Abstract
Background and objectives
Very few studies report acid base disorders in homozygous patients with sickle cell anemia (SCA) and describe incomplete renal acidosis rather than true metabolic acidosis, the prevalence of which is unknown and presumably low. This study aimed to assess the prevalence of metabolic acidosis and to identify its risk factors and mechanisms.
Design, setting, participants, & measurements
This study retrospectively analyzed 411 homozygous patients with SCA with a GFR≥60 ml/min per 1.73 m2, referred in a single center between 2007 and 2012. Acidosis and nonacidosis groups were compared for clinical and biologic data including SCA complications and hemolytic parameters. A subgroup of 65 patients with SCA, referred for a measured GFR evaluation in the setting of sickle cell–associated nephropathy, was further analyzed in order to better characterize metabolic acidosis.
Results
Metabolic acidosis was encountered in 42% of patients with SCA, with a higher prevalence in women (52% versus 27% in men; P<0.001). Several hemolytic biomarkers, such as lactate dehydrogenase, were different between the acidosis and nonacidosis groups (P=0.02 and P=0.03 in men and women, respectively), suggesting higher hemolytic activity in the former group. To note, fasting urine osmolality was low in the whole study population and was significantly lower in men with SCA in the acidosis group (392 versus 427 mOsm/kg; P=0.01). SCA subgroup analysis confirmed metabolic acidosis with a normal anion gap in 14 patients, characterized by a lower urinary pH (P<0.02) and no increase in urinary ammonium. Serum potassium, plasma renin, and aldosterone were similar between the two groups and thus could not explain impaired urinary ammonium excretion.
Conclusions
These results suggest that the prevalence of metabolic acidosis in patients with SCA is underestimated and related to impaired ammonium availability possibly due to an altered corticopapillary gradient. Future studies should evaluate whether chronic metabolic acidosis correction may be beneficial in this population, especially in bone remodeling.
Introduction
Sickle cell anemia (SCA), the homozygous form of sickle cell disease, is one of the most common worldwide severe monogenic diseases (1) and is responsible for anatomic and functional renal abnormalities leading to sickle cell–associated nephropathy (SCAN) in up to 70% of patients with SCA (2).
Very few studies report acid base disorders in patients with SCA (3). The prevalence of metabolic acidosis is unknown and is presumably low except in renal failure. Incomplete tubular acidosis was reported in several individuals (4–7), suggesting that this feature may be potentially frequent without acidemia. However, the links between this incomplete tubular acidosis and SCAN (e.g., hyperfiltration, albuminuria, or chronic renal failure) are unknown. Some authors speculated that this tubular dysfunction was related to papillary damage due to vasa recta occlusion (as a consequence of hemoglobin polymerization) (5), although no direct evidence was available.
This study aimed to determine the prevalence of metabolic acidosis in 421 patients and its potential link to SCAN, other SCA complications, and hemolysis. A well phenotyped subgroup of 65 patients with SCA allowed us to better characterize urinary acid excretion in this population.
Materials and Methods
Patient Population and Methods
Our study is divided into two parts. The first part is a retrospective study of patients with SCA with a regular follow-up in the Sickle Cell Centre of Tenon Hospital (Paris, France) between 2007 and 2012. During routine examination, clinical, biologic, and therapeutic parameters were collected and compared according to the presence of metabolic acidosis defined by a plasma CO2t<23 mmol/L. Data were recorded only if patients with SCA were in stable condition, with no recent acute SCA complication (e.g., acute chest syndrome, vaso-occlusion crisis, transfusion, or hydroxyurea onset). Among the 421 patients with recorded SCA, 10 patients with renal failure (8) defined by an eGFR<60 ml/min per 1.73 m2 (Modification of Diet in Renal Disease formula) were excluded. Two patients were treated with diuretics and 11 patients were taking a renin-angiotensin system inhibitor at the time of the evaluation. All patients of the Sickle Cell Centre of Tenon Hospital are advised not to use nonsteroidal anti-inflammatory drugs as a form of pain relief. No patients were treated with isoniazid or valproic acid. All data were collected from the first routine evaluation performed between 2007 and 2012. Renal hyperfiltration was defined as an eGFR>130 ml/min per 1.73 m2 for women and >140 ml/min per 1.73 m2 for men (9). Microalbuminuria was defined as an albumin excretion rate of 3–30 mg/mmol creatinine.
The second part of this study aims to further characterize metabolic acidosis among 65 well phenotyped SCA patients who were referred to the Department of Physiology for specific renal investigation in the setting of SCAN. All patients had a measured GFR≥60 ml/min per 1.73 m2 (renal 51Cr-EDTA clearance with radioactivity measurement performed with a LKB Gamma Counter 1282). In the phenotype SCA subgroup, acidosis was defined by plasma HCO3<23 mmol/L together with a venous pH≤7.36 in order to rule out a potential respiratory alkalosis. A normal plasma anion gap [(Na+K)–(Cl+CO2t)] threshold was defined as <20 mEq/L.
Venous pH, venous PCO2, and urinary pH were measured by selective electrodes (ABL 800; Radiometer). Plasma and urine assays for sodium, potassium, and chloride were also carried out on an ABL 800 analyzer by direct potentiometry using ion-selective electrodes, with plasma HCO3 deducted from the Henderson–Hasselbalch equation. The automatic blood gas analyzer also allowed plasma ionized calcium measurements. Ionogram, urea, serum creatinine, calcium, phosphorus, total calcium, lactate dehydrogenase, microalbuminuria, uric acid, and blood counts were analyzed at the Tenon Hospital central laboratory.
Plasma renin and aldosterone measurements were performed by RIA techniques. Urinary ammonium was assessed by an enzymatic assay using glutamate dehydrogenase. Serum and urinary creatinine were measured by enzymatic methods and the Jaffe method, respectively. Protein, phosphorus, urea, uric acid, and glucose were determined on a Konelab20 analyzer by a conventional colorimetric assay.
Plasma and urine osmolality were measured by freezing-point depression determination (Advanced Instruments Osmometer 3320). Fasting urine osmolality was measured on samples obtained after 10 hours of fluid restriction.
All patients with SCA gave their informed and written consent. This study was approved by the local ethics committee.
Statistical Analyses
Statistical analyses were performed by two different operators using StatView (SAS Institute, Inc.) and R software (R Project for Statistical Computing). Quantitative data were expressed as the mean±SD unless otherwise indicated and as a percentage for categorical variables. Because of sex differences for many biologic parameters especially related to hemolysis biomarkers, analyses were performed separately in women and men in study 1. Comparisons were performed using the t test or a nonparametric Wilcoxon and Mann–Whitney test whenever required. Comparisons of qualitative parameters were performed using a chi-squared test or Fisher’s exact test when necessary. Stepwise multivariate logistic regression analysis testing parameters associated with acidosis used as a dependent variable was performed. To be included in the multivariate analysis, factors had to be significantly associated with acidosis in the crude analysis (P<0.10 and noncolinear). P<0.05 was considered statistically significant.
Results
Prevalence of Metabolic Acidosis in the Whole SCA Study Population
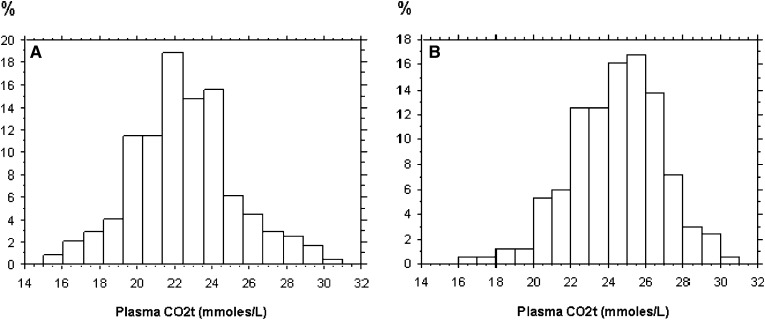
Among our population of 421 patients with SCA, 10 individuals (2.3%) had chronic renal failure (eGFR<60 ml/min per 1.73 m2) and were excluded from the analysis. Of the remaining individuals, 42% of SCA patients had a CO2t<23 mmol/L. Metabolic acidosis was present in half of the women and a quarter of the men. Table 1 provides a comparison between male and female SCA patients. Mean CO2t values appeared different according to sex (median CO2t was 24 mmol/L and 22 mmol/L, respectively, in men and women) with a Gaussian distribution as shown in Figure 1. Severe acidosis assessed by a CO2t≤20 mmol/L occurred in up to 16% of the whole SCA study population.
Table 1.
Baseline characteristics of men and women with SCA
| Characteristic | Women (n=244) | Men (n=167) | P Value |
|---|---|---|---|
| Age (yr) | 26.2±8.1 | 24.2±7.2 | 0.01 |
| Weight (kg) | 58.3±9.4 | 63.2±9.9 | <0.001 |
| Height (m) | 1.66±0.07 | 1.77±0.08 | <0.001 |
| Body mass index (kg/m2) | 21.1±3.1 | 20.1±2.8 | 0.001 |
| Systolic BP (mmHg) | 111±12 | 116±14 | <0.001 |
| Diastolic BP (mmHg) | 65±9 | 67±10 | 0.07 |
| eGFR (ml/min per 1.73 m2) | 134.3±35.5 | 157.7±44.2 | <0.001 |
| Serum creatinine (mg/dl) | 0.60±0.12 | 0.69±0.15 | <0.001 |
| Hyperfiltration (%) | 50 | 65 | 0.002 |
| Albuminuria (mg/mmol) | 2.8 (1.2–10.8) | 2.8 (1.2–8.9) | 0.50 |
| CO2t<23 mmol/L | 52 | 27 | <0.001 |
| CO2t (mmol/L) | 22.5±2.6 | 23.9±2.5 | <0.001 |
Data are presented as the mean±SD, a percentage, or the median (interquartile range).
Figure 1.
Plasma CO2t distribution in women (A) and men (B) with SCA.
Metabolic Acidosis and SCAN
Hyperfiltration was present in 65% of men and 50% of women, as previously reported (9). Although the acidosis and nonacidosis groups showed no differences in hyperfiltration prevalence, mean eGFR, and microalbuminuria values in women with SCA (Table 2), acidosis in men with SCA was associated with a higher prevalence of microalbuminuria and a lower fasting urine osmolality. To note, 29% of the study population had a low fasting urine osmolality<400 mOsm/kg H2O.
Table 2.
Renal parameters according to acid base status in women and men with SCA
| Parameter | Women with SCA | Men with SCA | ||||
|---|---|---|---|---|---|---|
| CO2t<23 mmol/L (n=126) | CO2t≥23 mmol/L (n=118) | P Value | CO2t< 23 mmol/L (n=46) | CO2t≥23 mmol/L (n=121) | P Value | |
| Plasma osmolality (mOsm/kg) | 295±13 | 295±5 | 0.95 | 293±4 | 295±5 | 0.16 |
| Urine osmolality (mOsm/kg) | 402±70 | 413±65 | 0.42 | 392±60 | 427±56 | 0.01 |
| Serum creatinine (mg/dl) | 0.61±0.12 | 0.59±0.11 | 0.25 | 0.70±0.17 | 0.69±0.14 | 0.47 |
| eGFR (ml/min per 1.73 m2) | 133.0±34.3 | 135.7±36.8 | 0.57 | 155.2±40.1 | 158.6±45.8 | 0.66 |
| Median albuminuria (mg/mmol) | 3.1 (1.6–16.0) | 2.5 (1.0–8.0) | 0.08 | 4.3 (2.4-13.4) | 2.1 (1.1-6.3) | 0.004 |
| Microalbuminuria (%) | 40 | 34 | 0.36 | 63 | 38 | 0.01 |
| Hyperfiltration (%) | 49 | 51 | 0.80 | 67 | 64 | 0.72 |
Data are presented as the mean±SD, a percentage, or the median (interquartile range).
Metabolic Acidosis and Other SCA Complications
The acidosis and nonacidosis groups were similar for age, body mass index, BP, and usual acute or chronic complications of SCA such as leg ulcers, acute chest syndrome, or retinopathy (Table 3). The prevalence of hydroxyurea treatment and transfusions was also similar in both groups.
Table 3.
Clinical and biologic characteristics according to acid base status in women and men with SCA
| Parameter | Women with SCA | Men with SCA | ||||
|---|---|---|---|---|---|---|
| CO2t<23 mmol/L (n=126) | CO2t≥23 mmol/L (n=118) | P Value | CO2t<23 mmol/L (n=46) | CO2t≥23 mmol/L (n=121) | P Value | |
| Age (yr) | 25.6±7.5 | 26.8±8.7 | 0.23 | 24.6±8.6 | 24.1±6.6 | 0.72 |
| Body mass index (kg/m2) | 21.1±3.0 | 21.2±3.2 | 0.8 | 20.3±2.8 | 20.1±2.8 | 0.69 |
| Systolic BP (mmHg) | 111±13 | 110±11 | 0.52 | 115±12 | 116±14 | 0.61 |
| Diastolic BP (mmHg) | 65±9 | 65±9 | 0.67 | 67±8 | 66±11 | 0.59 |
| CO2t (mmol/L) | 20.6±1.5 | 24.6±1.7 | <0.001 | 20.8±1.5 | 25.1±1.6 | <0.001 |
| Blood glucose (mmol/L) | 4.73±0.59 | 4.72±0.50 | 0.88 | 4.81±0.59 | 4.71±0.59 | 0.40 |
| Hemoglobin (g/dl) | 8.53±1.19 | 8.65±1.23 | 0.47 | 8.66±1.86 | 9.21±1.16 | 0.02 |
| Reticulocyte count (103/mm3 per/L) | 323±118 | 289±122 | 0.03 | 325±141 | 318±148 | 0.79 |
| Lactate dehydrogenase (IU/L) | 453±201 | 399±172 | 0.03 | 502±187 | 426±172 | 0.02 |
| Total bilirubin (mg/dl) | 3.0±1.9 | 2.7±1.6 | 0.13 | 3.7±2.4 | 3.4±2.1 | 0.4 |
| Plasma uric acid (mg/dl) | 4.8±1.4 | 4.4±1.3 | 0.04 | 6.6±1.7 | 5.9±1.4 | 0.01 |
| Hemoglobin F (%) | 8.3±5.1 | 8.0±5.3 | 0.73 | 5.9±4.8 | 5.4±4.5 | 0.53 |
| Leg ulcer | 6 | 9 | 0.45 | 18 | 15 | 0.64 |
| Retinopathy | 39 | 46 | 0.4 | 38 | 44 | 0.55 |
| Acute chest syndrome | 52 | 47 | 0.52 | 56 | 53 | 0.68 |
| Transfusion | 10 | 19 | 0.07 | 24 | 19 | 0.61 |
| Hydroxyurea | 19 | 22 | 0.63 | 17 | 21 | 0.76 |
| Priapism | 29 | 19 | 0.20 | |||
Data are presented as the mean±SD or a percentage.
Determinants of Metabolic Acidosis
As shown in Table 3, plasma lactate dehydrogenase and serum uric acid levels were significantly higher in the acidosis group in both sexes, suggesting stronger hemolytic activity. Accordingly, the reticulocyte count was higher in women with acidosis and the hemoglobin level was lower in men with acidosis.
Using logistic regression analysis, acidosis in male patients was independently associated with a fasting urine osmolality<400 mOsm/kg (odds ratio [OR], 3.5; 95% confidence interval [95% CI], 1.05 to 11.5; P=0.04) and a hemoglobin level below the lower quartile (8.1 g/dl; OR, 5.1; P=0.01). In female patients, a high reticulocyte count (above median values), but not fasting urine osmolality, was associated with acidosis (OR, 2.1; 95% CI, 1.26 to 3.49; P=0.004).
Acid Base Status and Urinary Acid Excretion in the SCA Subgroup
Fasting and 24-hour urine collection data from 65 patients referred to our Department of Physiology for a SCAN evaluation including an isotopic GFR measurement were retrospectively analyzed for acid base evaluation.
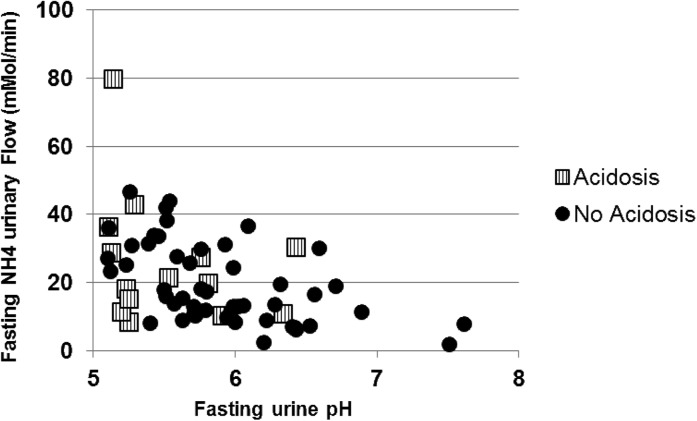
Fourteen patients (22%) had a fasting plasma HCO3−<23 mmol/L. All of these patients also had a venous pH≤7.36 and were thus considered to have metabolic acidosis. All 14 patients had a normal plasma anion gap. Sex and clinical parameters were similar between the acidosis and nonacidosis groups. Measured GFR values were similar between the two groups. Venous pH and PvCO2 were significantly lower in the acidosis group, with no difference in PvO2 values between the two groups (Table 4). Fasting urinary pH and urinary pH from the 24-hour urine collection were lower in the acidosis group. In this latter group, the fasting pH value was <5.5 for 9 of 14 patients and >6.0 for 2 patients. Only one patient, in the acidosis group, had an increased ammonium excretion value when plotted against urinary pH (Figure 2). Decreased ammonium availability was not explained by differences between the two groups for plasma potassium, plasma renin, and aldosterone levels (Table 4). No patient also had evidence of glycosuria, hypouricemia, or hypophosphatemia. Conversely, hyperphosphatemia was present in up to 40% of patients with no difference between the two groups.
Table 4.
Metabolic parameters according to acid base status in the SCA subgroup
| Parameter | Acidosis (n=14) | No Acidosis (n=51) | P Value |
|---|---|---|---|
| Plasma | |||
| Measured GFR (ml/min per 1.73 m2) | 115.4±22.1 | 121.3±30.2 | 0.43 |
| K+ (mmol/L) | 4.2±0.4 | 4.1±0.3 | 0.4 |
| Plasma phosphate (mg/dl) | 4.0±0.8 | 3.7±0.6 | 0.26 |
| Plasma uric acid (mg/dl) | 7.0±2.0 | 6.3±1.7 | 0.43 |
| Blood glucose (mg/dl) | 90.6±8.8 | 87.9±7.9 | 0.34 |
| HCO3− (mmol/L) | 21.7±0.9 | 25.6±1.6 | <0.001 |
| Venous pH | 7.31±0.03 | 7.34±0.04 | 0.03 |
| PvCO2 (mmHg) | 44.9±4.7 | 49.7±6.5 | 0.01 |
| PvO2 (mmHg) | 48.4±13.1 | 47.7±12.1 | 0.99 |
| Plasma renin (pg/ml) | 59.6±86.5 | 21.6±18.8 | 0.12 |
| Plasma aldosterone (pg/ml) | 77.0±63.6 | 79.3±71.2 | 0.67 |
| Aldosterone/renin ratio | 3.4±3.1 | 5.7±5.1 | 0.08 |
| Urine | |||
| Urine aldosterone (µg/24 h) | 7.4±6.0 | 8.0±7.1 | 0.94 |
| Fasting urine pH | 5.5±0.4 | 5.9±0.6 | 0.02 |
| Fasting urine PCO2 (mmHg) | 32.7±9.9 | 38.6±8.7 | <0.01 |
| Fasting bicarbonaturia (mmol/L) | 0.6±0.8 | 3±9.2 | 0.01 |
| Fasting ammonium excretion (µmol/min) | 25.8±18.7 | 19.4±11.5 | 0.26 |
| Sodium excretion (mmol/24 h) | 90±52 | 122±62 | 0.06 |
| Potassium excretion (mmol/24 h) | 27±12 | 35±18 | 0.19 |
| Urine phosphate (mg/24 h) | 47.1±20.5 | 48.4±24.6 | 0.84 |
| Urine uric acid (mg/24 h) | 49.9±20.0 | 59.8±33.4 | 0.36 |
| Estimated protein intake (g/kg per 24 h) | 0.85±0.39 | 0.85±0.39 | 0.95 |
| Ammonium excretion (mmol/24 h) | 22.2±7.4 | 19.1±12.6 | 0.12 |
| Estimated titratable acid (mmol/24 h) | 10.2±5.0 | 8.1±5.3 | 0.24 |
| Net acid excretion (mmol/24 h) | 32.5±10.3 | 27.3±16.7 | 0.12 |
| 24-h urine pH | 5.8±0.5 | 6.3±0.5 | 0.002 |
| Urine output (ml/24 h) | 1533±599 | 1771±709 | 0.22 |
Data are presented as the mean±SD.
Figure 2.
Association between fasting urine NH4 excretion and fasting urine pH in acidosis and nonacidosis groups with SCA (n=65).
No dietary differences for water intake, sodium chloride, and protein intake were detected between the acidosis and nonacidosis groups according to 24-hour urine natriuresis, and urea and 24-hour urinary net acid excretion (estimated by the sum of ammonium excretion and titratable acid) was not significantly different between the two groups.
Discussion
The prevalence of metabolic acidosis in this study was high in patients with SCA according to sex (27% and 52% in our male and female populations, respectively). Although metabolic acidosis was assessed by a venous CO2t threshold<23 mmol/L, more severe acidosis assessed by a CO2t≤20 mmol/L occurred in up to 16% of patients with SCA. Respiratory alkalosis was reasonably ruled out among the subgroup of 65 patients referred to our department for further evaluation of albuminuria in the context of SCAN: all SCA patients (n=14) with a blood bicarbonate<23 mmol/L had a venous pH value≤7.36. Although the presence of incomplete distal renal tubular acidosis (assessed by acidification tests) was previously investigated in several patients with SCA with no overt acidemia and is considered by some authors as a frequent feature (4–7), metabolic acidosis was only reported in rare cases generally associated with hyperkalemia and/or renal failure (3). Thus, such a high prevalence of metabolic acidosis in the SCA population with no renal failure is surprising.
The Gaussian distribution of CO2t values with a median 23 mmol/L suggests that CO2t below this value may be a general feature of SCA. Our finding that the prevalence of acidosis is different according to sex (more than half of women and a quarter of men) is also a new finding with no obvious explanation to date. Interestingly, this between-sex difference is also observed in a sickle cell hemoglobin C (SC) disease population, although the prevalence of metabolic acidosis is much lower (27% and 8% in female and male patients with SC, respectively; P=0.001; personal data, F. Lionnet). Thus, the high prevalence of metabolic acidosis both in SCA and SC patients suggests a similar pathologic process: the milder phenotype in the SC population could be explained by lower hemolysis activity (10). This different order of magnitude between SCA and SC patients was highlighted by Goossens et al. in 1972: acidification tests showed a normal acid excretion in patients with sickle cell trait, impaired acid excretion in patients with SCA, and an intermediate decrease in patients with SC (5).
The second part of our study aimed to characterize metabolic acidosis in a selected subgroup of 65 patients with SCA. Fourteen patients with metabolic acidosis with a normal anion gap had a significantly lower PvCO2, suggesting expected hyperventilation related to respiratory regulation. Urinalysis provides interesting findings in order to characterize renal acidosis: fasting urinary pH values<5.5 are present in most patients with acidosis despite the lack of ammonium excretion increase in 24-hour urine collection. Moreover, fasting ammonium excretion expressed according to urine pH is not increased in the acidosis group compared with the nonacidosis group (Figure 2), whereas NH4+ synthesis is expected to be maximal in acidosis patients. Altogether, these data are in accordance with renal tubular acidosis and demonstrate an impairment of ammonium buffer availability, rather than a primary defect in collecting duct H+ secretion in most patients (except two with a reported urinary pH>6.0). These data are further strengthened by a lower 24-hour urine pH value in the acidosis group compared with the nonacidosis group, whereas 24-hour urinary net acid excretion was similar, with no evidence for any proximal tubular dysfunction because plasma phosphate, potassium, and uric acid were comparable in the two groups. Decreased availability of the ammonia buffer was not associated with hyperkalemia or low plasma renin and aldosterone in accordance with one previous report (7).
In patients with incomplete tubular acidosis, impaired ammonium excretion was reported only once (7), and the view of a mild reversible H+ secretion defect in the collecting duct is rather prevailing: the capacity of urine acidification during the acidification test was restored after increasing the trans-tubular gradient by sodium sulfate administration (4–6). The reported defect of vasa recta vascularization was suggested to explain incomplete acidosis in this population (5,11). In agreement with this latter hypothesis, our data in patients with SCA with true acidosis suggest that a more pronounced defect (e.g., increased vasa recta rarefaction) could explain corticopapillary gradient impairment leading both to a water concentrating defect (low fasting urinary osmolality) and decreased NH3/NH4+ availability. This corticopapillary gradient defect hypothesis is further supported by the fact that patients with SC who were reported to have fewer vasa recta lesions (11) also had a lower prevalence of metabolic acidosis and a higher fasting urine osmolality (12,13). Accordingly, our data suggest that hemolysis with possible chronic heme and iron toxicity (14), rather than viscosity, is more likely to be involved in this process. Further work is needed to study whether an impaired NH3/NH4+ gradient could also be explained by the occurrence of downregulation of Na+/H+ exchangers within the medullary thick ascending limb of epithelial cells in the loop of Henle (15). Alternatively, other pathogenic processes may explain renal acidosis such as a decreased NH4+ production in the proximal convoluted tubule (secondary to a defect in the synthesis pathway of ammonium) or a decreased ammonium channel expression in collecting duct cells (16). These hypotheses are not mutually exclusive and deserve to be specifically addressed in experimental models because no current data are available to obtain further insight.
The prevalence of metabolic acidosis in adults with SCA is underestimated and is unexpectedly more frequent in women. In our view, the prevalence of metabolic acidosis deserves special attention in routine follow-up because chronic metabolic acidosis may worsen several frequent pathologic features of SCA related to CKD, bone marrow turnover, and especially bone mineral density, which is reported to be low in this population (17). Our data point out that renal acidosis is due to an impaired ammonium excretion in most cases, rather than a distal tubular defect, and support the view that low ammonium availability is due to a hemolysis-related impaired corticopapillary gradient.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rees DC, Williams TN, Gladwin MT: Sickle-cell disease. Lancet 376: 2018–2031, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Guasch A, Navarrete J, Nass K, Zayas CF: Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 17: 2228–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA: Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 72: 188–192, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Kong HH, Alleyne GA: Studies on acid excretion in adults with sickle-cell anaemia. Clin Sci 41: 505–518, 1971 [DOI] [PubMed] [Google Scholar]
- 5.Goossens JP, Statius van Eps LW, Schouten H, Giterson AL: Incomplete renal tubular acidosis in sickle cell disease. Clin Chim Acta 41: 149–156, 1972 [DOI] [PubMed] [Google Scholar]
- 6.Oster JR, Lespier LE, Lee SM, Pellegrini EL, Vaamonde CA: Renal acidification in sickle-cell disease. J Lab Clin Med 88: 389–401, 1976 [PubMed] [Google Scholar]
- 7.DeFronzo RA, Taufield PA, Black H, McPhedran P, Cooke CR: Impaired renal tubular potassium secretion in sickle cell disease. Ann Intern Med 90: 310–316, 1979 [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 9.Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, Grateau G, Baud L, Girot R, Lionnet F: Glomerular hyperfiltration in adult sickle cell anemia: A frequent hemolysis associated feature. Clin J Am Soc Nephrol 5: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballas SK, Lewis CN, Noone AM, Krasnow SH, Kamarulzaman E, Burka ER: Clinical, hematological, and biochemical features of Hb SC disease. Am J Hematol 13: 37–51, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J: Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet 1: 450–452, 1970 [DOI] [PubMed] [Google Scholar]
- 12.Statius van Eps LW, Schouten H, Haar Romeny-Wachter CC, La Porte-Wijsman LW: The relation between age and renal concentrating capacity in sickle cell disease and hemoglobin C disease. Clin Chim Acta 27: 501–511, 1970 [DOI] [PubMed] [Google Scholar]
- 13.Francis YF, Worthen HG: Hyposthenuria in sickle cell disease. J Natl Med Assoc 60: 266–270, 1968 [PMC free article] [PubMed] [Google Scholar]
- 14.Tracz MJ, Alam J, Nath KA: Physiology and pathophysiology of heme: Implications for kidney disease. J Am Soc Nephrol 18: 414–420, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P: NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest 120: 1895–1904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner ID, Verlander JW: Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldanzi G, Traina F, Marques Neto JF, Santos AO, Ramos CD, Saad ST: Low bone mass density is associated with hemolysis in Brazilian patients with sickle cell disease. Clinics (Sao Paulo) 66: 801–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]