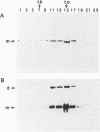
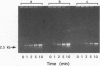
Abstract
A covalently cross-linked dimer of yeast DNA topoisomerase II was created by fusing the enzyme with the GCN4 leucine zipper followed by two glycines and a cysteine. Upon oxidation of the chimeric protein, a disulfide bond forms between the two carboxyl termini, covalently and intradimerically cross-linking the two protomers. In addition, all nine of the cysteines naturally occurring in topoisomerase II have been changed to alanines in this construct. This cross-linked, cysteine-less topoisomerase II is catalytically active in DNA duplex passage as indicated by ATP-dependent DNA supercoil relaxation and kinetoplast DNA decatenation assays. However, these experiments do not directly distinguish between a "one-gate" and a "two-gate" mechanism for the enzyme.
Full text
PDF


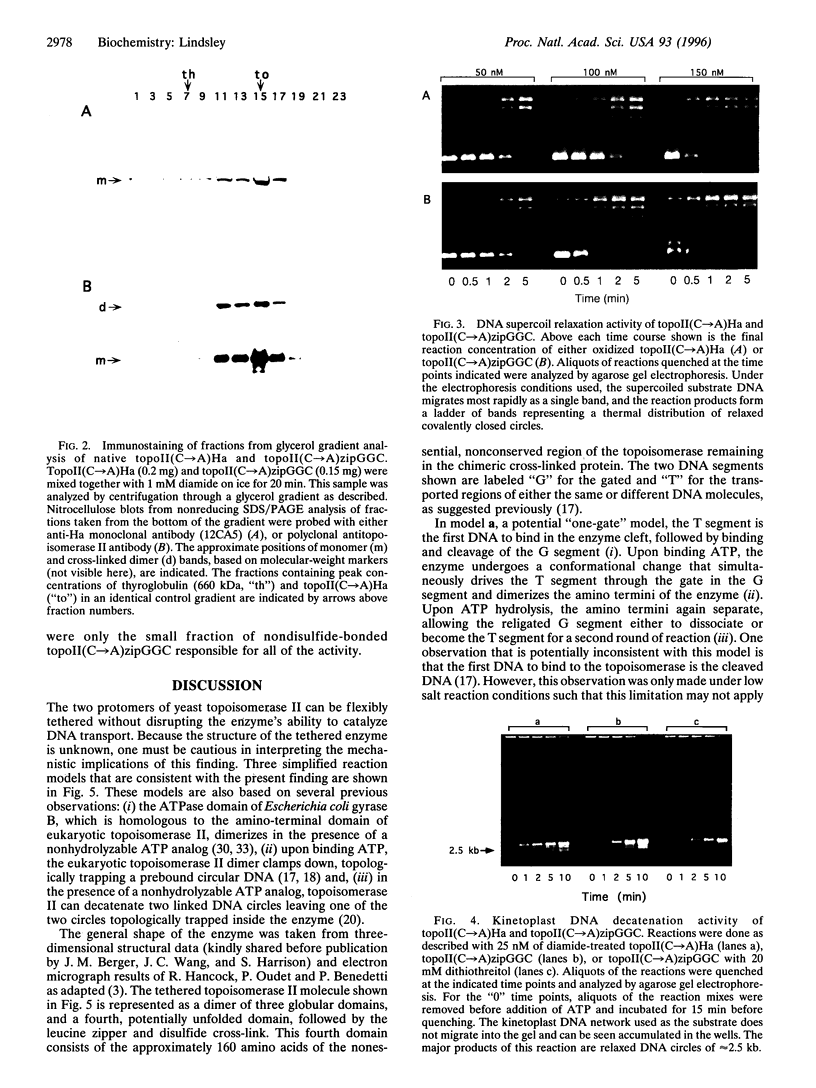
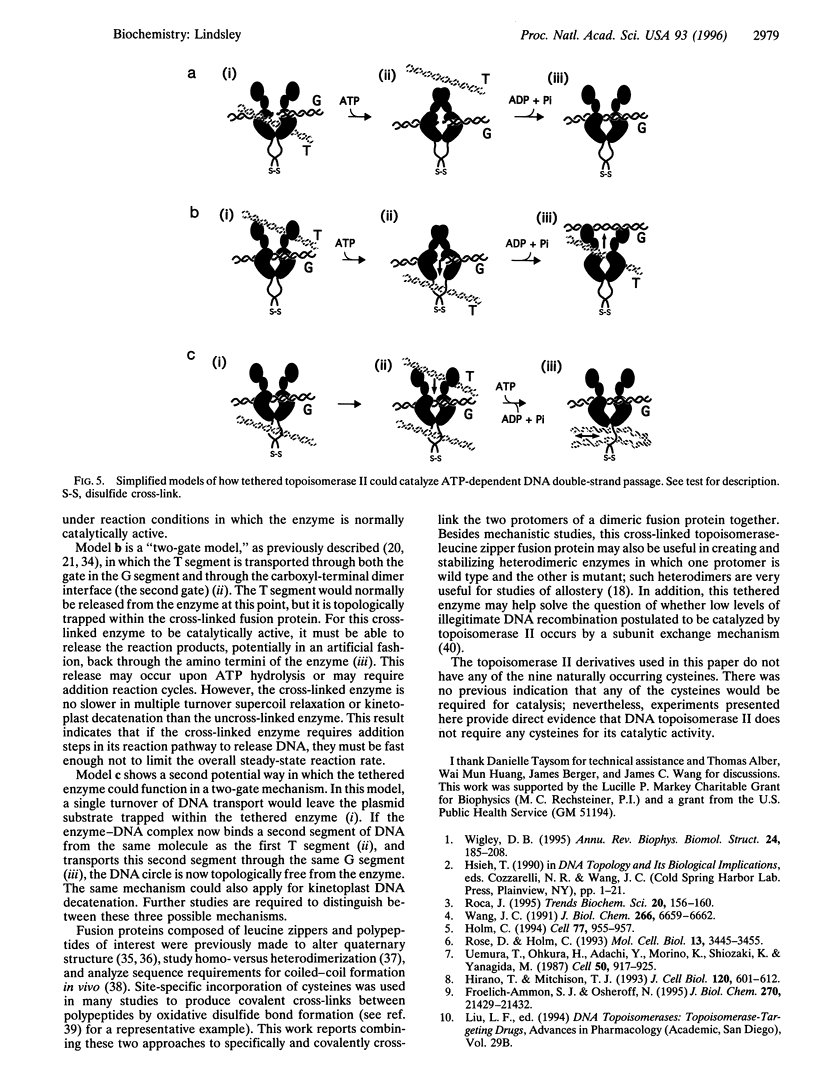

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali J. A., Jackson A. P., Howells A. J., Maxwell A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry. 1993 Mar 16;32(10):2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Kawasaki I., Ikeda H., Liu L. F. Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2076–2080. doi: 10.1073/pnas.85.7.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A., Bedouelle H. Engineering the quaternary structure of an exported protein with a leucine zipper. Protein Eng. 1991 Apr;4(4):457–461. doi: 10.1093/protein/4.4.457. [DOI] [PubMed] [Google Scholar]
- Caron P. R., Watt P., Wang J. C. The C-terminal domain of Saccharomyces cerevisiae DNA topoisomerase II. Mol Cell Biol. 1994 May;14(5):3197–3207. doi: 10.1128/mcb.14.5.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils: more facts and better predictions. Science. 1994 Jan 28;263(5146):488–489. doi: 10.1126/science.8290957. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Froelich-Ammon S. J., Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem. 1995 Sep 15;270(37):21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- Gale K. C., Osheroff N. Intrinsic intermolecular DNA ligation activity of eukaryotic topoisomerase II. Potential roles in recombination. J Biol Chem. 1992 Jun 15;267(17):12090–12097. [PubMed] [Google Scholar]
- Hu J. C., O'Shea E. K., Kim P. S., Sauer R. T. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science. 1990 Dec 7;250(4986):1400–1403. doi: 10.1126/science.2147779. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. Formation of disulfides with diamide. Methods Enzymol. 1987;143:264–270. doi: 10.1016/0076-6879(87)43050-4. [DOI] [PubMed] [Google Scholar]
- Lima C. D., Mondragón A. Mechanism of type II DNA topoisomerases: a tale of two gates. Structure. 1994 Jun 15;2(6):559–560. doi: 10.1016/s0969-2126(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. On the coupling between ATP usage and DNA transport by yeast DNA topoisomerase II. J Biol Chem. 1993 Apr 15;268(11):8096–8104. [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. Proteolysis patterns of epitopically labeled yeast DNA topoisomerase II suggest an allosteric transition in the enzyme induced by ATP binding. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10485–10489. doi: 10.1073/pnas.88.23.10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. Study of allosteric communication between protomers by immunotagging. Nature. 1993 Feb 25;361(6414):749–750. doi: 10.1038/361749a0. [DOI] [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1988 May 5;263(13):6268–6275. [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Orphanides G., Maxwell A. Topoisomerases. In one gate, out the other. Curr Biol. 1994 Nov 1;4(11):1006–1009. doi: 10.1016/s0960-9822(00)00227-x. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Pack P., Müller K., Zahn R., Plückthun A. Tetravalent miniantibodies with high avidity assembling in Escherichia coli. J Mol Biol. 1995 Feb 10;246(1):28–34. doi: 10.1006/jmbi.1994.0062. [DOI] [PubMed] [Google Scholar]
- Roca J. The mechanisms of DNA topoisomerases. Trends Biochem Sci. 1995 Apr;20(4):156–160. doi: 10.1016/s0968-0004(00)88993-8. [DOI] [PubMed] [Google Scholar]
- Roca J., Wang J. C. DNA transport by a type II DNA topoisomerase: evidence in favor of a two-gate mechanism. Cell. 1994 May 20;77(4):609–616. doi: 10.1016/0092-8674(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Roca J., Wang J. C. The capture of a DNA double helix by an ATP-dependent protein clamp: a key step in DNA transport by type II DNA topoisomerases. Cell. 1992 Nov 27;71(5):833–840. doi: 10.1016/0092-8674(92)90558-t. [DOI] [PubMed] [Google Scholar]
- Schmidt-Dörr T., Oertel-Buchheit P., Pernelle C., Bracco L., Schnarr M., Granger-Schnarr M. Construction, purification, and characterization of a hybrid protein comprising the DNA binding domain of the LexA repressor and the Jun leucine zipper: a circular dichroism and mutagenesis study. Biochemistry. 1991 Oct 8;30(40):9657–9664. doi: 10.1021/bi00104a013. [DOI] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Sci Am. 1982 Jul;247(1):94-7, 100-9. doi: 10.1038/scientificamerican0782-94. [DOI] [PubMed] [Google Scholar]
- Wasserman R. A., Wang J. C. Mechanistic studies of amsacrine-resistant derivatives of DNA topoisomerase II. Implications in resistance to multiple antitumor drugs targeting the enzyme. J Biol Chem. 1994 Aug 19;269(33):20943–20951. [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- Wigley D. B. Structure and mechanism of DNA topoisomerases. Annu Rev Biophys Biomol Struct. 1995;24:185–208. doi: 10.1146/annurev.bb.24.060195.001153. [DOI] [PubMed] [Google Scholar]
- Worland S. T., Wang J. C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989 Mar 15;264(8):4412–4416. [PubMed] [Google Scholar]
- Zechiedrich E. L., Christiansen K., Andersen A. H., Westergaard O., Osheroff N. Double-stranded DNA cleavage/religation reaction of eukaryotic topoisomerase II: evidence for a nicked DNA intermediate. Biochemistry. 1989 Jul 25;28(15):6229–6236. doi: 10.1021/bi00441a014. [DOI] [PubMed] [Google Scholar]