Abstract
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed cancers in elderly men, and accounts for 30% of all newly diagnosed cases of cancer. The development of the ‘clinically insignificant’ prostate cancer into its invasive form is still unclear, and it is believed that chronic inflammation may play its role, as proposed by De Marzo in 1999. However, there is no clear opinion on the subject of existence of dependencies between changes of the inflammatory type and PCa.
Material and methods
The study involved 1,010 men, suspected of prostate cancer development by positive digital rectal examination (DRE) and/or elevated PSA value. The 10 cores, TRUS guided biopsy was performed. In those with ASAP, HG–PIN or inflammation the second biopsy was proposed.
Results
In the first biopsy PCa was diagnosed in 336 patients (33.27%). ASAP was found in 58 (5.74%), HG–PIN in 82 (8.11%), and the coexistence of both was found in 19 (1.88%). Chronic prostatitis was diagnosed in 101 (10%) men. Of those who underwent second biopsy, PCa was diagnosed in six of 19 patients (31.57%) who were diagnosed with HG–PIN in the first biopsy, in four of 40 (10%) with BPH in the first biopsy, in four of 18 (22.22%) with ASAP or LG–PIN together with ASAP, and in two out of five (40%) with the coexistence of ASAP and HG–PIN. Malignancy was not confirmed in any of the patients in whom the diagnosis of BPH, HG–PIN, or ASAP was accompanied by chronic prostatitis.
Conclusions
Chronic prostatitis does not significantly increase the value of PSA in patients with benign changes (BPH). The presence of prostatitis in the first biopsy did not predict cancer in subsequent biopsy, because the second biopsy did not reveal prostate cancer in any of the patients in whom prostatitis was diagnosed in the first biopsy.
Keywords: chronic inflammation, prostate cancer development
INTRODUCTION
Prostate cancer (PCa) is one of the most commonly diagnosed cancers in elderly men. In Western Europe and the U.S.A. it is the most common malignancy in men and accounts for 30% of all newly diagnosed cases [1]. In Poland PCa is the third most common after cancers of the lung and stomach. It accounts for about 5% of all cancer deaths in men with a mean incidence of 28.3 per 100,000 people [2]. Data on the development of PCa is not clear. It is a unique type of cancer that can present as either overt, covert (latent), or clinically insignificant. The development of the ‘clinically insignificant’ prostate cancer into its invasive form is still unclear.
It is believed that chronic inflammation may stimulate the development of cancer, which has been confirmed in the pathophysiology of other cancers such as those of the stomach, liver, and colon. In 1999, De Marzo proposed a model for the development of PCa through its activation by chronic inflammation (proliferative inflammatory atrophy – PIA) [3]. It seems that the cells of the inflammatory process may be an important source of free radicals that detrimentally affect the DNA of prostate cells and stimulate reduction in the synthesis of glutathione transferase, one of the most important antioxidants [4]. This hypothesis is confirmed in numerous retrospective studies that involve chronic inflammation in the development of PCa [5–7].
At the moment, however, there is no unanimous opinion on the recognition of inflammatory changes in the prostate as a precancerous condition. There are a number of factors suggesting that the change recognized in the histological preparation can be classified as precancerous. There must be an epidemiological relationship between cancer and its precancerous condition, which should occur at a slightly earlier age with the existence of some dependencies (cellular, histological, structural) in the morphology of the two states. There is no clear opinion on the subject of existence of such dependencies between changes of the inflammatory type and PCa.
MATERIALS AND METHODS
The aim of this study was to assess the incidence of inflammatory changes (prostatitis) in patients undergoing biopsy for suspected PCa, the impact of inflammation on changing prostate specific antigen (PSA) levels, and to assess the role of inflammation in the prediction of the risk of PCa diagnosis in the second biopsy.
The study involved 1,010 men from the period of April 2006 to September 2007. The criterion for inclusion in the study was a suspicion of PCa on the basis of elevated PSA level (cut–off value was set at 4 ng/ml), changes found on digital rectal exam (DRE), and/or the presence of changes in the transrectal ultrasound (TRUS) image.
Patients enrolled in the study were sent to the Department of General Pediatric & Oncological Urology at the Medical College in Nicolaus Copernicus University, Bydgoszcz or the Department of General and Oncological Urology at the Specialist Municipal Hospital in Toruń. Under local anesthesia and prophylactic quinolone chemotherapy, a core needle biopsy was performed with a Tru Cut Cook 18G needle and TRUS guidance. Ten specimens were most often collected, but the protocol was sometimes extended to include additional segments of areas found to be suspicious in TRUS imaging.
Histopathological evaluation of the preparations was carried out by four pathologists: two from the Department of Pathology Medical College of Nicolaus Copernicus University in Bydgoszcz and two from the Department of Pathology Specialist Municipal Hospital in Toruń. If PCa was diagnosed in the biopsy then the patient was eligible for further treatment or observation, depending on the severity of the disease. If prostatitis was diagnosed in the biopsy then the patients were included in the observation program in an outpatient setting. After anti–inflammatory treatment (which included a quinolone chemotherapeutic and a non–steroidal anti–inflammatory drug), PSA studies were repeated and, if elevated above baseline, then prostate biopsy was repeated after three months. This was also performed in patients with precancerous lesions (atypical small acinar proliferation – ASAP; high–grade prostatic intraepithelial neoplasia – HG–PIN; or their co–existence) identified in the first biopsy.
RESULTS
I. Results of first biopsy
1. Overall results of biopsy
Among the 1,010 men who underwent primary biopsy, PCa was diagnosed in 336 patients (33.27%). The presence of precancerous lesions was found in 159 men (15.47%): ASAP was found in 58 (5.74%), HG–PIN in 82 (8.11%), and the coexistence of both was found in 19 (1.88%). Prostatitis occurring spontaneously or from benign changes (BPH) was diagnosed in 101 (10%) men. Prostatitis together with PCa were diagnosed in three (0.89%), together with ASAP in four (0.39%), and with HG–PIN in six (0.59%) patients (Table 1).
Table 1.
Results of prostate biopsy in the group of 1,010 patients with suspected prostate cancer
| Results of first biopsy | n | % |
|---|---|---|
| PCa | 336 | 33.27 |
| BPH | 387 | 38.32 |
| ASAP | 46 | 4.55 |
| ASAP + LG–PIN | 8 | 0.79 |
| ASAP + prostatitis | 4 | 0.40 |
| ASAP + HG–PIN | 19 | 1.88 |
| HG–PIN | 76 | 7.52 |
| HG–PIN + prostatitis | 6 | 0.59 |
| LG–PIN | 23 | 2.28 |
| Prostatitis | 101 | 10 |
| Fibrous atrophy of the prostate | 4 | 0.40 |
| Total | 1,010 |
2. Prostate–specific antigen in the study group
The average concentration of PSA in male subjects was 19.17 ng/ml (median 9.16 ng/ml). The highest values of PSA were found in patients with PCa (mean 34.93 ng/ml, median 13.5 ng/ml) and the lowest were found in the group of patients with low–grade prostatic intraepithelial neoplasia (LG–PIN) (mean 9.3 ng/ml, median 6.90 ng/ml) (Table 2). The only statistically significant difference in PSA values among the patients was found between the patients with PCa and those with LG–PIN (p = 0.036614).
Table 2.
PSA values in the group of studied patients
| PSA | Mean (ng/ml) | Median (ng/ml) |
|---|---|---|
| Mean of the whole group | 19.17 | 9.165 |
| in the group with PCa | 34.93 | 13.5 |
| in the group with ASAP | 12.06 | 11.18 |
| in the group with ASAP & HG–PIN | 12.47 | 8 |
| in the group with ASAP & LG–PIN | 13.06 | 11.15 |
| in the group with ASAP & prostatitis | 13.05 | 15.15 |
| in the group with HG–PIN | 11.3 | 9.915 |
| in the group with HG–PIN & prostatitis | 13.16 | 12.9 |
| in the group with LG–PIN | 9.3 | 6.98 |
| in the group with BPH | 10.83 | 8 |
| in the group with prostatitis | 12.68 | 9.09 |
3. PSA values for patients with prostatitis compared to BPH
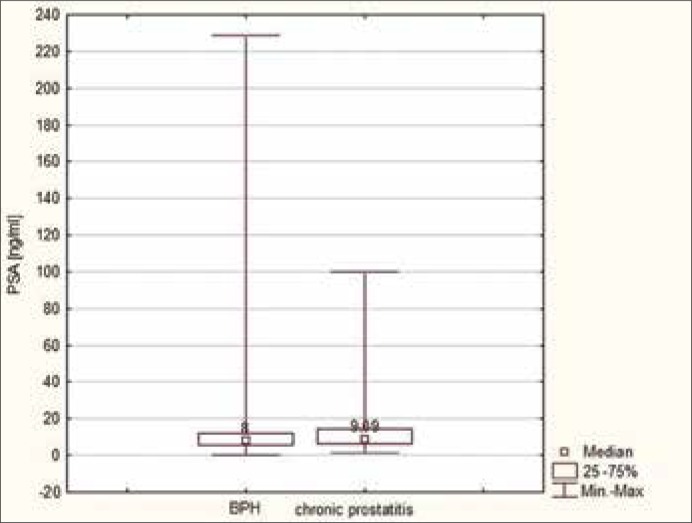
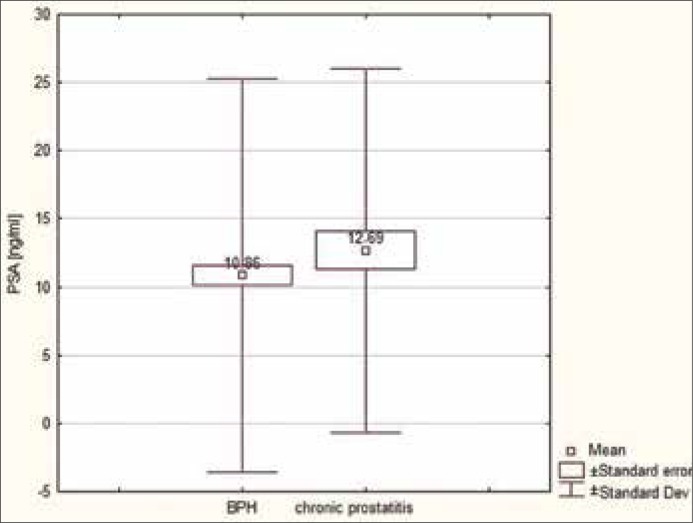
The PSA values of patients with prostatitis were compared to those of patients with benign changes (benign prostatic hyperplasia – BPH). The results demonstrated a higher mean and median in the group of men with prostatitis (average 10.83 ng/ml, median 8 ng/ml) in comparison to the group with BPH (mean 12.68 ng/ml, median 9.09 ng/ml), but the difference was not statistically significant (p = 0.7319) (Figures 1, 2).
Figure 1.
Median values of PSA in patients with prostatitis in comparison to those with BPH.
Figure 2.
Mean values of PSA in patients with prostatitis in comparison to those with benign changes.
Furthermore, the average values of PSA in patients diagnosed with prostatitis together with ASAP or HG–PIN were slightly higher than in those with only prostatitis. Also, the patients whose PCa coexisted with prostatitis had lower PSA values than those patients with only prostatitis (Table 3). These differences, however, were not statistically significant.
Table 3.
PSA values in patients with a diagnosis of prostatitis and other accompanying changes
| Mean (ng/ml) | Median (ng/ml) | |
|---|---|---|
| Prostatitis + ASAP | 13.05 | 15.15 |
| Prostatitis + HG–PIN | 13.16 | 12.9 |
| Prostatitis + PCa | 10.97 | 9.43 |
| Prostatitis | 12.68 | 9.09 |
| BPH | 10.86 | 8 |
| PSA for the studied group | 19.17 | 9.16 |
II. The results of second prostate biopsy
1. The overall results of second prostate biopsy
The most common diagnosis made in the course of second biopsy was BPH (32 patients – 32.65%) and PCa was confirmed in 16 (16.33%) patients in whom it was not previously found. In the second biopsy, ASAP was more frequently diagnosed (17 men – 17.34%) than HG–PIN (14 patients – 14.28%). Among the patients who underwent second biopsy, the presence of chronic prostatitis was found in 11 (11.22%) and LG–PIN was found in four (spontaneously occurring in two – 2.04%; and associated with prostatitis in two (2.04%). One patient demonstrated fibrous atrophy of the prostate (Table 4).
Table 4.
Results of the second prostate biopsy
| Diagnosis | N | % |
|---|---|---|
| PCa | 16 | 16.33 |
| ASAP | 17 | 17.34 |
| ASAP + HG–PIN | 3 | 3.06 |
| HG–PIN | 14 | 14.28 |
| BPH | 32 | 32.65 |
| Prostatitis | 11 | 11.22 |
| LG–PIN | 4 | 4.08 |
| Fibrous atrophy of the prostate | 1 | 1.02 |
| Total | 98 |
2. Dependence of the result of second prostate biopsy on the value of PSA
The PSA levels were compared in patients who underwent second biopsy depending on the result. The highest mean and median values were found in the group of men who were diagnosed with LG–PIN in the second biopsy (Table 5).
Table 5.
PSA values depending on the result of the second biopsy
| The result of second biopsy | n | PSA (ng/ml) | |
|---|---|---|---|
| Mean | Median | ||
| PCa | 16 | 11.95 | 9.92 |
| ASAP | 17 | 9.12 | 8.89 |
| ASAP + HG–PIN | 3 | 11.47 | 11.90 |
| HG–PIN | 14 | 11.81 | 9.50 |
| BPH | 32 | 10.82 | 8.13 |
| Prostatitis | 11 | 11.79 | 11.62 |
| LG–PIN | 4 | 13.79 | 15.65 |
| Fibrous atrophy of the prostate | 1 | 11.00 | 11.00 |
| Total | 98 | ||
The values of PSA did not reveal any statistically significant differences between the diagnoses of the second biopsy in comparison to each other.
3. Dependence of the result of second prostate biopsy on the diagnosis made in the first
Of those who underwent second biopsy, PCa was diagnosed in six of 19 patients (31.57%) who were diagnosed with HG–PIN in the first biopsy, in four of 40 (10%) with BPH in the first biopsy, in four of 18 (22.22%) with ASAP or LG–PIN together with ASAP, and in two out of five (40%) with the coexistence of ASAP and HG–PIN.
Malignancy was not confirmed in any of the patients in whom the diagnosis of BPH, HG–PIN, or ASAP was accompanied by prostatitis (Table 6 ).
Table 6.
Results of the first biopsy in patients diagnosed with prostate cancer in the second biopsy
| Diagnosis in the first biopsy | Number of people subjected to the second biopsy | Number of cancers diagnosed in the second biopsy | |
|---|---|---|---|
| ASAP | 13 | ASAP→ PCa | 2 |
| ASAP + HG–PIN | 5 | ASAP + HG–PIN→PCa | 2 |
| ASAP + LG–PIN | 5 | ASAP + LGPIN→ PCa | 2 |
| ASAP + prostatitis | 1 | ASAP + prostatitis→ PCa | 0 |
| HG–PIN | 19 | HG–PIN→ PCa | 6 |
| HG–PIN + prostatitis | 3 | HGPIN + prostatitis→ PCa | 0 |
| LG–PIN | 2 | LG–PIN→ PCa | 0 |
| BPH | 40 | BPH→ PCa | 4 |
| BPH + prostatitis | 10 | BPH + prostatitis→ PCa | 0 |
| Total | 98 | 16 |
DISCUSSION
Recurrent inflammation has been considered as the cause of many human cancers, including those of the esophagus, stomach, liver, and colon [8]. Most cancers developing in these organs have been related to a specific inflammatory state, which stimulates the migration of inflammatory cells and increases the local concentration of cytokines and growth factors that promote angiogenesis, cell replication, and tissue repair. The ongoing inflammatory process may result in damage to the genome of the tissue building cells and thus promote carcinogenesis [9–11]. In 1999, DeMarzo suggested that prostatitis could be associated with the development of PCa [3]. It is believed that ongoing chronic prostatitis, progressing to inflammatory prostatic atrophy (Prostate Inflammatory Atrophy–PIA), could be a promoter of change leading to the development of the PIN that is followed by PCa [4–7].
In men, especially in the elderly, inflammation of the prostate (prostatitis) can develop without the apparent presence of a pathogen, sometimes even without clinical signs and the absence of inflammatory cells in the prostatic secretions (type IV inflammation of the prostate according to the National Institutes of Health Classification of Prostatitis). Due to its asymptomatic nature, this condition can be overlooked by the patient who is unaware of the possibility for PCa development in the future [4]. The localized focus of epithelial atrophy that is observed in these patients is commonly found in the peripheral area of the prostate. Many of these foci, which are probably related to the acute or chronic inflammatory state, have proliferating epithelial cells and are idsentified at the most frequent site of PCa occurrence [12–14]. Such places also frequently contain HG–PIN and clinically insignificant PCa. In connecting prostatitis with the development of PCa, they could also indicate changes found in chromosome 8 thanks to in situ hybridization, which are analogous to the changes observed in HG–PIN and PCa [15].
It seems that since prostatitis, HG–PIN, and PCa have similar changes in the genotype of cells, their coexistence and association in development appears to be possible. Also proposed is the following sequence of events – foci of PIA, exposed to oxidative stress during the inflammatory process, can progress to PCa, either directly or via an intermediate phase that is HG–PIN.
If the inflammatory changes are associated with the development of PCa, it seems that their coexistence should be significantly more frequent. In the analyzed study this fact cannot be confirmed, because the prostatitis occurred independently or in coexistence with BPH in 101 (10%) men subjected to biopsy and these changes coexisted with PCa in only three (0.89%) men, with ASAP in four (0.39%), and with HG–PIN in six (0.59%).
Therefore, at the present time there is still no clear evidence for the association of PCa with chronic prostatitis and clinical studies on this topic should be continued.
Acute prostatitis, resulting in the destruction of its cellular structures, allows the movement of large quantities of PSA into the blood. However, the impact of chronic inflammatory changes on the increase in PSA level is ambiguous. Among the older studies, the generally dominating opinion is that acute prostatitis causes a statistically significant increase in PSA levels in all patients regardless of the severity of the disease [16–18]. Potts performed prostate biopsies in 122 men with elevated PSA levels and found symptoms of prostatitis in 51 of them (42%) [19]. Among the more recent studies that used the methodologies proposed by the NIH Classification of Prostatitis, an increase in PSA was observed in only about 58% of patients with acute symptoms of bacterial prostatitis, in 15.5% with chronic bacterial prostatitis, and in 9% with chronic prostatitis type IIIb and IV according to the NIH (National Institutes of Health Classification of Prostatitis) [20]. Similarly, a number of studies highlighted the fact that the occurrence of chronic prostatitis, particularly type IIIb and IV (NIH), cannot cause a rise in PSA levels [21]. The only increase in PSA values can be observed in men with type IIIa prostatitis [22]. Stancik biopsied 404 men with elevated PSA values and identified 34% of patients with NIH type IV prostatitis and 24% with PCa. The values of total PSA (tPSA) and free PSA (fPSA) in both groups were similar and amounted to 11.94 ng/mL in both groups (tPSA) and 1.31 ng/mL and 1.54 ng/mL (fPSA) respectively [23]. Although these data may suggest that chronic prostatitis may increase PSA values, other authors did not confirm such relationships. Kwak's study did not confirm a relationship between PSA value and the presence of chronic prostatitis. Although patients with PSA levels greater than 2.5 ng/ml experienced greater subjective symptoms associated with the presence of prostatitis than those with lower values of PSA, both of these groups were found in the group of patients with PSA values considered to be normal [24]. Carver, in his work from 2003, found that patients with NIH type IV prostatitis had a mean PSA value of 2.3 ng/ml [25]. Nadler confirmed the findings of the abovementioned studies by comparing NIH types IIIa and IIIb prostatitis to healthy men in his study, which was conducted within the realms of the Chronic Prostatitis Collaborative Research Network. A statistically significant elevation in PSA value was confirmed among men with prostatitis (1.97 ng/ml) when compared to healthy controls (1.72 ng/ml), but both values still remained within values considered as normal [26].
It is difficult to clearly identify the impact of prostatitis, or prostatitis with the coexistence of other diseases of the prostate, on PSA concentration. Among the men included in this study, the mean and median values of PSA in the group with prostatitis (average – 12.68 ng/ml, median – 9.09 ng/ml) were significantly higher than the values in the group of men with BPH (mean – 10.86 ng/ml, median – 8.01 ng/ml), but the difference was not statistically significant (p = 0.7319). In comparison to other groups, PSA was the lowest in the group in which PCa and prostatitis coexisted, since the values were 10.97 ng/ml, while the value of PSA in the group of patients with known prostatitis and associated states of ASAP or HG–PIN were slightly higher than independently occurring prostatitis. Prostatitis caused a statistically insignificant rise in PSA value, raising the level slightly in cases of coexisting ASAP (from 12.47 ng/mL to 13.05 ng/ml – p = 0.3082) or coexisting HG–PIN (from 11.35 ng/ml to 12.29 ng/ml – p = 0.4874). This is consistent with the opinion of most authors who believe that neither ASAP nor HG–PIN will increase the value of the PSA, and that the only factor able to increase its level is PCa [27–29]. Di Silverio made similar observations in his evaluation of 3,942 patients subjected to biopsy, which found that the only factor elevating the concentration of PSA was coexisting PCa pT1b. Moreover, neither the coexistence of precancerous lesions, prostatitis, nor even PCa pT1a was found to elevate PSA levels. In all the groups, the mean concentration of PSA did not exceed 5.1 ng/ml to 5.9 ng/ml [30].
Asymptomatic patients, with chronic inflammation confirmed in biopsy, are a challenge to most of urologists, and require close follow–up. It seems that during follow up, elevated PSA value should be a warning sign, and should lead to biopsy or just follow up–protocol. Therefore, in our opinion, it should be a standard procedure to administer anti–inflammatory and antibiotic treatment to every asymptomatic prostatitis patients with elevated PSA values, but not with chronic inflammation and normal (or decreased during observation) PSA. The risk of developed but not diagnosed neoplasmatic disease is high, therefore such protocol seems to be justified. In about 60% of such patients in our study group, there was a decrease of PSA value observed after that treatment, so they can be omitted in re–biopsy protocol. Therefore patients with raised PSA values and inflammation on biopsy, should be re–biopsied.
Free/total PSA ratio, and PSA velocity can be a helpful tool. It was not used in our study, because of patients enrollment in few different centers, some of them not using this valuable tool. A large cohort study, enrolling above 1000 patients should be started, to clarify a role of free/total PSA and PSA velocity in patients with prosatitis on biopsy, but suspected with prostate cancer.
CONCLUSIONS
Prostatitis is diagnosed in one in every ten patients subjected to biopsy for suspected prostate cancer.
Chronic prostatitis does not significantly increase the value of PSA in patients with benign changes (BPH).
The coexistence of prostatitis with precancerous lesions causes a statistically insignificant elevation in PSA levels in relation to prostatitis that occurs independently.
The persistent elevation of PSA level that qualifies for subsequent biopsy cannot reliably determine its outcome (cancer), because PSA was the highest in patients who had no evidence of cancer in subsequent biopsies (LG–PIN).
The presence of prostatitis in the first biopsy did not predict cancer in subsequent biopsy, because the second biopsy did not reveal prostate cancer in any of the patients in whom prostatitis was diagnosed in the first biopsy.
References
- 1.Jensen OM, Esteve J, Moller H, Renard H. Cancer in European community and its member states. Eur J Cancer. 1990;26:1167–1256. doi: 10.1016/0277-5379(90)90278-2. [DOI] [PubMed] [Google Scholar]
- 2.Chwaliński T. Prostate cancer: diagnosis and treatment. Nowa Medycyna. 2001;113:124–127. [Google Scholar]
- 3.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 5.Sciarra A, Mariotti G, Salciccia S, Gomez AA, Monti S, Toscano V, Di Silverio F. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Wagenlehner FM, Elkahwaji JE, Algaba F, Bjerklund–Johansen T, Naber KG, Hartung R, Weidner W. The role of inflammation and infection in the pathogenesis of prostate carcinoma. BJU Int. 2007;100:733–737. doi: 10.1111/j.1464-410X.2007.07091.x. [DOI] [PubMed] [Google Scholar]
- 7.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 10.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Nat Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerutti PA, Trump BF. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 12.Ruska KM, Sauvageot J, Epstein JI. Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol. 1998;22:1073–1077. doi: 10.1097/00000478-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Shah R, Mucci NR, Amin A, Macoska JA, Rubin MA. Postatrophic hyperplasia of the prostate gland: neoplastic precursor or innocent bystander? Am J Pathol. 2001;158:1767–1773. doi: 10.1016/S0002-9440(10)64132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high–grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 15.Macoska JA, Trybus TM, Wojno KJ. 8p22 loss concurrent with 8c gain is associated with poor outcome in prostate cancer. Urology. 2000;55:776–782. doi: 10.1016/s0090-4295(00)00468-4. [DOI] [PubMed] [Google Scholar]
- 16.Hasui Y, Marutsuka K, Asada Y, Ide H, Nishi S, Osada Y. Relationship between serum prostate specific antigen and histological prostatitis in patients with benign prostatic hyperplasia. Prostate. 1994;25:91–96. doi: 10.1002/pros.2990250206. [DOI] [PubMed] [Google Scholar]
- 17.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 18.Nadler RB, Humphrey PA, Smith DS, Catalona WJ, Ratliff TL. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154:407–413. doi: 10.1097/00005392-199508000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Potts JM. Prospective identification of National Institutes of Health category IV prostatitis in men with elevated prostate specific antigen. J Urol. 2000;164:1550–1553. [PubMed] [Google Scholar]
- 20.Battikhi MN, Ismail H, Battikhi Q. Effects of chronic bacterial prostatitis on prostate specific antigen levels total and free in patients with benign prostatic hyperplasia and prostate cancer. Int Urol Nephrol. 2006;38:21–26. doi: 10.1007/s11255-005-1662-6. [DOI] [PubMed] [Google Scholar]
- 21.Chang SG, Kim CS, Jeon SH, Kim YW, Choi BY. Is chronic inflammatory change in the prostate the major cause of rising serum prostate–specific antigen in patients with clinical suspicion of prostate cancer? Int J Urol. 2006;13:122–126. doi: 10.1111/j.1442-2042.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Hu WL, Yang H, Qiu XF, Zhang CZ. Effects of antibiotic and anti–inflammatory treatment on serum PSA and free PSA levels in patients with chronic prostatitis IIIA. Zhonghua Nan Ke Xue. 2006;12:787–790. [PubMed] [Google Scholar]
- 23.Stancik I, Luftenegger W, Klimpfinger M, Muller MM, Hoeltl W. Effect of NIH–IV prostatitis on free and free–to–total PSA. Eur Urol. 2000;46:760–764. doi: 10.1016/j.eururo.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Kwak C, Ku JH, Kim T, Park DW, Choi KY, Lee E, Lee SE, Lee C. Effect of subclinical prostatic inflammation on serum PSA levels in men with clinically undetectable prostate cancer. Urology. 2003;62:854–859. doi: 10.1016/s0090-4295(03)00688-5. [DOI] [PubMed] [Google Scholar]
- 25.Carver BS, Bozeman CB, Williams BJ, Venable DD. The prevalence of men with National Institutes of Health category IV prostatitis and association with serum prostate specific antigen. J Urol. 2003;169:589–591. doi: 10.1097/01.ju.0000042720.98483.08. [DOI] [PubMed] [Google Scholar]
- 26.Nadler RB, Collins MM, Propert KJ, Mikolajczyk SD, Knauss JS, Landis JR, et al. Prostate–specific antigen test in diagnostic evaluation of chronic prostatitis/chronic pelvic pain syndrome. Urology. 2006;67:337–342. doi: 10.1016/j.urology.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Minardi D, Galosi AB, Dell'Atti L, Hanitzsch H, Mario P, Muzzonigro G. Production of serum–free and total prostate–specific antigen due to prostatic intraepithelial neoplasia. Scand J Urol Nephrol. 2002;36:323–329. doi: 10.1080/003655902320783818. [DOI] [PubMed] [Google Scholar]
- 28.Ramos CG, Carvahal GF, Mager DE, Haberer B, Catalona WJ. The effect of high grade prostatic intraepithelial neoplasia on serum total and percentage of free prostate specific antigen levels. J Urol. 1999;162:1587–1590. [PubMed] [Google Scholar]
- 29.Hochreiter WW. The issue of prostate cancer evaluation in men with elevated prostate–specific antigen and chronic prostatitis. Andrologia. 2008;40:130–133. doi: 10.1111/j.1439-0272.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 30.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, Sciarra A. Distribution of inflammation, pre–malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164–175. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]