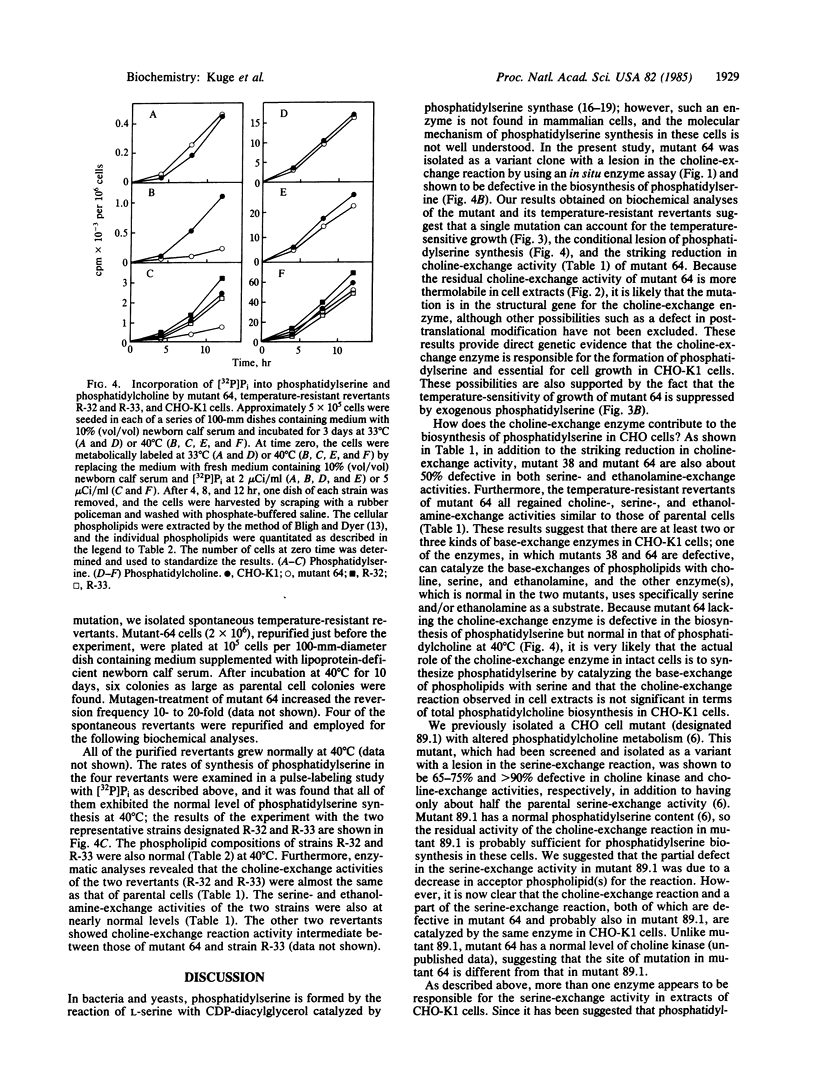
Abstract
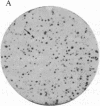
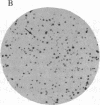
Mutant clones of Chinese hamster ovary (CHO) cells defective in the base-exchange reaction of phospholipids with choline were isolated by using an in situ enzymatic assay for the reaction in cell colonies immobilized on polyester cloth. The specific activities of the choline-exchange reaction in extracts of one of the mutants (designated 64) grown at 33 degrees C and 40 degrees C were 13% and 6% of those in parental (CHO-K1) cells, respectively. The choline-exchange activity in the mutant was more thermolabile in cell extracts than that in the parent, suggesting that a mutation in the structural gene for the choline-exchange enzyme might have been induced in this mutant. In culture medium supplemented with lipoprotein-deficient serum, mutant 64 grew almost normally at 33 degrees C but divided only twice at 40 degrees C and then stopped growing. Labeling of intact cells with [32P]Pi showed that mutant 64 was also strikingly defective in the biosynthesis of phosphatidylserine at 40 degrees C but was normal at 33 degrees C. Most temperature-resistant revertants of mutant 64 exhibited nearly normal ability to synthesize phosphatidylserine at 40 degrees C and also showed choline-exchange activity similar to that in parental cells. The addition of phosphatidylserine to medium supplemented with newborn calf serum, in which mutant 64 grew more slowly than parental cells at 40 degrees C, restored the growth rate of the mutant to the parental level. Our findings suggest that the choline-exchange enzyme functions as the major route for the formation of phosphatidylserine and that the temperature-sensitive growth of mutant 64 is due to a defect in phosphatidylserine biosynthesis at 40 degrees C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Diringer H. Phospholipid metabolism in mammalian cells. Kinetic data suggest a biosynthesis of phosphatidylserine via phosphatidylcholine. Hoppe Seylers Z Physiol Chem. 1973 May;354(5):577–582. [PubMed] [Google Scholar]
- Esko J. D., Matsuoka K. Y. Biosynthesis of phosphatidylcholine from serum phospholipids in Chinese hamster ovary cells deprived of choline. J Biol Chem. 1983 Mar 10;258(5):3051–3057. [PubMed] [Google Scholar]
- Esko J. D., Nishijima M., Raetz C. R. Animal cells dependent on exogenous phosphatidylcholine for membrane biogenesis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1698–1702. doi: 10.1073/pnas.79.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Autoradiographic detection of animal cell membrane mutants altered in phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5192–5196. doi: 10.1073/pnas.77.9.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Replica plating and in situ enzymatic assay of animal cell colonies established on filter paper. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1190–1193. doi: 10.1073/pnas.75.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marggraf W. D., Anderer F. A. Alternative pathways in the biosynthesis of phosphatidylserine in mouse cells,. Hoppe Seylers Z Physiol Chem. 1974 Oct;355(10):1299–1304. doi: 10.1515/bchm2.1974.355.2.1299. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Kuge O., Maeda M., Nakano A., Akamatsu Y. Regulation of phosphatidylcholine metabolism in mammalian cells. Isolation and characterization of a Chinese hamster ovary cell pleiotropic mutant defective in both choline kinase and choline-exchange reaction activities. J Biol Chem. 1984 Jun 10;259(11):7101–7108. [PubMed] [Google Scholar]
- Polokoff M. A., Wing D. C., Raetz C. R. Isolation of somatic cell mutants defective in the biosynthesis of phosphatidylethanolamine. J Biol Chem. 1981 Aug 10;256(15):7687–7690. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Wermuth M. M., McIntyre T. M., Esko J. D., Wing D. C. Somatic cell cloning in polyester stacks. Proc Natl Acad Sci U S A. 1982 May;79(10):3223–3227. doi: 10.1073/pnas.79.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Bourque E., Kanfer J. Studies on base-exchange reactions of phospholipids in rat brain particles and a "solubilized" system. Arch Biochem Biophys. 1975 Jul;169(1):304–317. doi: 10.1016/0003-9861(75)90345-8. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Voelker D. R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]