Abstract
Intradialytic hypotension is the most common adverse event that occurs during the hemodialysis procedure. Despite advances in machine technology, it remains a difficult management issue. The pathophysiology of intradialytic hypotension and measures to reduce its frequency are discussed. An accurate assessment of dry weight is crucial in all patients on dialysis and especially those patients prone to intradialytic hypotension. The presence of edema and hypertension has recently been shown to be a poor predictor of volume overload. Noninvasive methods to assess volume status, such as whole body and segmental bioimpedance, hold promise to more accurately assess fluid status. Reducing salt intake is key to limiting interdialytic weight gain. A common problem is that patients are often told to restrict fluid but not salt intake. Lowering the dialysate temperature, prohibiting food ingestion during hemodialysis, and midodrine administration are beneficial. Sodium modeling in the absence of ultrafiltration modeling should be abandoned. There is not enough data on the efficacy of l-carnitine to warrant its routine use.
Case Presentation
A 65-year-old man was on hemodialysis since 2006 as a result of long-standing type II diabetes mellitus. Other medical problems included hypertension, hyperthyroidism treated with total thyroidectomy, history of subtotal parathyroidectomy, sickle cell trait, gastroesophageal reflux disease, severe peripheral vascular disease, coronary artery disease, moderate concentric left ventricular hypertrophy (LVH), and diabetic retinopathy. His medications were metoprolol, lisinopril, gabapentin, cinacalcet, calcium acetate, lanthanum carbonate, levothyroxine, and omeprazole.
On physical examination, lungs were clear to auscultation, cardiac rhythm was regular, an S4 was audible, no edema was present, and there was a brachiobasilic fistula in the left arm. Relevant laboratory studies included sodium=139 mEq/L, potassium=4.6 mEq/L, calcium 8.9=mg/dl, phosphorus=6.5 mg/dl, parathyroid hormone=558 pg/ml, albumin=3.4 g/dl, and hemoglobin=11.5 g/dl. Dialysis duration was 4 hours. Dialysate composition was 2.0 mEq/L potassium and 2.5 mEq/L calcium with a citrate, and not acetate-based, acid concentrate. The single pool Kt/V on this prescription was 1.49. His average interdialytic weight gain was 4 kg per treatment, and his dry weight was 98.5 kg.
During a chronic outpatient dialysis session, he developed intradialytic hypotension (IDH). BPs during the treatment are shown in Table 1 (treatment 1). His predialysis temperature was 36.2°C. At the BP indicated in Table 1, he felt poorly and was diaphoretic. In response, saline was administered, ultrafiltration was stopped, and the patient was placed in a reclining position with resolution of the hypotension. He had a previous history of IDH and as a result, was already being dialyzed with cool dialysate (temperature=35.5°C) and ultrafiltration modeling. Given the apparent absence of signs of volume, his dry weight was increased to 99.5 kg. Despite this increase, 9 days later, he developed another episode of IDH at the BP indicated in Table 1 (treatment 2). On this day, his predialysis temperature was 35.8°C. Once again, his dry weight was increased (to 100.5 kg.) At that time, he received dietary counseling on limiting salt intake, which resulted in a reduction of his interdialytic weight gain from 4 to 2.5 kg. Over the subsequent 2 months, there were no additional episodes of IDH. His predialysis BP has been stable around 130/60 mmHg.
Table 1.
BP on dialysis
| Time into Treatment (min) | BP (mmHg) |
| Treatment 1 | |
| 0 | 164/72 |
| 5 | 163/72 |
| 35 | 154/69 |
| 65 | 151/69 |
| 95 | 158/69 |
| 125 | 139/62 |
| 140 | 131/60 |
| 169 | 129/62 |
| 186 | 126/62 |
| 200 | 109/55 |
| 215 | 88/39a |
| 225 | 115/54 |
| 230 | 108/50 |
| Treatment 2 | |
| 0 | 126/58 |
| 5 | 124/58 |
| 40 | 126/58 |
| 70 | 122/60 |
| 100 | 140/63 |
| 131 | 119/62 |
| 143 | 117/53 |
| 161 | 114/53 |
| 183 | 107/50 |
| 191 | 96/46 |
| 212 | 85/49a |
| 221 | 109/48 |
| 251 | 103/51 |
Intervention carried out. Treatment 1 stopped early at 230 minutes.
Introduction
When initially evaluating the patient with hypotension on hemodialysis, it is important to rule out acute conditions that can lower BP. These conditions include, but are not limited to, infections (especially involving the access if the patient has a permcath or a graft), pneumonia, cellulitis, and osteomyelitis; blood loss; new onset of cardiac arrhythmias, such as supraventricular tachycardia or atrial fibrillation; and pericardial effusion. The patient had none of these diagnoses. After these diagnoses were ruled out, evaluation and management became evaluation and management of IDH in an otherwise stable patient.
Background and Definition
IDH remains one of the most vexing management challenges for nephrologists. It has three essential components: a drop in BP generally defined as ≥20 mmHg systolic BP or ≥10 mmHg in mean arterial pressure; the presence of symptoms of end organ ischemia; and an intervention carried out by the dialysis staff (1). The intervention can be a reduction in ultrafiltration rate, administration of saline, albumin, or mannitol, or placement of the patient in a reclining position, all of which were done in this patient. Despite advances in hemodialysis technology over the last several decades, the incidence of IDH still remains unacceptably high. A variety of factors are responsible for this finding. Some factors are modifiable by either the patient or the nephrologist, and other factors, such as the presence of autonomic neuropathy, are not. Consequences of IDH can range from annoying (cramping and postdialysis fatigue) to devastating (bowel ischemia, stroke, myocardial infarction, and access thrombosis). In addition, IDH may compromise our ability to provide adequate dialysis from both a clearance and volume removal standpoint, which was seen in this patient when a hemodialysis treatment was stopped early at the patient’s request. To better understand how to manage patients with IDH, we need to understand why it occurs. Then, we will focus on interventions that were either considered or carried out in this patient to try and lessen its frequency (Table 2).
Table 2.
Interventions that may be used for intradialytic hypotension
| Establish an accurate dry weight |
| Reduce ultrafiltration rate |
| Limit interdialytic weight gain by reducing salt intake |
| Prohibit food ingestion during hemodialysis |
| Consider adjusting antihypertensive medications or timing |
| Cool dialysate |
| Ultrafiltration modeling |
| Dialysate sodium modeling |
| Increase dialysate calcium |
| l-Carnitine |
| Midodrine |
Pathophysiology
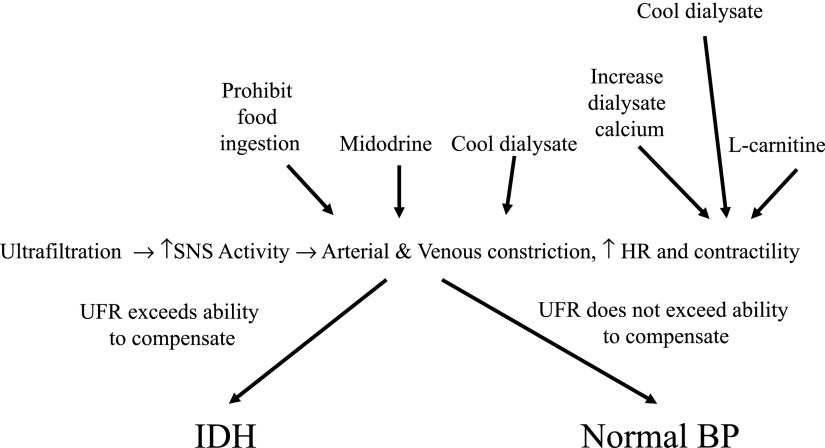
IDH complicates approximately 15%–30% of all hemodialysis treatments and occurs in >50% of treatments in a smaller number of patients (2). It results from two basic processes. The first process is the patient’s inability to tolerate, from a BP standpoint, hemodialysis-associated ultrafiltration. This is shown in Figure 1 along with interventions designed to mitigate it, which will be discussed. There is an imbalance between central hypovolemia and the adequacy of reflex-mediated hemodynamic responses. The body has several defenses against hypovolemia. These defenses include increasing vascular resistance to splanchnic and cutaneous beds, which results in shunting blood to the central circulation, and increasing heart rate and contractility. Decreased venous return is the most important factor that impairs the body’s ability to maintain cardiac output with volume depletion. Optimizing cardiac output by increasing heart rate or contractility is of little value in the absence of adequate venous return. Why do some ESRD patients not compensate appropriately to ultrafiltration? It can result from autonomic or baroreceptor failure or disturbed cardiac function. Diabetes, aging, and uremia can cause autonomic and baroreceptor dysfunction, leading to excessive venous pooling and aberrant vasodilation. Cardiac diseases, such as LVH, ischemic heart disease, and the recently appreciated concept of myocardial stunning, contribute to cardiac dysfunction with IDH (3). Our patient had both long-standing diabetes and moderate concentric LVH, which likely increased his susceptibility to IDH.
Figure 1.
Cardiovascular responses to plasma volume depletion. These adaptive responses can be either abnormal or overwhelmed by a high ultrafiltration rate. Interventions that affect various stages are shown and discussed. HR, heart rate; IDH, intradialytic hypotension; SNS, sympathetic nervous system; UFR, ultrafiltration rate.
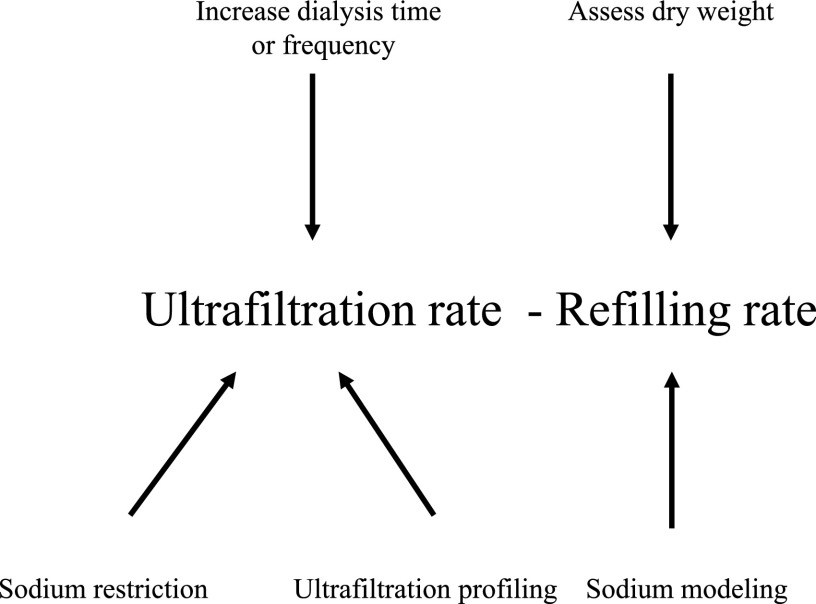
The second mechanism is related to the degree of fall in plasma volume that occurs during hemodialysis. With ultrafiltration, fluid removed from the vascular space is replaced by fluid from the interstitial space (plasma refilling). This is shown in Figure 2; also shown are mechanisms to attempt to address mismatches between a decline in plasma volume from ultrafiltration and plasma refilling, which are discussed subsequently. The rate at which refilling occurs is dependent on the size of the interstitial space. When the interstitial space is expanded, reduction in size of the plasma volume with ultrafiltration is relatively smaller. When the interstitial space is smaller, reduction in size of the plasma volume will be relatively larger, and IDH will be more likely to occur. In the latter case, IDH occurs because the dry weight has been set too low, which, in retrospect, was the case in our patient. In the former case, the patient is often hypertensive and has arrived for the dialysis session 3 or more kg above dry weight, which results in an ultrafiltration rate that may exceed the refilling rate and consequent IDH. In this situation, raising dry weight to minimize IDH would perpetuate a state of pressure–volume overload, resulting in hypertension, LVH, and prescription of antihypertensive medication that would likely be unnecessary if the appropriate dry weight was achieved. In this scenario, IDH limits the ability to achieve dry weight, because the ultrafiltration rate often needs to be reduced.
Figure 2.
Relationship between ultrafiltration rate and plasma refilling rate. An imbalance between these rates favoring a decrease in plasma volume can set the stage for intradialytic hypotension. Potential interventions are shown.
Assessment of Extracellular Fluid Volume
Establishing an accurate dry weight in hemodialysis patients is critical. Errors in dry weight assessment set the stage for IDH. Dry weight was recently defined by Sinha and Agarwal as “the lowest tolerated postdialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia” (4). Probing to achieve dry weight should be done with caution given the potential adverse consequences of hypotension in an ESRD population with multiple comorbidities that predispose to end organ ischemia. Achievement of even a relatively small reduction in dry weight (1 kg) in hypertensive hemodialysis patients reduced systolic and diastolic BPs by 6.6 and 3.3 mmHg, respectively, in the Dry Weight Reduction in Hypertensive Hemodialysis Patients trial (5).
The patient’s dry weight was raised, in part, because he did not have peripheral edema, which is commonly considered a sign of volume overload in hemodialysis patients. This concept, however, was recently challenged. Agarwal et al. (6) studied 150 hemodialysis patients from four different centers. Volume was assessed using intradialytic blood volume monitoring, echocardiographic measures (including inferior vena cava diameter), and biochemical markers, such as renin, aldosterone, and N-terminal pro–B-type natriuretic peptide. Although pedal edema correlated with age, left ventricular mass, and body mass index, it did not correlate with markers of intravascular volume. In addition, the presence of hypertension was also recently shown to be a poor predictor of the presence of volume overload. Wabel et al. (7) examined 500 hemodialysis patients from eight centers and measured predialysis BP and deviation of hydration status from normal using multiple frequency bioimpedance; 13% of patients had hypertension but were not hypervolemic, whereas 10% of patients were hypervolemic but did not have hypertension. This finding shows that hypertension does not always equal hypervolemia and vice versa in hemodialysis patients. It is not a complete surprise in that factors other than volume expansion play a role in the genesis of hypertension in hemodialysis patients, such as sympathetic nervous system activation, vascular stiffness, and cardiac function. Clearly, we need better tools to augment the physical examination to assess volume overload.
Several noninvasive methods to assess volume status in hemodialysis patients are either currently available or in development. Relative plasma volume monitoring is a hematocrit-based method in which an optical sensing device is placed at the arterial end of the dialyzer. A percent blood volume change is calculated based on change in hematocrit in real time during the hemodialysis session. It provides an estimate of the relative rates of decrease in plasma volume caused by ultrafiltration versus plasma refilling from the interstitial space. If the patient is relatively volume-expanded, then the refilling rate will match the decrease in plasma volume from ultrafiltration, and the curve of relative plasma volume over time will be relatively flat. If the plasma refilling rate does not match the loss from the plasma compartment caused by ultrafiltration, because the patient is closer to their dry weight or the ultrafiltration rate is excessive, then the relative plasma volume will decline over time. This method does not measure absolute blood volume but only relative changes. It was hoped that there would be a critical relative blood volume (the crash crit) for each patient that could be identified, below which IDH would be more likely to occur. Unfortunately, this concept has not been borne out in clinical trials. Although the concept is logical, enthusiasm for this method waned with the publication of the Crit-Line Intradialytic Monitoring Benefit study trial, which showed that patients dialyzed with blood volume monitoring over a 6-month period versus controls had higher mortality (8.7% versus 3.3%) (8). Hospitalization rates were also higher in the intervention group.
Measurement of inferior vena cava diameter has also been used as a measure of intravascular volume, but it is difficult to interpret in patients with heart failure and tricuspid regurgitation. There is also a significant degree of intra- and interobserver variability and, as a result, this technique has not been routinely used. Whole-body bioimpedance is the most promising of the techniques, but it is cumbersome. Single frequency devices can only measure total body water and are not that helpful, whereas multifrequency devices measure both total body water and extracellular water. Whole-body bioimpedance is based on the assumption that the body is a cylinder with uniform conductivity, which is not true given that the limbs provide 90% of total body resistance but only 30% of total body water. Segmental devices that measure bioimpedance in the calf do not suffer from this problem (9). In addition, manufacturers recommend that patients with pacemakers, stents, artificial joints, or amputation(s) and patients who are pregnant not undergo bioimpedance analysis for safety and/or performance issues. These patients constitute a significant percentage of the ESRD population.
Treatment
When the rate of fluid loss from the plasma caused by ultrafiltration exceeds the refilling rate and the patient’s cardiovascular system is unable to compensate for the decline in plasma volume, one approach is to reduce the need for a high ultrafiltration rate chronically. It can be accomplished by limiting interdialytic weight gain by sodium restriction or increasing the time or frequency of hemodialysis. A common misconception that exists is that reduction in interdialytic weight gain is achieved by restricting fluid, which ignores the most basic principles of salt and water homeostasis. The main determinant of extracellular fluid volume is sodium. In addition, salt intake and small increases in sodium concentration may increase thirst, driving fluid ingestion. Therefore, salt and not water restriction should be emphasized (10). This process involves making informed and healthy food choices, because only 15% of salt ingested in the United States is added during the cooking process or at the table (11). The rest is added during the processing of foods. There is evidence that centers that use a salt restriction approach have patients with lower interdialytic weight gains, less antihypertensive medication use, and lower left ventricular masses, despite comparable BPs (12). Increasing the dialysis time is often resisted by the patient, but IDH may be less frequent and BP may be better controlled with long dialysis treatments, nocturnal dialysis, or daily hemodialysis. More frequent hemodialysis schedules are also associated with fewer episodes of left ventricular regional wall motion abnormalities (13). Large interdialytic weight gains and high ultrafiltration rates are not just problematic from an IDH standpoint but also associated with increased cardiovascular morbidity and mortality (14).
Food ingestion is often prohibited during the hemodialysis procedure. After a meal, peripheral vascular resistance decreases, and blood flow to the splanchnic circulation and liver increase. In addition, baroreceptor responses are impaired after glucose ingestion. Patients with autonomic dysfunction are particularly prone to hypotension after meals. All of these factors predispose to IDH, which Sherman et al. (15) showed in a prospective controlled trial of 125 hemodialysis treatments in nine nondiabetic patients. Mean BP fell by 14.4 mmHg/h 45 minutes after a meal consumed between 1.5 and 2 hours after the start of dialysis versus 2.2 mmHg/h in the control period. The effect may be even more pronounced in diabetic patients.
One should consider adjusting antihypertensive medications or their timing in the patient with IDH. There is little hard data to guide us in this respect. Many nephrologists hold antihypertensive drugs before hemodialysis based on the rationale that poorer BP control in the short term is preferable to IDH, which is especially true for direct venous (nitrates) and arterial (hydralazine) dilators. β-Blockers and nondihydropyridine calcium channel blockers may be associated with a reduced incidence of IDH in patients with LVH and diastolic dysfunction. My practice is to use either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker as a first-line agent followed by a β-blocker or a combined β- and α-blocker, because most ESRD patients have cardiovascular disease. This patient was on lisinopril and metoprolol. Amlodipine is a reasonable third-line agent given its long half-life, and at least in one randomized prospective clinical trial in ESRD patients, it did not increase the incidence of IDH (16). We avoid short-acting drugs, such as clonidine, that need to be administered frequently whenever possible. One can also administer once daily antihypertensive medications at bedtime.
Core body temperature increases during the course of a hemodialysis treatment because of peripheral vasoconstriction. After core temperature rises to a critical level, peripheral vasodilation occurs. This results in heat dissipation, but at the expense of a decrease in peripheral vascular resistance and a fall in BP. The patient can either be cooled slightly or maintained at the same core temperature that was present at the start of hemodialysis (isothermic). In most cases, a dialysate temperature is chosen between 35°C and 35.5°C. Devices exist (blood temperature monitor) that can provide a set change in body temperature or maintain a fixed temperature, but they are not available in all units. Cooling the patient by 0.5°C may be slightly better than the isothermic approach (17). Cool dialysate has a variety of other beneficial effects that result in a reduction of IDH events, including reduction in left ventricular regional wall motion abnormalities, enhancement of peripheral vasopressor responses, and increase in baroreceptor sensitivity. A systematic review showed that IDH was 7.1 (95% confidence interval [95% CI], 5.3 to 8.9) times more frequent with standard dialysate temperatures than cool dialysis and that mean arterial pressure was 11.3 mmHg (95% CI, 7.7 to 15.0 mmHg) higher with cool dialysate (18). Side effects, an unpleasant cold feeling, were 2.0 (95% CI, 0.4 to 3.6) times more frequent with cool dialysate. This patient’s dialysis temperature was set at 35.5°C. It is important to recognize that many dialysis patients are hypothermic, which was the case with this patient. Before the second episode of IDH, his predialysis temperature was only 35.8°C, and the dialysate temperature may need to be lowered further if episodes of IDH recur. A dialysate temperature of 37°C in this patient would create the potential for core warming.
Modifications to the hemodialysis machine can be made to model ultrafiltration rate and dialysate sodium concentration during the treatment. Our patient’s machine was programmed to carry out ultrafiltration modeling. Ultrafiltration modeling was developed to provide higher ultrafiltration rates early in the hemodialysis procedure when the interstitial space is larger and plasma refilling is higher. As the treatment proceeds, the interstitial space decreases in size, refilling rates decline, and ultrafiltration rate is reduced. Intravascular volume during hemodialysis decreases as a result of two processes. The first process is ultrafiltration, and the second process is solute removal by diffusion. Solute removal reduces extracellular fluid osmolality and creates a driving force for fluid movement into cells, which is prevented by increasing dialysate sodium concentration. As sodium concentration is increased, fluid moves out of the intracellular space, the interstitial space increases in size, and plasma refilling is augmented. However, increasing dialysate sodium concentration stimulates thirst, results in larger interdialytic weight gains, and increases BP. Sodium modeling was developed to try and reduce these adverse effects. Sodium concentration in the dialysate is higher early in the treatment when rates of diffusive solute clearance are higher, and then, it is gradually reduced. However, the time-averaged sodium concentration can result in positive sodium balance, which over the long term, is detrimental. In addition, if a higher than mean dialysate sodium concentration is used early, it must then be balanced by a lower than mean sodium dialysate concentration later in the treatment, which may result in an increase in IDH during this period. Therefore, Kidney Disease Outcomes Quality Initiative recommends that “use of high dialysate sodium concentration and sodium profiling should be discouraged” (19). Ultrafiltration and sodium modeling seem to be most beneficial when combined together in a biofeedback system (20).
High dialysate calcium concentration is associated with increased stroke volume, systolic BP, and postdialysis calcium concentration (21). In a prospective, double-blind, randomized trial involving 240 hemodialysis treatments, 20 hemodialysis patients were dialyzed alternately with a dialysate calcium concentration of 2.5 or 3.5 mEq/L (22). At 1.5 hours, the systolic, diastolic, and mean BPs were 6, 3.6, and 4.6 mmHg higher with the 3.5 mEq/L calcium dialysate compared with the 2.5 mEq/L concentration, respectively. However, in a small subgroup of patients prone to IDH, change in mean arterial pressure was much smaller (2.3 mmHg). In this subgroup, there was no significant reduction in IDH frequency. Given the potential long-term adverse effects of positive calcium balance in ESRD patients and the relatively modest effect of increasing dialysate calcium concentration on BP in patients with IDH, in general, we do not increase dialysate calcium concentration in patients with IDH.
l-Carnitine was approved by the US Food and Drug Administration for use in hemodialysis patients in 1999. It plays a role in mitochondrial energy metabolism in skeletal and cardiac muscle, and its deficiency is common in ESRD patients. A meta-analysis of five studies totaling 162 patients failed to show a statistically significant reduction in IDH (23). The pooled odds ratio was 0.28, but the 95% CI was very wide (0.04 to 2.23) because of the small number of studies. The reduction in odds ratio was largely driven by one study. If that study was eliminated, the odds ratio increased to 0.84. The studies were also very heterogeneous, with doses of l-carnitine ranging from 2100 to 14,000 mg/wk. The route of administration was also not uniform; both intravenous and oral studies were included. We do not recommend using l-carnitine given the paucity of evidence regarding its efficacy.
Midodrine is a prodrug that is rapidly absorbed and transformed into the active metabolite desglymidodrine, which is a selective α-1 adrenergic agonist. One generally administers 2.5–10 mg 15–30 minutes before dialysis. A second smaller dose can be given halfway through the treatment. It has high bioavailability, with peak levels occurring at 60 minutes, and it is removed by hemodialysis (half-life is 3.0 hours on hemodialysis); therefore, the drug effect does not persist long in the postdialytic period. A meta-analysis that included 117 patients from 10 small studies showed an increase in postdialysis systolic BP of 12.4 mmHg (95% CI, 7.5 to 17.7) (24). There were no major adverse events. Six of seven studies that reported symptoms showed an improvement in IDH. These studies, however, were very heterogeneous, contained small numbers of patients, and were of limited quality. In addition, in a small crossover study of 11 hemodialysis patients, Cruz et al. (25) showed that there was no additional benefit when midodrine and cool dialysate were used combined versus separately. This patient was already receiving cool dialysate, and therefore, I would expect little additional benefit from adding midodrine.
Conclusion
IDH is and will continue to remain a management challenge for nephrologists given the cyclical nature of expansion and contraction of the extracellular fluid volume with three times a week hemodialysis and difficulties in restricting sodium intake. Manipulations of the hemodialysis prescription and pharmacologic agents can help reduce the frequency of IDH. We have a clear need for better noninvasive tools that can be easily used at the bedside to assess dry weight. The ideal solution to the problem, which is to increase dialysis time and/or frequency, is difficult because of patient preference, cost, and resource constraints. In our unit, we emphasize salt restriction in an attempt to limit interdialytic weight gain, frequently use cool dialysate, administer midodrine in those patients who cannot tolerate cool dialysate when appropriate, prohibit food ingestion during the dialysis procedure, and use ultrafiltration modeling. We do not use sodium modeling and l-carnitine.
Questions
Dr. Nishank Jain: What about the possibility of transitioning the patient with severe IDH to peritoneal dialysis?
R.F.R.: This was addressed in Guideline 3.7 of the European Best Practices Guidelines on Hemodialysis, which states: “A treatment change to peritoneal dialysis should be considered in patients who remain refractory to interventions for the prevention of intradialytic hypotension.” This was an opinion and not evidence-based guideline. We have occasionally transitioned patients with severe liver or cardiac disease to peritoneal dialysis, because they could not tolerate hemodialysis due to hemodynamic instability, and, for the most part, they have done well.
Dr. Vishal Jaikaransingh: The nurses in the dialysis unit tell me that they often look for symptoms, such as yawning or sighing, as an indicator of intradialytic hypotension. Is there any truth to this?
R.F.R.: Intradialytic hypotension may be preceded by a variety of symptoms. The two you mention are vagal symptoms and have been reported to precede intradialytic hypotension in a variety of review articles. Transient hoarseness may also be a result of IDH. This is thought to result from thinning of the vocal cords due to volume removal during the hemodialysis procedure.
Dr. Javier Alberto Neyra-Lozano: You did not mention the option of raising the bath potassium concentration in patients with IDH. Can you please comment on this?
R.F.R.: Rapid declines in serum potassium concentration can lower systolic and diastolic BP by decreasing systemic vascular resistance. I am aware of a small study that used a crossover design to study this issue. Although there were more frequent episodes of hypotension, defined as a systolic BP<90 mmHg with lower potassium baths, the authors did not report on symptoms or interventions. We do not routinely alter the potassium bath in our patients with IDH.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Agarwal R: How can we prevent intradialytic hypotension? Curr Opin Nephrol Hypertens 21: 593–599, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Santos SFF, Peixoto AJ, Perazella MA: How should we manage adverse intradialytic blood pressure changes? Adv Chronic Kidney Dis 19: 158–165, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Selby NM, McIntyre CW: The acute cardiac effects of dialysis. Semin Dial 20: 220–228, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Sinha AD, Agarwal R: Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial 22: 480–482, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V: Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 23: 2965–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Reddan DN, Szczech LA, Hasselblad V, Lowrie EG, Lindsay RM, Himmelfarb J, Toto RD, Stivelman J, Winchester JF, Zillman LA, Califf RM, Owen WF, Jr.: Intradialytic blood volume monitoring in ambulatory hemodialysis patients: A randomized trial. J Am Soc Nephrol 16: 2162–2169, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dou Y, Zhu F, Kotanko P: Assessment of extracellular fluid volume and fluid status in hemodialysis patients: Current status and technical advances. Semin Dial 25: 377–387, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Tomson CRV: Advising dialysis patients to restrict fluid intake without restricting sodium intake is not based on evidence and is a waste of time. Nephrol Dial Transplant 16: 1538–1542, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD, Yoshita K, Elliott P, Yamamoto ME, Stamler J: Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J Am Diet Assoc 110: 736–745, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E: The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24: 956–962, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW: Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 6: 1326–1332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman RA, Torres F, Cody RP: Postprandial blood pressure changes during hemodialysis. Am J Kidney Dis 12: 37–39, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W: Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant 23: 3605–3612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Sande FM, Wystrychowski G, Kooman JP, Rosales L, Raimann J, Kotanko P, Carter M, Chan CT, Leunissen KM, Levin NW: Control of core temperature and blood pressure stability during hemodialysis. Clin J Am Soc Nephrol 4: 93–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby NM, McIntyre CW: A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant 21: 1883–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for 2006. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Nesrallah GE, Suri RS, Guyatt G, Mustafa RA, Walter SD, Lindsay RM, Akl EA: Biofeedback dialysis for hypotension and hypervolemia: A systematic review and meta-analysis. Nephrol Dial Transplant 28: 182–191, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Gabutti L, Bianchi G, Soldini D, Marone C, Burnier M: Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol Dial Transplant 24: 973–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman RA, Bialy GB, Gazinski B, Bernholc AS, Eisinger RP: The effect of dialysate calcium levels on blood pressure during hemodialysis. Am J Kidney Dis 8: 244–247, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Lynch KE, Feldman HI, Berlin JA, Flory J, Rowan CG, Brunelli SM: Effects of L-carnitine on dialysis-related hypotension and muscle cramps: A meta-analysis. Am J Kidney Dis 52: 962–971, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Prakash S, Garg AX, Heidenheim AP, House AA: Midodrine appears to be safe and effective for dialysis-induced hypotension: A systematic review. Nephrol Dial Transplant 19: 2553–2558, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA: Midodrine and cool dialysate are effective therapies for symptomatic intradialytic hypotension. Am J Kidney Dis 33: 920–926, 1999 [DOI] [PubMed] [Google Scholar]