Abstract
Several recent studies have demonstrated that T-helper cell-dependent events during the initial priming period are required for the generation of CD8+ T cell-mediated protective immunity. The underlying mechanisms of this phenomenon have not yet been determined, mostly because of difficulties in studying memory T cells or their precursor populations at early stages during immune responses. We identified IL-7 receptor (CD127) surface expression as a marker for long-living memory T cells, most importantly allowing the distinction between memory and effector T cells early after in vivo priming. The combination of surface staining for CD127 and CD62L further separates between two functionally distinct memory cell subsets, which are similar (if not identical) to cell subsets recently described as central memory T cells (CD127high and CD62Lhigh) and peripheral effector memory T cells (CD127high and CD62Llow). Using this new tool of memory T cell analysis, we demonstrate that CD8+ T cell priming in the absence of T cell help or CD40L specifically alters the generation of the effector memory T cell subset, which appears to be crucial for immediate memory responses and long-term maintenance of effective protective immunity. Our data reveal a unique strategy to obtain information about the quality of long-term protective immunity early during an immune response, a finding that may be applied in a variety of clinical settings, including the rapid monitoring of vaccination success.
It is well established that CD4+ T cell help can modulate or shape pathogen-specific CD8+ memory T cell responses (1). The quality of protective immunity gradually decreases after T cell priming in the absence of T cell help (2–4), and “unhelped” CD8+ T cells respond poorly to antigen rechallenge. CD8+ T cells generated in a CD4+ T cell-deficient environment maintain this altered memory phenotype after transfer into recipient mice with a normal CD4+ T cell compartment, indicating that “instructive” events during the priming period determine the quality of T cell memory (2–4). CD40–CD40L interactions have been shown to be involved in crucial steps of T cell help (5–7); however, the exact nature of the memory deficiency in the absence of CD4+ T cells or CD40–CD40L interactions has not yet been determined. Identification of the mechanisms underlying this phenomenon is of general importance for the understanding of how protective immunity is generated. For example, insufficient recruitment of helper mechanisms might explain the failure of most T cell-based vaccines to achieve long-term maintenance of effective antigen-specific memory T cell populations, although initial T cell responses to these vaccines are often quite vigorous. Furthermore, missing T cell help could be responsible for ineffective CD8+ T cell responses in HIV patients and other immunocompromised individuals.
It is unclear whether “unhelped” CD8+ memory T cells are not maintained over time, or if memory T cells with true functional differences are generated in the absence of CD4+ T cells. We decided to characterize in more detail the CD8+ memory T cells generated in vivo in the presence or absence of CD4+ T cell help. We chose murine infection with Listeria monocytogenes (Lm) as a model, because several recent reports have demonstrated the importance of T cell help for memory T cell development in this experimental system (3, 4). Analysis of memory T cells in the mouse is, however, complicated by the lack of markers that distinguish between effector and memory T cell phenotypes. Furthermore, the memory T cell compartment itself appears to be heterogeneous. A central memory T cell (TCM) subpopulation resides preferentially in lymphoid organs, lacks immediate effector functions, and is characterized by vigorous homeostatic and antigen-driven proliferation; the maintenance of TCM appears to depend on signaling by T cell growth factors, such as IL-2, IL-15, or IL-7, through common γ-chain receptors (8–10). On the other hand, a peripheral effector memory T cell (TEM) subpopulation exists that is characterized by immediate effector function on antigen reencounter (11–13). One of the main problems in studying in vivo memory T cell development is that, to date, memory T cells with effector function cannot be definitively distinguished from “post”-effector T cells, which are thought to be rapidly deleted after the primary T cell response; both effector T cells (TE) and effector memory T cells migrate preferentially to nonlymphoid organs, where their maintenance depends on survival factors provided by the local “micromilieu.” Although this model, consisting of three main subsets of antigen-experienced T cells (TE,TEM, and TCM), might have its limitations in describing the in vivo status of memory T cell differentiation (even our own data in this study indicate that additional heterogeneity may exist within the subsets), the general concept of two main subsets of memory T cells (TEM and TCM) with distinct migration patterns was recently convincingly confirmed by “whole-body” T cell analyses in mice (11, 12). In humans, the different memory T cell subsets can be phenotypically discriminated by CCR7 expression in combination with CD45RA (13), although some recent studies indicated that these markers do not always correlate with the same functional differentiation pattern (14). In the mouse, l-selectin (CD62L) expression, which mediates T cell migration into lymphoid organs, has been used as a marker to distinguish TCM from TEM (15, 16), but neither CCR7 nor CD62L expression correlated exactly with the functional characteristics described for TCM (15, 17).
In this study, we identified surface expression of IL-7 receptor α-chain as a memory T cell marker, especially allowing us to distinguish between effector and memory T cells at early time points of in vivo immune responses.
Materials and Methods
Mice and Bacteria. BALB/c and C57BL/6 mice were obtained from Harlan Winkelmann (Borchen, Germany); MHC class II (MHC II)–/– mice (B6/I-Aβ–/–), BALB/c Thy1.1, and CD40L–/– mice (BALB/c background, kindly provided by R. Flavell, Yale University) were derived from in-house breeding under specific pathogen-free conditions. Infection experiments were performed by i.v. injection of WT Lm strain 10403s (BALB/c mice) or Listeria expressing recombinant ovalbumin (kindly provided by H. Shen, University of Pennsylvania, Philadelphia) for experiments with C57BL/6 as described (18). A dose of ≈2 × 104 i.v.-injected Lm 10403s represents the LD50 for BALB/c mice.
Phenotypical T Cell Analysis by Using MHC Multimer Reagents. H2-Kd/LLO91–99 and H2-Kb/SIINFEKL multimer reagents were generated as described (19). For live/dead discrimination, cells were incubated with ethidium monazide (Molecular Probes) followed by MHC multimer and surface marker staining for 45 min at 4°C. The following mAbs were used: anti-CD8α (clone 53-5.8), anti-CD4 (clone RM4-5), anti-CD62L (clone MEL-14), anti-CD127 (clone SB/14; blocking Ab), all from Pharmingen, and anti-CD127 (clone A7R34, eBioscience, San Diego; stimulating Ab). For costaining with H2-Kb/SIINFEKL tetramers, anti-CD8α-specific mAb was used (clone CD8α; Caltag, South San Francisco, CA). Data were acquired on a FACSCalibur (Becton Dickinson) or a CyAn analyzer (DakoCytomation). Acquired data were further analyzed with cellquest or flowjo (Tree Star) software.
Intracellular Cytokine Staining, Cytotoxicity Measurement, and Proliferation Assay. Antigen-specific T cells were stained with reversible MHC multimer reagents (Streptamers) and were subsequently sorted with a MoFlo (DakoCytomation) directly into d-biotin-containing buffer to remove MHC multimers from the cell surface (all done at 4°C). Sorted antigen-specific T cells from Listeria-infected mice were incubated briefly (5 h) in the presence of irradiated, autologous splenocytes pulsed with the relevant epitope (10–6 M); for the last 3 h, Brefeldin A (GolgiPlug, Pharmingen) was added. Intracellular cytokine staining for IL-2, IFNγ, and tumor necrosis factor α (Pharmingen) was performed by using the Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer's recommendations. For cytotoxicity experiments, sorted T cell subpopulations from BALB/c mice were incubated for 8 h in the presence of 51Cr-labeled, peptide-pulsed P815 target cells. Cell proliferation was determined by in vitro incubation of ex vivo sorted, carboxy fluorescein succinimidyl ester-labeled, antigen-specific T cell populations for 3 days in the presence of anti-CD3-stimulating Ab and irradiated autologous splenocytes; cells divisions were visualized by flow cytometry.
Bacterial Clearance. The quality of Listeria-specific protection was determined by measuring the bacterial load in the spleen 3 days after infection with a high dose (≈10 × LD50) of Lm by plating out serial dilutions on brain heart infusion broth agarose as described (18).
In Vivo BrdUrd Uptake. For in vivo proliferation assays, BrdUrd (Sigma) was added to the drinking water (0.8 mg/ml) for 2–5 days after reinfection with Lm. Splenocytes were harvested on day 3 or 5 after reinfection and stained for BrdUrd as described (20). FITC-conjugated anti-BrdUrd mAb (Becton Dickinson) was used as a detection reagent for flow cytometric analysis.
Adoptive Cell Transfer. LLO91–99-specific CD8+ T cells from Listeria-infected Thy1.1 BALB/c mice were isolated after staining with APC-conjugated reversible MHC multimers by flow cytometrybased cell sorting on a MoFlo (DakoCytomation); surface-bound MHC multimers were removed after purification in the presence of 1mM d-biotin (21). Then 5 × 105 cells were injected i.v. into Thy1.2 BALB/c mice; 3 wk after transfer mice were killed, and lymphocytes from spleen, lung, and lymph nodes (LN) were stained for Thy1.1 and CD8α. For infection experiments after adoptive cell transfer, mice were infected with 103 Lm, and transferred cells were traced in different organs by staining for Thy1.1-positive CD8+ T cells as described above.
Results
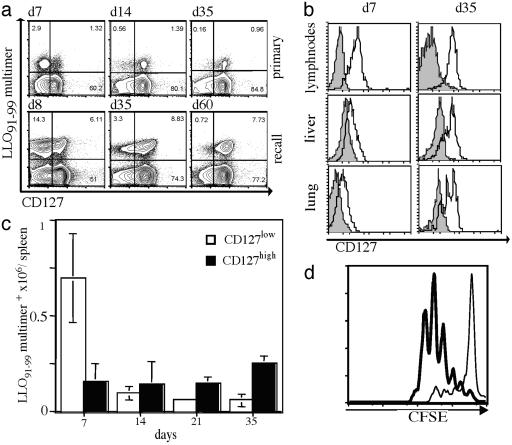
Identification of IL-7 Receptor α Chain (CD127) as a Marker for Memory T Cells. While searching for new markers to define effector and memory T cell subsets in the mouse, we analyzed surface expression of the IL-7 receptor α-chain/CD127 (Fig. 1a). During primary responses to Lm, distinct subpopulations of antigen-specific splenic T cell populations can be distinguished based on CD127 expression levels. CD127 low expressing cells (CD127low) predominate during the acute effector phase. During the transition into the memory T cell phase, however, CD127low cells gradually disappear, whereas populations expressing high levels (CD127high) remain remarkably stable in size over time (Fig. 1 a and c). These distinct kinetic values strongly suggest that CD127 expression is a selective characteristic for long-living memory T cells. During the effector phase, CD127low CD8+ T cells migrate preferentially to nonlymphoid organs, whereas only CD127high CD8+ T cells are detected in LN (Fig. 1b). The spleen represents an “intermediate organ” where all different subpopulations are found early after immunization (22, 23). Later during the memory phase, antigen-specific T cells in all organs are characterized by a CD127high phenotype (Fig. 1 b and c). Comparison of the capabilities of CD127low and CD127high antigen-specific T cells to proliferate in response to stimulation revealed striking differences between the two subsets. As shown in Fig. 1d, purified Listeria-specific CD127high T cells proliferate rapidly after anti-CD3 stimulation, whereas CD127low T cells proliferate poorly. It is unlikely that these differences in proliferation reflect an advantage of CD127-positive cells through Ab-mediated IL-7R stimulation because different mAb clones with known CD127 activating or blocking activity demonstrated the same effect in short-term in vitro stimulation assays (data not shown).
Fig. 1.
CD127 (IL-7R) surface expression as a marker for memory T cells. (a) A cohort of BALB/c mice was infected with a sublethal dose (≈0.1 × LD50) of WT Lm, followed by secondary infection (≈10 × LD50) 5 wk later. Representative dot plots of splenocytes (gated on live CD8+ T cells) stained with H2-Kd/ LLO91–99 tetramers (y axis) and for CD127 surface expression (x axis) are shown for the indicated time points during primary (Upper) and recall (Lower) responses. (b) Lymphocytes from different organs were isolated during the primary effector (7 days after infection) and memory (35 days after infection) phases. Representative histograms are gated on CD8+ and H2-Kd/ LLO91–99 tetramer-positive cells and show CD127 staining (open) and unstained controls (filled). (c) Kinetics of the absolute numbers (y axis) of CD127 high-(filled) and low (open)-expressing CD8+ and H2-Kd/LLO91–99 tetramer-positive cells; six individual mice per time point. (d) CD127 high- and low-expressing CD8+, H2-Kd/LLO91–99 multimer-positive cells were sorted directly ex vivo 10 days after Listeria infection. Proliferation of carboxy fluorescein succinimidyl ester-labeled cells was analyzed after anti-CD3 stimulation; CD127high (bold line) or CD127low (thin line) cells.
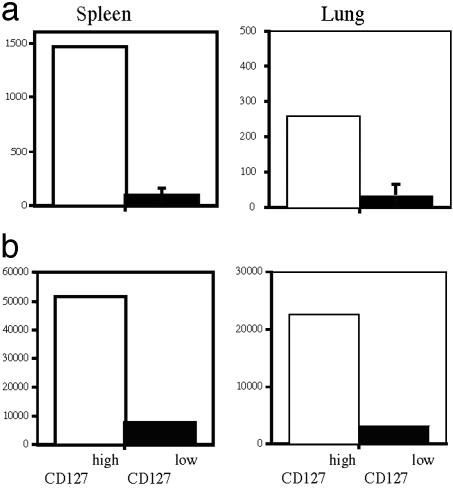
To more directly demonstrate that long-living memory T cells are exclusively present in the CD127 high expressing compartment early after in vivo priming, we performed adoptive transfer studies by using a recently developed reversible MHC multimer-staining technique (21) to functionally isolate antigen-specific T cells directly ex vivo. After adoptive transfer of CD127high LLO91–99-specific T cells from mice infected for 10 days with Lm, the transferred cells were still detectable several weeks later in the spleen of naive recipient mice (Fig. 2a), whereas the cell numbers after transfer of CD127low cells were at the detection limit. This observation was not due to differences of in vivo migration of transferred CD127 high and CD127low cells, because we found similar differences for the lung (Fig. 2a) and other organs (LN and liver, data not shown). To further support the interpretation that CD127high T cells exclusively maintain antigen-specific memory cells, recipient mice were challenged after adoptive transfer with Listeria infection. Again, only in mice that had received CD127high T cells before infection was rapid expansion of transferred LLO91–99-specific T cells detectable (Fig. 2b). Again, this result was not due to differences in migration because maintenance or expansion of transferred CD127low T cells was not detectable in any other organ (Fig. 2b and data not shown). Although these results from adoptive transfer studies strongly support the hypothesis that long-living memory T cells are exclusively present in the CD127high T cells compartment, we also observed substantial limitations of this experimental approach. In particular, memory T cells with immediate effector function seem to be quite sensitive to adoptive transfer experiments and die rapidly after purification and i.v. application; although they survive for months to years if they are left untouched in vivo (11). Thus, although our results from adoptive transfer conform with the interpretation that CD127 is a marker for long-living memory T cells, we cannot exclude that intrinsic problems regarding survival of distinct T cell subpopulations also contribute to the outcome of these experiments.
Fig. 2.
Adoptive transfer studies indicate that long-living memory T cells are exclusively present in the CD127-positive compartment. CD127 high- and low-expressing CD8+, H2-Kd/LLO91–99 multimer-positive cells from BALB/c Thy1.1 mice were sorted directly ex vivo 10 days after Listeria infection. Cells were transferred into naive BALB/c (Thy1.2)-recipient mice, and 3 wk later spleens and lungs were stained for donor cells (CD8+ and Thy1.1+). (a) Bar graphs summarize data for absolute numbers of Thy1.1+ cells per organ after transfer of CD127high cells (open) or CD127low (filled) T cells. (b) Same data acquisition and data presentation as described in a, 5 days after infection with 103 Lm.
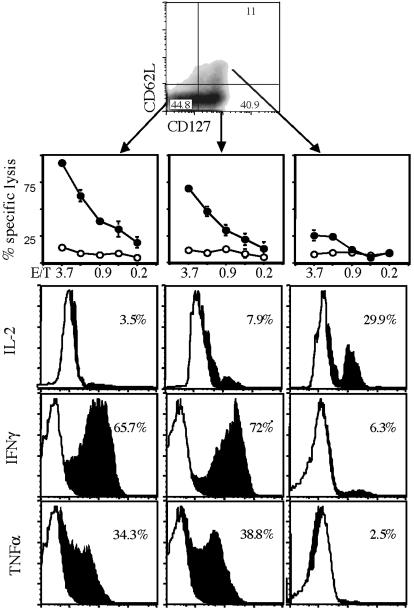
Costaining of Antigen-Specific T Cell Populations with CD127 and CD62L Allows Discrimination Between Memory T Cell Subpopulations. Further characterization of the CD127high T cell population showed that it can be subdivided into subpopulations expressing high and low levels of CD62L (Fig. 3). Direct ex vivo functional analysis (21) of purified T cells (10–12 days after infection, when all subpopulations are simultaneously detectable in the spleen) uncovered further functional differences. Whereas CD62Llow T cell subsets show vigorous and immediate ex vivo cytolytic activity, CD127high/ CD62Lhigh T cells demonstrate very poor lysis of peptide-coated target cells (Fig. 3). Intracellular cytokine staining of sorted subpopulations revealed that CD127high/CD62Llow T cells, like the CD127low/CD62Llow subset, produce the effector cytokines INF-γ and tumor necrosis factor α; in contrast, the CD127high/CD62Lhigh subset produces IL-2 but not IFN-γ and tumor necrosis factor α. Thus, the marker combination of CD127 and CD62L enables identification of T cell subpopulations with characteristics very similar to those described previously in humans. CD127low/ CD62Llow T cells demonstrate all known characteristics of a real effector T cell population (TE, poor proliferative activity, limited in vivo survival, and immediate effector function). The CD127high/ CD62Lhigh subset matches the characteristics of TCM, and the CD127high/CD62Llow T cells fit the description of TEM.
Fig. 3.
CD127/CD62L double staining distinguishes between distinct CD8+ T cell subsets; similar results can be found in humans. Double staining of CD8+, H2-Kd/LLO91–99 multimer-positive cells for surface expression of CD62L (y axis) and CD127 (x axis) after infection with Lm; relative percentages of subpopulations in quadrants are indicated. CD127low/CD62Llow, CD127high/CD62Llow, and CD127high/CD62Lhigh subpopulations were sorted directly ex vivo 12 days after Listeria infection and transferred into different functional assays. Cytolytic activity was assessed after incubation of sorted cells in the presence of target cells and LLO91–99 peptide (squares) or no peptide (circles). Intracellular cytokine staining of ex vivo sorted LLO91–99-specific subpopulations (as indicated above) was performed for IL-2 (Top), IFN-γ (Middle), and tumor necrosis factor α (Bottom) after brief in vitro restimulation with LLO91–99 peptide (black areas; white areas represent unstimulated controls); percentages of cytokine-positive cells are indicated.
Generation of Distinct Memory T Cell Subpopulations Depends on CD40L-Mediated T Cell Help. Because we felt that it might be problematic to exclusively base the interpretation that CD127 expression can be used as a marker to distinguish between effector and long-living memory T cells on adoptive cell transfer experiments, we decided to analyze mouse mutant lines with defects in the generation of T cell memory. If CD127 is indeed an “early” memory T cell marker, we should be able to identify differences during early and late time points within the CD127-positive subsets in mice with memory defects.
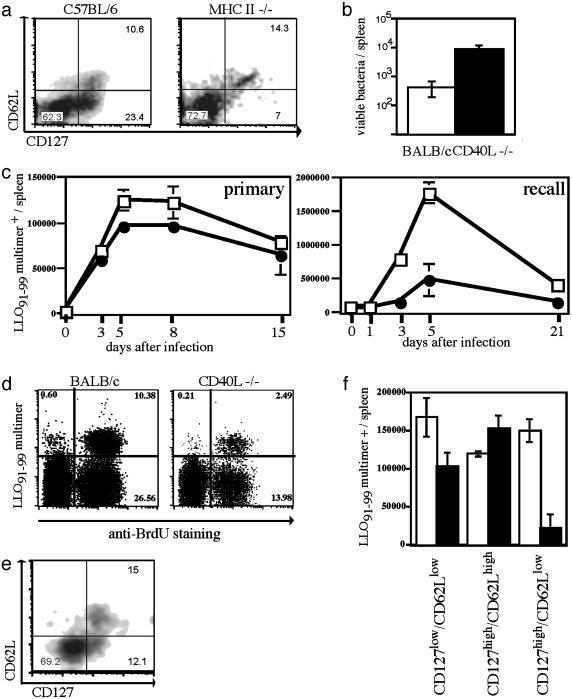
Because recent studies demonstrated the importance of T cell help for the generation of long-lasting memory responses (2–4), we decided to determine whether the presence or absence of CD4+ T cell help during CD8+ T cell priming leads to differences in the antigen-specific T cell subset compositions detected in the early posteffector phase. MHC II-deficient mice, which lack conventional CD4+ T cells, have a defective CD8+ memory response, and the quality of protection decreases over time (2–4). Staining for T cell subpopulations 10 days after primary infection revealed that the CD127high/CD62Llow subset is almost completely absent in MHC II–/– mice (Fig. 4a); the other subpopulations are present at frequencies similar to those in WT C57BL/6 mice. These data demonstrate that the absence of T cell help prevents efficient generation of a T cell subset with an effector memory phenotype, which seems to be crucial for rapid in vivo expansion and effector function.
Fig. 4.
Impaired memory responses in MHC II–/– and CD40L–/– mice correlate with early defects in the generation of distinct T cell subsets. (a) WT C57BL/6 (Left) and MHC II-deficient (Right) mice were infected with a sublethal dose of ovalbumin-expressing Lm; dot plots of CD8+, H2-Kb/SIINFEKL-positive T cells stained for CD62L (y axis) and CD127 (x axis) 10 days after primary infection are shown. (b) Numbers of viable bacteria in the spleen 3 days after reinfection (≈10 × LD50; 5 wk after primary infection) with Listeria in CD40L–/– (filled) and WT BALB/c (open); n = 3 per group. (c) Kinetics of LLO91–99-specific T cells determined by H2-Kd/LLO91–99 tetramer staining in CD40L–/– (•) or WT BALB/c mice (□) after primary (0.1 × LD50) and recall infection (2.5 × LD50; 5 wk after primary infection) with Lm; three mice per time point. (d) WT BALB/c mice and CD40L–/– mice received BrdUrd by drinking water during recall infection with Lm (2.5 × LD50; 5 wk after primary infection) during the entire expansion phase (days 1–5); dot plots show BrdUrd incorporation (x axis) in CD8+ T cells stained with H2-Kd/LLO91–99 tetramers (y axis), staining was performed on day 5. (e) Double staining of CD8+, H2-Kd/ LLO91–99 tetramer-positive cells from a CD40L–/– BALB/c mouse for CD62L (y axis) and CD127 (x axis) 10 days after primary infection (WT BALB/c control see Fig. 2c). (f) Absolute numbers of LLO91–99 tetramer-positive T cell subsets in the spleen determined 10 days after primary infection by staining for CD62L and CD127.
Because important mechanisms of CD4+ T cell help are mediated through the interaction of CD40 and CD40L (24–26), we investigated whether CD40L–/– mice show a phenotype similar to that of MHC II–/– mice. In accordance with other recent studies (27), CD40L–/– mice demonstrate no differences in bacterial load or bacterial clearance after primary Listeria infection (data not shown) and have normal primary CD8+ T cell responses to a sublethal dose of Lm (Fig. 4c). However, their ability to rapidly clear reinfection with a higher dose of Lm (≈10 × LD50) is reduced (Fig. 4b), correlating with impaired CD8+ memory T cell responses (Fig. 4c) and a much reduced CD127high/CD62Llow subset (Fig. 4 e and f) during the early posteffector phase. During reinfection with Listeria, proliferation of responding T cells in CD40L–/– mice is equivalent to that of cell populations in WT mice, indicating that the phenotype is not due to a general defect in the proliferative capacity of memory T cells (Fig. 4d) but rather to reduced numbers of memory T cells capable of responding immediately to antigen reencounter. This phenotype was demonstrated by in vivo-labeling experiments in which BrdUrd was incorporated during the entire expansion phase (Fig. 4d) and by in vivo BrdUrd pulse–chase studies monitoring the loss of label on proliferation (data not shown).
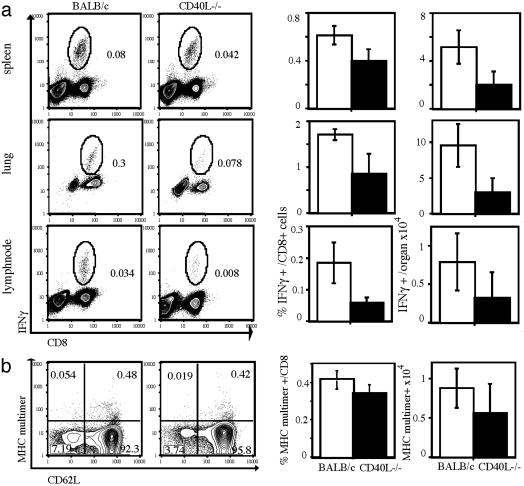
Reduced Generation of T Cell Subsets with an Effector Memory Phenotype Early After Priming Is Transmitted into the Late Memory T Cell Pool. Our data show that in the absence of CD4+ T cells or CD40L, the CD127high/CD62Llow T cell subset is significantly reduced early after priming. Furthermore, we (Fig. 4b) and others (3–5) demonstrated that impaired T cell help during T cell priming leads to changes in the quality of protective immunity toward reinfection with Lm or lymphocytic choriomeningitis virus. To more clearly link the observed defect in early generation of CD127high/ CD62Llow T cells with the quality of subsequent T cell memory in these mice, we analyzed antigen-specific T cell populations during the memory phase (5 wk after primary Listeria infection) in more detail. At this time point, analysis of antigen-specific T cells must be performed on cells derived from different tissues because of their distinct migratory behavior. In addition to the spleen, we chose LN as a representative tissue for lymphoid organs and the lung as a typical peripheral, nonlymphoid compartment. At this time point, the antigen-specific T cells in these organs are nearly all CD127high (Fig. 1b); the cells in the lung are CD62Llow (data not shown), whereas most antigen-specific memory T cells in the LN are CD62Lhigh (Fig. 5b). To determine the numbers of antigen-specific memory T cells with clear immediate effector function in the different organs, we performed intracellular cytokine staining for IFN-γ. As shown in Fig. 5a, CD40L–/– mice are characterized by substantially diminished numbers of antigen-specific memory T cells with immediate effector function as compared with WT mice. This result is true on the level of frequencies (percentage) within the CD8+ T cell compartment and in absolute numbers (Fig. 5a). However, MHC tetramer staining of LN-derived lymphocytes (Fig. 5b) revealed almost identical numbers of antigen-specific T cells with a CD62Lhigh phenotype. Some CD62Llow antigen-specific T cells also are detectable in LNs of WT mice, correlating with the frequencies of IFN-γ-producing cells (Fig. 5a). This population is basically absent in LNs of CD40L–/– mice; the entire CD8+/ CD62Llow subset is reduced in CD40L–/– mice, suggesting a general defect in the generation of this T cell subset. In summary, our data show that the absence of CD40L-dependent CD4+ T cell help during the priming period results in quantitative differences in antigen-specific CD8+ T cell subpopulations, with greatly reduced numbers of cells exhibiting an effector memory phenotype (CD127high/CD62Llow). These quantitative differences in cells with immediate early effector function are transmitted into the memory phase and affect the quality of protective immunity.
Fig. 5.
CD40L-dependent changes in T cell subsets early after T cell priming are transmitted into the subsequent memory T cell pool. (a) Lymphocytes were isolated from lungs, spleens, and LN derived from WT BALB/c mice or CD40L–/– 35 days after primary infection with Lm (0.1 × LD50). Intracellular IFN-γ staining was performed after brief in vitro restimulation in the presence of LLO91–99 peptide to identify T cells with immediate effector function. Dot plots show representative results for different organs (as indicated); numbers indicate the overall frequencies of IFN-γ-producing cells. The first row of bar graphs summarizes the data for frequencies of IFN-γ-producing, LLO91–99-specific T cells within the CD8+ compartment; the row of bar graphs to the right summarizes the data for absolute numbers of IFN-γ-producing, LLO91–99-specific T cells in different organs [CD40L–/– (filled) and WT BALB/c (open); n = 3 per group]. (b) LN cells were taken 35 days after primary infection as described in a; LLO91–99-specific T cells were detected by MHC multimer staining (dot plot, y axis) together with costaining for CD8 and CD62L (dot plot, x axis) surface expression. Representative dot plots are shown for cells gated on CD8+ T cells; frequencies within each quadrant are indicated. Bar graph to the left summarizes data for frequencies of MHCS multimer- and CD62L-positive T cells within the CD8+ compartment; the row of bar graph to the right summarizes the data for absolute numbers of LLO91–99/H2-Kd multimer-positive T cells in LN (CD40L–/– (filled) and WT BALB/c (open); n = 3 per group).
Discussion
These studies have revealed important aspects of in vivo memory T cell development. We identified IL-7R/CD127 expression as a memory T cell marker, supporting recent data characterizing IL-7 as an important in vivo maintenance factor for memory T cells (9). Most strikingly, the detection of CD127 expression allows for the first time to distinguish between effector and memory T cells early during immune responses and therefore, to analyze early memory T cell generation in vivo. Using this new tool, we analyzed the defect of CD8+ memory T cell generation in the absence of T cell help in more detail. We show that this defect is not due to a general lack of memory T cells; the absence of T cell help specifically affects the generation of a subtype of memory T cells with immediate early effector function. These new findings also provide an explanation for the somewhat puzzling data obtained from MHC II–/– and CD4–/– mice, which were known to be able to generate and maintain long-term protective immunity toward intracellular pathogens, although the quality of protection is inferior as compared to WT mice (1, 27, 28). A similar situation might also occur after immunization with heat-killed bacteria, a protocol which results in the generation of memory T cells but fails to induce immediate protective immunity (16).
Several studies on human memory T cell subsets suggest a progressive model of effector and memory T cell development (29) in which memory T cells with a “central memory-like” phenotype can differentiate into memory T cells with immediate effector function. The exact conditions and costimulatory factors required for each differentiation/transition step are not known. As shown in this study, interactions through CD40 and CD40L appear to be crucial for the in vivo generation of effector memory T cells. Whether T cell help for CD8+ memory T cell differentiation early during the priming period is mainly achieved through CD40L-mediated crosstalk with APCs or through CD40 expression on activated CD8+ T cells (7) still needs to be determined. Furthermore, some experimental infection models indicate that the requirements for antigen responsiveness of memory T cell subsets might also depend on the location of the cells, demonstrating CD40-dependent differences between the splenic and mucosal compartment (30). Because CD127 expression is down-regulated on contact with antigen, the observed differential segregation into subpopulations based on CD127 expression in MHC II–/– and CD40L–/– mice could also be due to differences in antigen prevalence. Although this interpretation would be in accordance with a model of antigen-driven progression of memory T cells into effector T cells, we and others (27) could not observe substantial differences in bacterial load and bacterial clearance in MHC II–/– and CD40L–/– mice after primary Listeria infection, indicating that more direct mechanisms of T cell help cause this phenotype. The progressive differentiation model of memory T cells has recently been questioned by adoptive transfer experiments in mice (15), in which memory T cell subsets were defined exclusively based on CD62L expression profiles. Our data show that the CD62Llow antigen-specific T cell population is very heterogeneous, indicating that results obtained from these adoptive transfer experiments have to be interpreted with caution. Although our data favor a linear model of memory T cell differentiation, it will probably require sophisticated genetic in vivo approaches to uncover the exact developmental dynamics of these subpopulations.
Recent studies have also indicated that the generation of memory T cells might be a dynamic process, in which some posteffector T cells “mature” to a memory phenotype over time (31). These data were generated by determining the kinetics of gene expression on entire antigen-specific T cell populations purified from the spleen by using microarray technology. Our data demonstrate an early phenotypical and functional segregation of T cell subsets. Because these cell subsets have different migration patterns and maintenance requirements, the dynamic changes described could also be interpreted as a reflection of differences in the relative prevalence of distinct subpopulations at certain time points, especially when looking at the spleen (Fig. 1). Interestingly, the same group that originally proposed the “dynamic” model of memory generation recently published data on early segregation of memory T cell subsets (32), very similar to the results of our study. They also used CD127 as a marker for memory T cells; however, all their conclusions were exclusively based on adoptive transfer studies, which have to be interpreted with caution. We, therefore, decided to more clearly demonstrate that CD127 is indeed a marker, which allows the ability to distinguish between long-living memory T cells and effector T cells. This was achieved by further phenotypical analysis of memory T cell subsets within the CD127 positive population, and the clear demonstration that exclusively subsets within the CD127 positive cell compartment are affected in mice with defective T cell memory.
The most important result of our studies may be the observation that segregation into distinct T cell subsets can be monitored early after the priming period. This result is in accordance with studies showing that antigen rechallenge during the effector phase can induce a characteristic memory response, indicating that at this early time point T cells with all characteristics of memory T cells are already present (33). The identification of new phenotypic markers to distinguish between effector and memory T cell subsets allowed us to directly visualize the early development, differentiation, and segregation of memory T cell subpopulations. This finding is of special clinical interest because it might make it possible to obtain information about the quality of long-term protective immunity early during an immune response, e.g., after vaccination against a pathogen. Furthermore, the ability to monitor the development of T cell subsets early after immunization is likely to improve vaccine development. Certain immunization strategies might favor the generation of effector T cells, but these cells are not maintained in the long term; other protocols may give rise to memory T cells with immediate early effector function or may be dominated by memory T cells with a CD127high/CD62Lhigh (“central memory”-like) phenotype. Each of these vaccine types will have distinct advantages over others for certain clinical applications. A better understanding of the requirements for T cell subset generation in vivo will provide the rationale for design of more efficient vaccines.
Acknowledgments
We thank Hao Shen for providing ovalbumin-expressing Listeria; Gerd Holzapfel and Thomas Schmidt from IBA, Göttingen, for providing Streptactin-APC for reversible multimer sorting; and M. Koffler, A. Wulf, and A. Heit for technical support. D.H.B., M.S., and K.L. are members of the Clinical Cooperation Group “Vaccinology.” This work was supported by the Sonderforschungsbereich 576 (Teilprojekt A8) and a Gerhard Hess fellowship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Lm, Listeria monocytogenes; LN, lymph nodes; TE, effector T cell; TCM, central memory T cell; MHC II, MHC class II; TEM, effector memory T cell; APC, allophycocyanin.
References
- 1.Herrath, M. v., Yokoyama, M., Dockter, J., Oldstone, M. B. A. & Whitton, J. L. (1996) J. Virol. 70, 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen, E. M., Lemmens, E. E., Wolfe, T., Christen, U., von Herrath, M. G. & Schoenberger, S. P. (2003) Nature 421, 852–856. [DOI] [PubMed] [Google Scholar]
- 3.Sun, J. C. & Bevan, M. J. (2003) Science 300, 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shedlock, D. J. & Shen, H. (2003) Science 300, 337–339. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., Tshon, A., Lee, S., Xu, J., Grewal, I. S., Oldstone, M. B. A. & Flavell, R. A. (1996) J. Exp. Med. 183, 2129–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgeois, C., Veiga-Fernandes, H., Joret, A. M., Rocha, B. & Tanchot, C. (2002) Eur. J. Immunol. 32, 2199–2207. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois, C., Rocha, B. & Tanchot, C. (2002) Science 297, 2060–2063. [DOI] [PubMed] [Google Scholar]
- 8.Judge, A. D., Zhang, X., Fujii, H., Surh, C. D. & Sprent, J. (2002) J. Exp. Med. 196, 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prlic, M., Lefrancois, L. & Jameson, S. C. (2002) J. Exp. Med. 195, F49–F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yajima, T., Nishimura, H., Ishimitsu, R., Watase, T., Busch, D. H., Pamer, E. G., Kuwano, H. & Yoshikai, Y. (2002) J. Immunol. 168, 1198–1203. [DOI] [PubMed] [Google Scholar]
- 11.Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. (2001) Science 291, 2413–2417. [DOI] [PubMed] [Google Scholar]
- 12.Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. (2001) Nature 410, 101–105. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708–712. [DOI] [PubMed] [Google Scholar]
- 14.Ravkov, E. V., Myrick, C. M. & Altman, J. D. (2003) J. Immunol. 170, 2461–2468. [DOI] [PubMed] [Google Scholar]
- 15.Wherry, E. J., Teichgraber, V., Becker, T. C., Masopust, D., Kaech, S. M., Antia, R., Von Andrian, U. H. & Ahmed, R. (2003) Nat Immunol. 4, 225–234. [DOI] [PubMed] [Google Scholar]
- 16.Lauvau, G., Vijh, S., Kong, P., Horng, T., Kerksiek, K., Serbina, N., Tuma, R. A. & Pamer, E. G. (2001) Science 294, 1735–1739. [DOI] [PubMed] [Google Scholar]
- 17.Unsoeld, H., Krautwald, S., Voehringer, D., Kunzendorf, U. & Pircher, H. (2002) J. Immunol. 169, 638–641. [DOI] [PubMed] [Google Scholar]
- 18.Busch, D. H., Vijh, S. & Pamer, E. G. (1998) Curr. Methods Immunol. 8. 353–362. [Google Scholar]
- 19.Busch, D. H., Pilip, I. & Pamer, E. G. (1998) J. Exp. Med. 188, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tough, D. F. & Sprent, J. (1994) J. Exp. Med. 179, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knabel, M., Franz, T. J., Schiemann, M., Wulf, A., Villmow, B., Schmidt, B., Bernhard, H., Wagner, H. & Busch, D. H. (2002) Nat. Med. 8, 631–637. [DOI] [PubMed] [Google Scholar]
- 22.Busch, D. H., Pilip, I. M., Vijh, S. & Pamer, E. G. (1998) Immunity 8, 353–362. [DOI] [PubMed] [Google Scholar]
- 23.Murali-Krishna, M., Altman, J. D., Suresh, M., Sourdive, D. J. D., Zajac, A. J., Miller, J. D., Slansky, J. & Ahmed, R. (1998) Immunity 8, 177–188. [DOI] [PubMed] [Google Scholar]
- 24.Grewal, I. S. & Flavell, R. A. (1998) Annu. Rev. Immunol. 16, 111–135. [DOI] [PubMed] [Google Scholar]
- 25.Diehl, L., Den Boer, A. T., van der Voort, E. I., Melief, C. J., Offringa, R. & Toes, R. E. (2000) J. Mol. Med. 78, 363–371. [DOI] [PubMed] [Google Scholar]
- 26.Tuma, R. A. & Pamer, E. G. (2002) Curr. Opin. Immunol. 14, 348–353. [DOI] [PubMed] [Google Scholar]
- 27.Shedlock, D. J., Whitmire, J. K., Tan, J., MacDonald, A. S., Ahmed, R. & Shen, H. (2003) J. Immunol. 170, 2053–2063. [DOI] [PubMed] [Google Scholar]
- 28.Belz, G. T., Wodarz, D., Diaz, G., Nowak, M. A. & Doherty, P. C. (2002) J. Virol. 76, 12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzavecchia, A. & Sallusto, F. (2002) Nat. Rev. Immunol. 2, 982–987. [DOI] [PubMed] [Google Scholar]
- 30.Masopust, D., Jiang, J., Shen, H. & Lefrancois, L. (2001) J. Immunol. 166, 2348–2356. [DOI] [PubMed] [Google Scholar]
- 31.Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. (2002) Cell 111, 837–851. [DOI] [PubMed] [Google Scholar]
- 32.Kaech, S. M., Tan, J. T., Wherry, E. J., Konieczny, B. T., Surh, C. D. & Ahmed, R. (2003) Nat. Immunol. 4, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 33.Busch, D., Kerksiek, K. & Pamer, E. (2000) J. Immunol. 164, 4063–4070. [DOI] [PubMed] [Google Scholar]