher levels of endothelial activation markers. In addition, a significant correlation between perfused boundary region, angiopoietin-2, and eGFR was observed (perfused boundary region versus GFR: Spearman’s ρ=0.31; P<0.05; perfused boundary region versus angiopoietin-2: Spearman’s ρ=−0.33; P<0.05).
Conclusion
Reduced renal function is strongly associated with low endothelial surface layer dimensions. After successful kidney transplantation, the endothelial surface layer is indistinguishable from control.
Introduction
Patients with ESRD have a dysfunctional endothelium and increased morbidity and mortality, mainly because of their increased risk for cardiovascular disease (1,2). The mechanisms responsible for activation of the endothelium in chronic renal failure are multifactorial and include the presence of uremic toxins (particularly asymmetric dimethyl arginine), low-grade inflammation, and hypertension (3). One of the earliest changes on this endothelial activation has been postulated to be compositional and dimensional alterations of the endothelial surface layer (ESL) (4).
The ESL is a carbohydrate gel-like layer composed of proteoglycans, glycoproteins, and loosely bound plasma molecules (5–7). It has been postulated to govern the interaction between blood and the vessel wall. Heparan sulfates, the main constituent of the ESL, have been shown to activate antithrombin III and prevent leukocyte interaction with the endothelium (8–11). In addition, the ESL regulates endothelial permeability and acts as a shear stress sensor in the control of vasomotor function (12,13). It is, therefore, very likely that alterations in or loss of ESL function can predispose to the development of vascular disease (14,15). Under inflammatory conditions, a changed heparan sulfate composition in the ESL facilitates the recruitment of inflammatory cells (14). With sustained inflammation, the production of heparanase is induced in the glomerulus, which may partially degrade the ESL. Prevention of this ESL loss in diabetic heparanase knockout mice also prevented the development of proteinuria and kidney injury (16). Thus, ESL function and dimensions are also an important determinant of progression of kidney disease.
Because the ESL is being produced and shed continuously and its dimensions are, among others, based on BP and plasma proteins, it is very challenging to analyze (5–7). Most methods that aim to detect changes in ESL are based on the measurement of shed ESL components, like proteoglycans, hyaluronan, or heparan sulfates in the plasma. The only direct measurements for ESL properties are currently done in animals only using perfusion staining or intravital techniques (13,17,18). Until recently, ESL changes were measured using an invasive and time-consuming method using the distribution of labeled red blood cells (RBCs) and low-molecular weight dextrans (19). Recently, a noninvasive tool for analyzing the ESL was developed using sidestream–darkfield imaging. ESL dimensions were calculated as the difference in RBC column width before (functionally perfused capillary diameter) and after (anatomic capillary diameter) leukocyte passage. This technology correlates well with the invasive technique based on tracer dilution to assess the volume of the ESL, which is very labor-intensive and subject to investigator bias (20). We use a new approach, in which spatiotemporal changes in the ability of RBCs to penetrate the ESL can be detected (21). This method is fully automated and blinded to the investigator. In contrast to previous methods, in which the ESL quantity was measured in volume or thickness, this method analyzes ESL quality: a larger perfused boundary region (PBR) or increased accessibility to RBCs reflects a disturbance of the ESL structure.
In the present study, we used this method to investigate the hypothesis that renal failure is associated with perturbation of the endothelial ESL (i.e., larger PBR) and its relation to the presence of shed ESL components syndecan-1 and thrombomodulin (sTM) and the endothelial activation marker angiopoietin-2 (Ang2). In addition, we measured the ESL in patients who received kidney transplantation (KTx).
Material and Methods
Study Population
In a previous study, endothelial function has been shown to be estrogen-dependent and thus, varies during the menstrual cycle (22). Furthermore, in a currently ongoing study, we could show a small but significant difference in baseline PBRs between man and woman (unpublished data). Because the exact reason for these differences is still unclear, we only studied men in the current study to exclude sex as a possible confounding factor. In total, 53 men and 10 age-matched controls were enrolled in a cross-sectional study in which PBR was measured (Table 1); 31 patients were included after being diagnosed with ESRD (eGFR<15 ml/min per 1.73 m2), 12 patients were included with stable renal function after KTx (eGFR=30 ml/min per 1.73 m2 or higher; stable group), and 10 patients were included with interstitial fibrosis and tubular atrophy (IFTA) that developed after KTx, (eGFR<30 ml/min per 1.73 m2 or less and histopathologic proof; IFTA group). This latter group was included to investigate whether loss of function of the transplanted kidney would result in perturbation of the ESL. All procedures were approved by the Leiden University Medical Center (LUMC) Institution’s Medical Ethical Committee and complied with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients.
Table 1.
Baseline characteristics of study participants
| Characteristic | Control (n=10) | ESRD (n=23) | Stable (n=12) | IFTA (n=10) |
|---|---|---|---|---|
| Age (yr) | 44.8 (±10.3) | 50.6 (±12.4) | 54.1 (±13.9) | 56.0 (±9.4) |
| Smoking, N (%) | 0 | 1 | 2 (17) | 2 (20) |
| Time since transplant, median yr (IQR) | — | — | 5.0 (1.0–7.5) | 8.0 (3.0–14.5) |
| Body mass index (kg/m2) | 25.6 (±3.7) | 25.92 (±4.1) | 26.2 (±3.4) | 23.8 (±3.0) |
| eGFR (ml/min per 1.73 m2) | 86.8 (±17.2) | 8.2 (±2.7)a | 61.2 (±16.8)a | 22.3 (±9.03)a |
| Systolic BP (mmHg) | 132.8 (±11.6) | 139.5 (±12.9) | 132.4 (±9.1) | 141.9 (±20.5) |
| Diastolic BP (mmHg) | 80.6 (±7.1) | 83.0 (±12.54) | 78.1 (±6.2) | 81.5 (±6.9) |
| Hemoglobin (g/dl) | 14.9 (±0.72) | 12.4 (±1.6)a | 14.4 (±2.0) | 11.6 (±1.9)a |
| Hematocrit (%) | 43 (±3) | 38 (±5) | 44 (±5) | 37 (±5)a |
| Mean corpuscular volume (fl) | 88.8 (±2.6) | 92.9 (±4.1) | 89.7 (±3.4) | 95.4 (±5.5)a |
| Median proteinuria, g/24 h (IQR) | — | 2.04 (±1.81) | 0.23 (±0.10) | 2.27 (±2.38) |
| Anticoagulation, N (%) | — | 5 (22) | 0 (0) | 6 (60) |
| Antihypertensives, N (%) | — | 23 (100) | 8 (67) | 10 (100) |
| Statins, N (%) | — | 12 (52) | 2 (17) | 6 (60) |
| Immunosuppressive, N (%) | — | 4 (17) | 12 (100) | 10 (100) |
Categorical variables are summarized by frequency (percent). Continuous variables are summarized by the median and interquartile range (IQR) of the measurement distribution. All data are mean±SD unless otherwise specified.
P<0.05 versus control.
Transplantation
All patients received KTx at the LUMC between 1984 and 2012. Transplantations were performed as described previously (23). Patients were treated with prednisone (tapered to a dose of 10 mg by 6 weeks and a dose of 5–7.5 mg by 3 months), cyclosporine (area under the curve [AUC]=5400 ng⋅h/ml for the first 6 weeks and then 3250 ng⋅h/ml), or tacrolimus (AUC=210 ng⋅h/ml for first 6 weeks and then 125 ng⋅h/ml) and mycophenolate mofetil (AUC=30–60 ng⋅h/ml). From 2000 on, these patients received induction treatment with basiliximab (40 mg at days 0 and 4) or daclizumab (100 mg/d on the day of transplantation and 10 days after transplantation). Patients were treated routinely with oral val-ganciclovir prophylaxis for 3 months, except for a cytomegalovirus–negative donor recipient status patient. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Imaging of the Sublingual Microvasculature
Imaging of the sublingual microvasculature was performed using an sidestream–darkfield MicroScan Video Microscope (MicroVision Medical, Inc., Wallingford, PA) (24) connected to Glycocheck acquisition and analysis software (Glycocheck BV, Maastricht, The Netherlands). This software has been developed to calculate the PBR within 5 minutes after measurements. The method of calculation is based on the method published before and is fully automated to ensure unbiased analysis of the ESL (21). The method is described in full detail in Supplemental Material 1. In short, the camera is placed under the tongue for approximately 2 minutes. The software then automatically identifies all measurable microvessels and defines small vascular segments every 10 μm along the length of these vessels. For each vascular segment, 840 radial intensity profiles are tested for the presence of RBCs and signal quality. The program only selects vessels that are well perfused, thereby eliminating the influences of hematocrit on the PBR. From these intensity profiles, RBC column widths are determined. This determination results in an RBC width (RBCw) distribution of each individual vascular segment, from which the median RBCw and the outer edge of the RBC perfused lumen are determined. Because the PBR is present at both sides of the RBC column, it is calculated using the equation: (outer edge of the RBC perfused lumen−RBCw)/2. Next, the calculated PBR values, classified according to their corresponding RBCw values (between 5 and 25 µm), are averaged to provide a single PBR value for each patient. Although the method is not fully validated as a diagnostic tool, we tested whether loss of glycocalyx dimension is reflected by outward radial displacement of circulating RBCs in mice. Using intravital microscopy in enhanced green fluorescence protein transgenic mice, the effect of enzymatic degradation of the ESL on RBCw was studied. These validation experiments are described in detail in Supplemental Material 2.
Laboratory and Urinary Assessments
All persons enrolled in this study underwent blood sampling before the morning intake of immunosuppression. Creatinine, hematocrit, and hemoglobin were measured. For all patients, the eGFR was calculated from the plasma creatinine concentration using the Modification of Diet in Renal Disease formula. Simultaneously, blood was collected for analysis of Ang2 and sTM in serum and syndecan-1 in plasma. Ang2 (ELISA; R&D Systems, Minneapolis, MN), sTM (ELISA; Diaclone Research, Besancon, France), and syndecan-1 concentrations were measured by their respective ELISAs according to the protocol supplied by the manufacturer.
Statistical Analyses
Continuous normally distributed data are presented as mean±SD unless stated otherwise.
Differences between the groups were tested by ANOVA and shown as difference compared with control; 95% confidence interval (95% CI); P value. When criteria for parametric testing were not met, median and interquartile range were presented and tested with the Mann–Whitney test. Correlations between interval variables were calculated using the Spearman rank correlation coefficient. Differences were considered statistically significant if P<0.05. Data analysis was performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism, version 5.0 (GraphPad Software, Inc., San Diego, CA).
Results
Method Validation in Mice
To validate the PBR measurements, the gap between endothelial cells and RBC column (RBC-EC gap) was measured in mice before and 30 minutes after intravenous administration of the glycocalyx-degrading enzyme hyaluronidase (Supplemental Material). RBC-EC gaps ranged from 0.3 to 2.6 μm (vessel diameters=5–35 μm). Hyaluronidase treatment reduced the RBC-EC gap from 1.30 to 0.52 μm (average vessel diameter=16.0 μm) (Supplemental Figure 3). To exclude that changes in the RBC-EC gap originate from changes in the vessel diameter, the vessel diameter was measured before and after hyaluronidase treatment (Supplemental Figure 4). Because no changes could be observed in these paired measurements (P=0.91), the influence of vessel diameter can be excluded, thereby confirming that the observed changes originate from the changes in RBC column size as a result of the degradation of the ESL.
Patient Characteristics
Clinical characteristics of all patients included in this study are described in Table 1. No differences were observed in age, BP, or body mass index. The eGFR was significantly lower in all patient groups compared with controls (P<0.05). Hemoglobin and mean corpuscular volume levels were significantly lower in patients with ESRD and IFTA compared with controls (Table 1) (P<0.05).
Comparison of ESL among Patient Groups
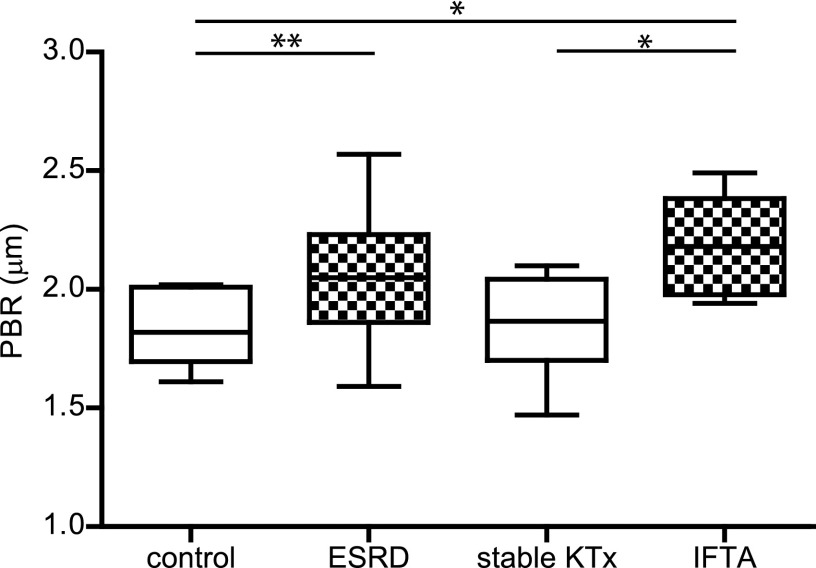
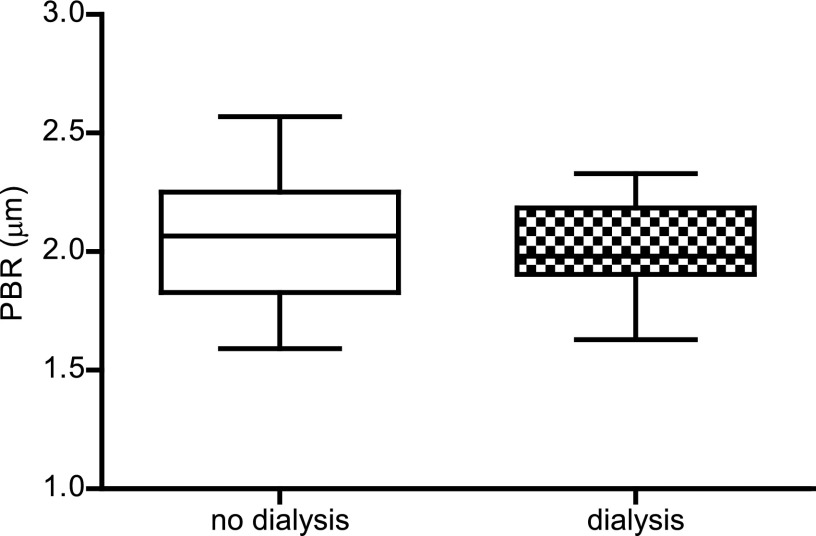
Changes in ESL related to endothelial activation are hypothesized to be the first changes in the development of cardiovascular disease (4,14). Therefore, we measured the distance that the RBCs can penetrate the ESL, and the calculated PBR reflects the ESL barrier properties. In this study, the PBR in controls was comparable with the PBR measured in controls in an earlier published study with lacunar stroke patients (21). Compared with healthy controls (1.82±0.16 µm), the PBR in patients with ESRD was significantly different (+0.23; 95% CI, 0.46 to <0.01; P<0.05). Patients who developed IFTA also showed a different PBR (+0.36; 95% CI, 0.09 to 0.63; P<0.01). Interestingly, the PBR in stable transplanted participants was larger but statistically indistinguishable from healthy controls (1.85±0.20 µm; P=1.00) (Figure 1). To determine the influence of dialysis on the PBR, we compared patients who received dialysis (n=9) with patients without dialysis (n=14) within the ESRD group. No difference was observed between these patient groups (2.02±0.21 versus 2.07±0.29 µm, respectively; P=0.62), which suggests that dialysis does not contribute to the observed differences in PBR (Figure 2).
Figure 1.
Measurements of the perfused boundary region (PBR) in participants with and without loss of renal function. PBR in healthy control participants (control; n=10) and patients with ESRD (n=23), stable kidney transplantation (stable KTx; n=12), and interstitial fibrosis and tubular atrophy (IFTA; n=10). Box plot whiskers indicate 1st and 99th percentiles. *P<0.05; **P<0.01.
Figure 2.
Measurements of the PBR in ESRD patients with and without dialysis. PBR in patients who did (n=9) and did not receive dialysis (n=14) within the ESRD group. Box plot whiskers indicate 1st and 99th percentiles.
Comparison of Endothelial Dysfunction and Shed Proteoglycans among Patient Groups
The endothelial activation state was determined by measuring serum Ang2 levels. In agreement with increasing PBR, the Ang2 level was higher, although not significant, compared with control levels (4.21±3.23 and 2.09±1.16 ng/ml; P=0.19) in ESRD serum samples (Figure 3A). There was no difference in Ang2 levels in the successfully transplanted and IFTA groups (1.68±0.85 and 3.59±2.34 ng/ml; both P=1.00). Next, markers of shed ESL compounds were measured. Serum sTM levels between control participants (7.06±1.17 ng/ml) and ESRD patients were significantly different (+12.88; 95% CI, 0.29 to 25.46; P<0.001). Patients diagnosed with IFTA had even higher serum sTM levels (+34.86; 95% CI, 19.87 to 49.86; P<0.001). In participants with stable KTx, sTM levels were indistinguishable from control (12.98±3.01 ng/ml; P=1.00) (Figure 3B). In accordance, shed syndecan-1 levels were high compared with control (49.8±17.4 ng/ml) in ESRD patients (+57.71; 95% CI, 17.38 to 98.04; P<0.01). However, shed syndecan-1 levels were not significantly different from controls in both IFTA and stable KTx samples (54.8±32.7 and 64.0±15.2 ng/ml, respectively) (Figure 3C).
Figure 3.
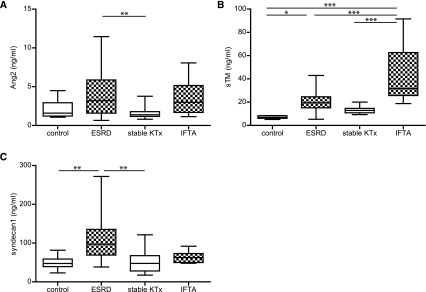
Measurements of circulatory endothelial and endothelial surface layer damage markers in participants with and without loss of renal function. Markers were measured in healthy controls (n=10) and patients with ESRD (n=23), stable KTx (n=12), and IFTA (n=10). (A) Serum angiopoietin-2 (Ang2). (B) Serum soluble thrombomodulin (sTM). (C) Plasma shed syndecan-1. Box plot whiskers indicate 1st and 99th percentiles. *P<0.05; **P<0.01; **P<0.001.
Association between Renal Function and the ESL
To examine any relation between renal failure, health status of the microcirculation, and ESL composition, the following correlations were achieved (Table 2). First, the endothelial cell activation marker Ang2 inversely correlated with renal function, which was assessed by the eGFR (Spearman’s ρ=−0.40; P<0.01), suggesting a relation between endothelial dysfunction and renal failure. Second, PBR was inversely correlated with the eGFR (Spearman’s ρ=−0.33; P<0.05) and positively correlated with serum Ang2 levels (Spearman’s ρ=0.31; P<0.05). A comparable inverse correlation of sTM and syndecan-1 with eGFR (Spearman’s ρ=−0.53 and −0.67, respectively; both P<0.001) supports the indication that endothelial activation and a perturbed ESL are associated with global renal function.
Table 2.
Correlations between renal function (eGFR), perfused boundary region, and endothelial and endothelial surface layer damage markers
| Parameter | eGFR (ml/min per 1.73 m2) | PBR (µm) |
|---|---|---|
| PBR | ||
| Spearman’s ρ | −0.33 | — |
| P value | <0.05 | — |
| Ang2 | ||
| Spearman’s ρ | −0.40 | 0.31 |
| P value | <0.01 | <0.05 |
| sTM | ||
| Spearman’s ρ | −0.57 | 0.33 |
| P value | <0.001 | <0.05 |
| Syndecan-1 | ||
| Spearman’s ρ | −0.67 | 0.13 |
| P value | <0.001 | 0.35 |
Spearman’s rank correlation coefficient to identify relationships between the PBR, eGFR, and endothelial and endothelial surface layer damage markers. PBR, perfused boundary region; Ang2, angiopoietin-2; sTM, soluble thrombomodulin.
Association between PBR and Shed ESL Markers
Because both PBR and shed ESL markers associated with the observed differences in renal function, we tested the possible relation between the endothelial surface markers (Table 2). Although we were able to find a positive correlation between PBR and sTM (Spearman’s ρ=0.33; P<0.05) and between sTM and shed syndecan-1 (Spearman’s ρ=0.45; P<0.001), no significant correlation between PBR and shed syndecan-1 was found (Spearman’s ρ=0.1; P=0.35).
Discussion
We show that patients with impaired kidney function have a larger PBR (i.e., loss of ESL). Interestingly, ESL dimensions in patients with a stable KTx were indistinguishable from healthy controls, whereas loss of renal function in patients developing IFTA resulted again in perturbation of the ESL. These changes in PBR are reflected by the presence of circulating ESL components, sTM, and syndecan-1.
In the current study, we used a new noninvasive, automated, and easy to apply approach to measure the ability of RBC to penetrate the ESL, which is quantitatively defined as the PBR. The automated software analysis results in an average PBR of 1.82 µm in healthy control participants, which is lower than the previously published PBR of 3.3 µm (25); however, it is far more reliable, because this new method uses over 3000 measurement sites and stringently removes artifacts and background signal. Moreover, it ensures unbiased data analysis.
Ang2 is released by endothelial cells on their activation (26,27). The paracrine release of Ang2 interferes with Ang1 signaling from perivascular cells to the endothelium, resulting in capillary destabilization and angiogenic responses (28,29). The correlation that we show between Ang2, eGFR, and PBR indicates that the endothelial activation during renal failure is associated with a loss of ESL dimensions.
TM is an anticoagulant cell surface proteoglycan that is shed from the endothelial cell surface layer after inflammatory stimulation, resulting in the sTM that we measured in serum (30). Although it is also expressed in low amounts by dendritic cells and monocytes, it is mainly expressed by endothelial cells, thereby making it a reasonable marker for shedding of proteoglycans from the ESL. In this study, we show a negative correlation between serum sTM and renal function (eGFR), which has also been shown in type 1 diabetic patients after simultaneous pancreas–kidney transplantation (23). Although shedding from the ESL most likely explains higher sTM, reduced clearance by the kidneys needs to be considered as well. A study in diabetic patients, however, showed that urinary sTM was not influenced by GFR (31). Also, the size of sTM would preclude filtration by the kidney. Together, these data indicate that the high levels of sTM are a direct result of increased TM shedding from the ESL.
In addition to TM, syndecan-1 is also shown to be expressed at the luminal endothelial surface (32,33). Its shedding from the ESL under inflammatory conditions is thought to contribute to plasma levels of soluble syndecan-1 (34). In agreement, syndecan-1 shedding has been shown previously to be associated with ischemia-reperfusion injury, septic shock, and postcardiac arrest syndrome (32,35,36). In addition, patients with early diabetic nephropathy have higher shed syndecan-1 levels compared with healthy controls (37). Alternatively, reduced renal clearance of syndecan-1 could also be involved in elevated plasma levels in patients with renal failure. Although little is known about the exact clearance mechanism of syndecan-1, its large molecular weight excludes a direct straightforward relationship with GFR. Although the large PBR and sTM levels coincided with high syndecan-1 levels in ESRD patients, transplant recipients with IFTA and reduced renal function still showed low levels of syndecan-1.
Interestingly, in patients who were stable after KTx, sTM and PBR were not similar but also not significantly different from the measured PBR in ESRD patients or healthy controls. This position between healthy controls and ESRD patients might be explained by the fact that kidney function is still not optimal compared with healthy controls. The higher level of sTM in IFTA patients compared with ESRD patients is absent for syndecan-1, which suggests that the various pathologies that underlie renal failure in these patients affect the shedding of ESL components differently.
All together, the plasma and serum biomarkers and PBR measurements corroborate the observation that loss of renal function is associated with endothelial activation and ensuing loss of ESL thickness. A question is whether mucosal ESL thickness is representative of systemic changes. However, like with other endothelial function measurements, systemic factors will induce representative functional endothelial changes throughout the circulation. Using dextran distribution, we previously validated in patients with diabetes that such mucosal changes in ESL thickness are, indeed, accompanied by changes in systemic ESL thickness (38). In addition, ESL changes have also been shown to coincide in both the retinal and the sublingual microcirculation in patients with type 2 diabetes (39).
In this study, we cannot completely exclude the effect of immunosuppression in the transplant patients on the mechanical properties of the ESL. Nonetheless, a clear change in PBR was observed between the kidney recipients with stable function and the IFTA group while both groups were treated with immunosuppressive medication, suggesting that renal function per se is a more important determinant of PBR. Another question is whether changes in RBC deformability may have contributed to the observed PBR measurements. A study by Martínez et al. (40) did not observe any alteration in erythrocyte deformability between control participants and patients with CKD with or without dialysis, which makes a contribution of RBC deformability to the observed differences in PBR less likely for these patients.
Changes in the ESL have been postulated to precede vascular (and renal) damage (4). Because measuring PBR in the microcirculation is a noninvasive and fast method to assess changes in the ESL in patients, it is a promising new method for clinical monitoring of the systemic microvasculature to predict the risk for cardiovascular disease and follow the vascular effect of interventions, such as KTx.
Disclosures
H.V. is commercially involved with the sidestream–darkfield acquisition and analysis technique and is Chief Scientific Officer at GlycoCheck BV (Maastricht, The Netherlands).
Supplementary Material
Acknowledgments
The study was supported by Dutch Kidney Foundation Grant C08.2265 and Glycoren Consortium Grant CP09.03.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08160813/-/DCSupplemental.
References
- 1.Santoro D, Bellinghieri G, Conti G, Pazzano D, Satta E, Costantino G, Savica V: Endothelial dysfunction in chronic renal failure. J Ren Nutr 20[Suppl]: S103–S108, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Moody WE, Edwards NC, Madhani M, Chue CD, Steeds RP, Ferro CJ, Townend JN: Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: Cause or association? Atherosclerosis 223: 86–94, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Rabelink TJ, de Boer HC, van Zonneveld AJ: Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol 6: 404–414, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG: The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinbaum S, Tarbell JM, Damiano ER: The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Pries AR, Secomb TW, Gaehtgens P: The endothelial surface layer. Pflugers Arch 440: 653–666, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Boels MG, Lee DH, van den Berg BM, Dane MJ, van der Vlag J, Rabelink TJ: The endothelial glycocalyx as a potential modifier of the hemolytic uremic syndrome. Eur J Intern Med 24: 503–509, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G: Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem 267: 20435–20443, 1992 [PubMed] [Google Scholar]
- 10.Rops AL, van den Hoven MJ, Baselmans MM, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J: Heparan sulfate domains on cultured activated glomerular endothelial cells mediate leukocyte trafficking. Kidney Int 73: 52–62, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Constantinescu AA, Vink H, Spaan JA: Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23: 1541–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC: Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A 100: 7988–7995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ: Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol 182: 1532–1540, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M: Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc Res 87: 300–310, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Broekhuizen LN, Mooij HL, Kastelein JJ, Stroes ES, Vink H, Nieuwdorp M: Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol 20: 57–62, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, van der Vlag J, Vlodavsky I, Elkin M: Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes 61: 208–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J: Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 23: 1339–1350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith ML, Long DS, Damiano ER, Ley K: Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J 85: 637–645, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES: Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Nieuwdorp M, Meuwese MC, Mooij HL, Ince C, Broekhuizen LN, Kastelein JJ, Stroes ES, Vink H: Measuring endothelial glycocalyx dimensions in humans: A potential novel tool to monitor vascular vulnerability. J Appl Physiol (1985) 104: 845–852, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Martens RJ, Vink H, van Oostenbrugge RJ, Staals J: Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis 35: 451–454, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Lambert J, Stehouwer CD: Modulation of endothelium-dependent, flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 94: 2319–2320, 1996 [PubMed] [Google Scholar]
- 23.Khairoun M, de Koning EJ, van den Berg BM, Lievers E, de Boer HC, Schaapherder AF, Mallat MJ, Rotmans JI, van der Boog PJ, van Zonneveld AJ, de Fijter JW, Rabelink TJ, Reinders ME: Microvascular damage in type 1 diabetic patients is reversed in the first year after simultaneous pancreas-kidney transplantation. Am J Transplant 13: 1272–1281, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C: Sidestream Dark Field (SDF) imaging: A novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 15: 15101–15114, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H: Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 23: 1900–1908, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 27.David S, Kümpers P, Hellpap J, Horn R, Leitolf H, Haller H, Kielstein JT: Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am J Kidney Dis 53: 770–778, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Khairoun M, van der Pol P, de Vries DK, Lievers E, Schlagwein N, de Boer HC, Bajema IM, Rotmans JI, van Zonneveld AJ, Rabelink TJ, van Kooten C, Reinders ME: Renal ischemia-reperfusion induces a dysbalance of angiopoietins, accompanied by proliferation of pericytes and fibrosis. Am J Physiol Renal Physiol 305: F901–F910, 2013 [DOI] [PubMed] [Google Scholar]
- 29.de Vries DK, Khairoun M, Lindeman JH, Bajema IM, de Heer E, Roest M, van Zonneveld AJ, van Kooten C, Rabelink TJ, Schaapherder AF, Reinders ME: Renal ischemia-reperfusion induces release of angiopoietin-2 from human grafts of living and deceased donors. Transplantation 96: 282–289, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Boehme MW, Deng Y, Raeth U, Bierhaus A, Ziegler R, Stremmel W, Nawroth PP: Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: In vivo and in vitro studies. Immunology 87: 134–140, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, Inukai T, Takemura Y: Mechanisms of elevation of serum and urinary concentrations of soluble thrombomodulin in diabetic patients: Possible application as a marker for vascular endothelial injury. Metabolism 47: 362–365, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF: Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116: 1896–1906, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF: The glycocalyx of the human umbilical vein endothelial cell: An impressive structure ex vivo but not in culture. Circ Res 104: 1313–1317, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Bartlett AH, Hayashida K, Park PW: Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells 24: 153–166, 2007 [PubMed] [Google Scholar]
- 35.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ: Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation 83: 715–720, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettilä V: Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand 56: 316–322, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Svennevig K, Kolset SO, Bangstad HJ: Increased syndecan-1 in serum is related to early nephropathy in type 1 diabetes mellitus patients. Diabetologia 49: 2214–2216, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H: Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H: Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53: 2646–2655, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez M, Vayá A, Alvariño J, Barberá JL, Ramos D, López A, Aznar J: Hemorheological alterations in patients with chronic renal failure. Effect of hemodialysis. Clin Hemorheol Microcirc 21: 1–6, 1999 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.