Abstract
Murine epidermal growth factor (EGF) is synthesized as part of a large precursor (pro-EGF), which is thought to span the cell membrane. Comparison of the published pro-EGF sequence with the sequences of the translation products of viral oncogenes reveals that pro-EGF is related to the translation product of mos, the oncogene of Moloney murine sarcoma virus. Similarity is greatest between the COOH-terminal region of v-mos (residues 317-360) and part of the cytoplasmic domain of pro-EGF (residues 1127-1174). Statistical comparison of these sequences indicates that the probability of the similarity arising by chance is less than 2 X 10(-8). This similarity extends to the corresponding regions of the translation products of the cellular homologues (c-mos) of the v-mos gene present in normal murine and human DNA. Similarities are also observed between two other regions of the murine c-mos sequence (residues 48-134 and 196-275) and parts of the extracellular domain of pro-EGF (residues 565-651 and 741-817, respectively). All three mos genes are members of the tyrosine kinase family of oncogenes, as is erbB, the oncogene of avian erythroblastosis virus. Since the sequences of the erbB translation product and the EGF receptor are closely related, the relationship between mos and pro-EGF suggests that pro-EGF and the EGF receptor have evolved from a common ancestor.
Full text
PDF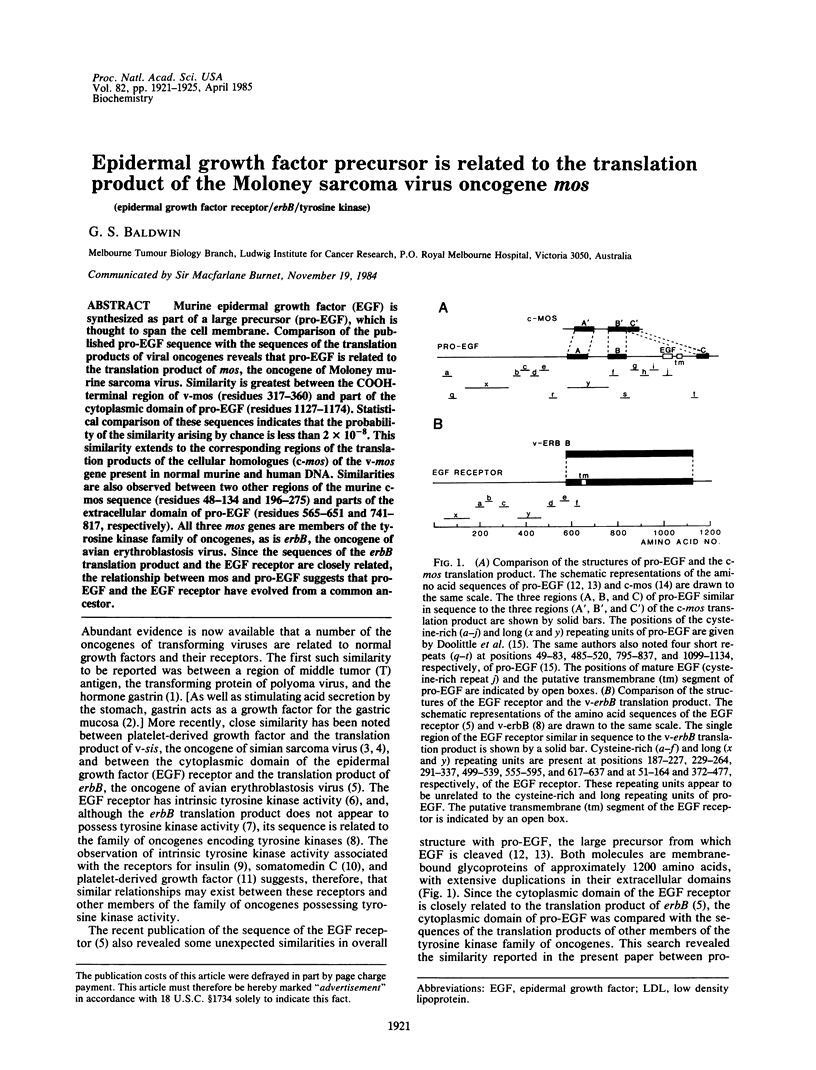




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. S. Gastrin and the transforming protein of polyoma virus have evolved from a common ancestor. FEBS Lett. 1982 Jan 11;137(1):1–5. doi: 10.1016/0014-5793(82)80302-5. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Rylatt D. B., Nimmo G. A. The hormonal control of glycogen metabolism: the amino acid sequence at the phosphorylation site of protein phosphatase inhibitor-1. FEBS Lett. 1977 Apr 15;76(2):182–186. doi: 10.1016/0014-5793(77)80147-6. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. Corrections to the nucleotide sequence of the src gene of Rous sarcoma virus. Nature. 1983 Feb 24;301(5902):736–738. doi: 10.1038/301736b0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S. Computer-based characterization of epidermal growth factor precursor. Nature. 1984 Feb 9;307(5951):558–560. doi: 10.1038/307558a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Gattoni S., Kirschmeier P., Weinstein I. B., Escobedo J., Dina D. Cellular Moloney murine sarcoma (c-mos) sequences are hypermethylated and transcriptionally silent in normal and transformed rodent cells. Mol Cell Biol. 1982 Jan;2(1):42–51. doi: 10.1128/mcb.2.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Johnson L. R. The trophic action of gastrointestinal hormones. Gastroenterology. 1976 Feb;70(2):278–288. [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Primary structure of v-raf: relatedness to the src family of oncogenes. Science. 1984 Apr 20;224(4646):285–289. doi: 10.1126/science.6324342. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]


