Abstract
The Kidney Research National Dialogue represents a novel effort by the National Institute of Diabetes and Digestive and Kidney Diseases to solicit and prioritize research objectives from the renal research and clinical communities. The present commentary highlights selected scientific opportunities specific to the study of renal development, physiology, and cell biology. Describing such fundamental kidney biology serves as a necessary foundation for translational and clinical studies that will advance disease care and prevention. It is intended that these objectives foster and focus scientific efforts in these areas in the coming decade and beyond.
Commentary
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) recently asked the community to identify research objectives, which, if addressed, would improve our understanding of basic kidney function and aid in the prevention, treatment, and reversal of kidney disease. The Kidney Research National Dialogue (KRND) welcomed all interested parties to submit, discuss, and prioritize ideas through an interactive website (1). Over 1600 participants posted ideas covering all areas of kidney disease. This commentary represents one important component of the KRND and highlights selected scientific opportunities for studies of renal development, physiology, and cell biology that are expected to provide important contributions to maintaining and improving kidney health and regenerating kidney tissues.
Renal research has undergone a decade-long shift from the study of fundamental kidney biology to the study of renal pathophysiology and translational development of treatment strategies for renal disease. This evolution may have been driven by the perception that continued description of basic kidney biology is unlikely to have direct impact on clinical management of kidney disease. However, the study of kidney biology remains the foundation for understanding renal disease and serves as an engine for driving translational and clinical advances.
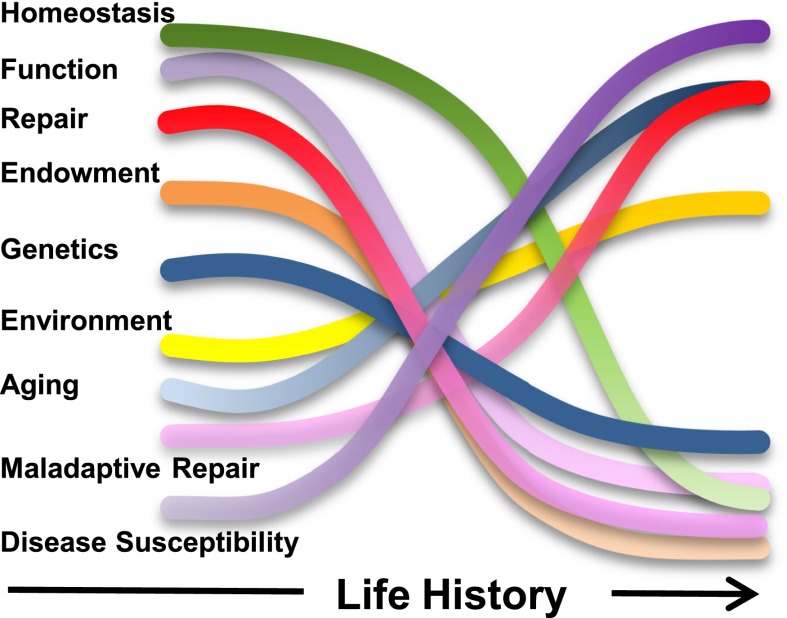
There are many examples of fundamental research insights leading to improved patient care. Perhaps the most striking is the molecular characterization of the renin-angiotensin system leading to the development of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for the treatment of renal and cardiovascular disease. Understanding the biology of water reabsorption provided a foundation for the vasopressin-receptor antagonist treatment of hyponatremia and, perhaps, polycystic kidney disease. Cellular signaling studies have identified targets ranging from Nrf2 to Jak/Stat that are currently being tested in human studies. Hence, understanding basic kidney biology remains central to advancing health and driving discoveries for translation to clinical progress in the next decade and beyond. It is clear from these studies that many interrelated factors contribute to normal biology and the progression over time to the development of pathophysiology (Figure 1). Below are a number of fruitful areas of investigation identified in the KRND.
Figure 1.
Untangling the knot. Schematic representation of the influence of interrelated environmental and biologic considerations over the life history of the kidney that underlie kidney health.
Renal Embryogenesis
Recent studies have advanced our understanding of renal embryogenesis from the identification of molecular subcompartments and genetic circuitries of developing renal structures to the mechanism of uretic bud branching to the identification of the Six2-expressing cap mesenchyme as the multipotent nephron progenitor population. The next critical step is to place the genetic and cellular basis of renal development in a wider context, which includes the determination of kidney endowment/number of nephrons and reaching out to neglected topics (such as the innervation of the kidney), the patterning of the two major circulatory systems (blood/vascular and lymphatic), the differentiation of the tubular segments, and the regional, functional, and anatomic heterogeneity of nephrons within the kidney. Advances in these topics may allow generation of a functional nephron (with correct vascular, tubular, and neural integration) that will improve kidney function and serve as a foundation for novel physiologic and tissue regeneration studies. They will also underpin potential advances in bioengineering and nephrotoxicity screening.
Environmental Effects
A suboptimal prenatal environment, including placental insufficiency, maternal undernutrition, and glucocorticoid exposure, reduces nephron number and can increase the risk of diseases, including hypertension and hydronephrosis, in offspring. However, the underlying mechanisms by which these factors affect fetal programming and reduce nephron number and kidney efficiency are unknown. To fully understand fetal reprogramming of the kidney and the regulation of kidney size, we will require additional advances in our ability to image and accurately quantitate the size of individual cellular compartments during development as well as quantify nephron number in vivo. Given the relevance of in utero challenge to the community, we also need to apply advanced genetics and epigenetics to the analysis of animal models of fetal environmental perturbation.
Renal Physiology and Cell Biology
It is clear that defining normal physiology and cellular function drives additional discovery and informs clinical management. Over the past several years, our understanding of renal cell function has taken advantage of progress in several fields, including, but not limited to, ion channel and transporters, cell matrix interactions, receptor biology, and signaling cascades. The advent of genetically engineered animals offers exciting opportunities to understand the physiologic effect of over- or underexpression of such proteins in renal function, including those proteins that are mutated in human diseases. Such functional studies provide unique data and the ability to test novel therapeutics for both beneficial effects and unanticipated negative effects. Understanding the pathways and mechanisms that govern sorting and trafficking of proteins essential for kidney function will inform therapeutic strategies for kidney disorders caused by mutations in individual proteins. The use of diverse model systems and humanized mice offers tremendous opportunities, including contributions of cellular mediators to organ physiology. New renal cellular models can elucidate the signaling pathways regulating transport and explore mechanisms, such as protein–protein interactions, protein phosphorylation, protein trafficking, and the role of degradation pathways (ubiquitination and lysosomal degradation). As one example, studies of the primary cilium, whether in algae, zebrafish, or mammals, have led to important insights into polycystic kidney disease and nephronophthisis.
Systems Biology
There has been an explosion of new genetic, proteomic, and imaging techniques applicable to analysis at the organ, cell, or biochemical level. A better grasp is needed of how regulatory mechanisms (like ubiquitination, sumoylation, DNA methylation, histone modifications, and noncoding RNAs) modulate kidney cell functions. Opportunities exist in kidney research for integrating genotype, RNA expression, promoter analysis, proteome expression, and metabolic profiles to fully understand the complexities of normal kidney function. There is also the opportunity to integrate data in three and four dimensions both in vitro and in vivo. Determining the most efficient way to leverage the growing amount of such complex “omics” data into a better understanding of kidney function and new therapeutic strategies represents an additional critical next step. It will require advances in analytical tools, such as predictive mathematical models, and increased access to large sets of data in a variety of formats.
Kidney Function Across the Lifespan
Understanding the structure and function of the kidney throughout its life history (from early development to aged adult) remains a neglected field of study. The basic science research community is poised to undertake these studies using an integrated approach that draws on genetics, physiology, and developmental, molecular, and cellular biology. Little is known about the changes in kidney in response to organismal life history, including the cellular and molecular basis involved in maintaining or resetting physiologic homeostasis to adjust BP between the juvenile and adult or respond to circulating hormones (puberty and menopause) or diet (weaning). The molecular basis for the increased susceptibility of the aged kidney to acute and chronic damage is also largely unknown. This area holds enormous and largely untapped potential to inform clinical considerations.
Loss of Homeostasis
We encourage traditional fields of normal kidney biology to consider the study of kidney disease as a loss of homeostasis. An unrecognized concept is the idea of homeostatic productive repair that maintains kidney and nephron health on a day-to-day basis in the absence of dramatic challenges. Indeed, the loss of homeostatic repair may be part of the etiology of increased kidney disease observed in the elderly. Current kidney injury models largely focus on the response to severe or catastrophic injury, such as those responses triggered by ischemia, sepsis, or nephrotoxins. However, debilitating kidney injury in humans is generally superimposed on an underlying chronic disorder and/or occurs in the elderly. This dichotomy emphasizes the need to understand the changes in the physiologic regulation and the homeostatic repair processes throughout the life history of the kidney, especially the aged kidney. It is relevant to one of the most common kidney diseases (diabetic nephropathy), which can take many years to develop in humans, and, perhaps, because of this fact, it has been very difficult to model in mice. If the effectiveness of targeted therapies proves to be too limited in the future, more comprehensive strategies should be considered (i.e., efforts involving system biology, integrative biology, and system medicine).
Conclusion
The study of kidney physiology, cell biology, and development has been, and remains, the foundation for understanding renal disease. Research in these areas provides opportunities to further characterize largely neglected cell types, define contributions of other physiologic systems tightly integrated with the functioning kidney, and incorporate normal variables, such as fetal nutrition, aging, and the life history of the kidney, into ongoing and future studies. Initial data may be available from Murine Atlas of Genitourinary Development, an NIDDK-supported consortium designed to define the molecular and cellular anatomy of the murine kidney through developmental time. Insights from such efforts provide essential baselines for understanding normal changes in renal structure/function, response to injury, and, ultimately, the broad spectrum of kidney diseases. The increased ability to develop targeted genetic and pharmacologic interventions to correct individual pathways and proteins defective in disease offers an extraordinary opportunity to treat or cure these disorders.
The six areas of investigation mentioned above suggest important crosscutting themes and opportunities for broadly informing kidney research. Basic physiologic and cell biologic studies of hormone receptors, signal transduction pathways, regulation by microRNA, and mechanisms (such as protein–protein interactions, protein phosphorylation, protein trafficking, and the role of degradation) will elucidate novel regulatory pathways and identify previously unrecognized therapeutic targets. Such crosscutting fundamental studies are especially important in designing therapies that may compensate for a mutant gene or predict an otherwise unexpected complication from a therapy aimed at a particular target. These studies may also contribute to novel strategies for maintenance of better kidney health over its life history.
Disclosures
B.D.H. is funded by a grant from Evotec with the goal of discovering new therapeutic targets to treat acute kidney disease and CKD. A.P.M. is in collaboration around kidney disease with Evotec, for which the laboratory of A.P.M. receives research support from Evotec.
Acknowledgments
The Kidney Research National Dialogue was developed and implemented by the National Institute of Diabetes and Digestive and Kidney Diseases/Division of Kidney, Urologic and Hematologic Diseases (KUH) staff and directed by Dr. Krystyna Rys-Sikora. The Normal Kidney Development, Cell Biology, and Physiology topic was facilitated by C.M. and D.H. Please visit the Kidney Research National Dialogue website http://www2.niddk.nih.gov/KUH/KUHHome/KRND.htm for full details or to post comments about this review.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Reference
- 1.Rys-Sikora KE, Ketchum CJ, Star RA, Kidney Research National Dialogue (KRND) Editorial Board : Kidney research national dialogue overview and commentary. Clin J Am Soc Nephrol 8: 1599–1602, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]