Abstract
INTRODUCTION:
Consistent, moderate-to-vigorous intensity exercise has been associated with a lower risk of upper respiratory tract infection (URI). However, the molecular basis for this apparent protection has not yet been fully resolved. Host-derived lipids such as cholesteryl esters (CEs) have emerged as important effector molecules of innate defense against infections. Here, we compared antimicrobial CEs in nasal fluid before and after moderate-to-vigorous exercise between active and inactive subjects.
METHODS:
Nasal fluid was collected from fourteen healthy, recreationally-active subjects (32 ± 11 yr, 7 males, 7 females) and 14 healthy, inactive subjects (25 ± 3 yr, 7 males, 7 females) before and after treadmill exercise at 70% heart rate reserve. Nasal fluid was analyzed for lysozyme, cholesteryl linoleate (CL), cholesteryl arachidonate (CA), and albumin (Alb) concentrations.
RESULTS:
Baseline concentrations (means ± SEM, inactive vs. active) of lysozyme (117.7±31.1 μg/mL vs. 122.9±15.5 μg/mL), CL+CA (15.3±1.8 μg/mL vs.26.2±10.05 μg/mL), and albumin (156.6± 54.5μg/mL vs.126.9±32.8 μg/mL) were similar to previously reported levels and did not differ significantly between study groups. However, post-exercise, CL+CA concentration was significantly lower in inactive compared to active subjects (7.8 ± 1.5 μg/mL vs. 20.1 ± 4.8 μg/mL, p = 0.036) dropping below the antimicrobial effective range. Once adjusted to albumin concentrations the changes were no longer significant, suggesting that plasma transudation accounted for the increased CA+CL concentration post-exercise in the active group relative to the inactive group.
CONCLUSION:
Moderate-to-vigorous aerobic exercise acutely decreases the antimicrobial CE response in inactive subjects, but does not modify baseline levels of CEs between active and inactive subjects. This suggests that compared to active individuals, inactive individuals may be at greater risk for URI immediately post-exercise.
Keywords: Mucosal immunity, antimicrobial peptides, epithelial cells, free fatty acids, cholesteryl esters, aerobic fitness
INTRODUCTION
Current research on exercise and upper respiratory tract infection (URI) suggests consistent, moderate-vigorous aerobic exercise at intensities and duration recommended by the American College for Sports Medicine (ACSM) may provide a protective effect against infectious disease (16,21). The relationship between exercise and URI risk has been represented in the form of a “J”-shaped curve, implying that the individuals who perform moderate-to-vigorous levels of exercise may lower their risk of URI, while those who engage in prolonged, high-intensity exercise or no exercise at all are more susceptible to infection (13,23,24,29). However, the molecular immunological basis for the protective effect of moderate exercise against URI has not yet been resolved.
The first line of defense against infection is mediated by the innate immune system (27). Recent investigations about the acute effects of exercise on innate mucosal immunity have been primarily directed towards salivary antimicrobial (poly)peptides , such as the human cathelicidin LL-37 or lysozyme (1,7,32,33). Yet, other effector molecules, such as antimicrobial lipids, are also present in mucosal secretions, and contribute to the first line of defense against microbial invasion at mucosal surfaces (9).
Lipids have been identified in a wide range of biological functions including energy storage, structural units of cells, precursors of steroidal hormones and cell signaling (4). Increasing evidence suggests an additional role for lipids as antimicrobial agents in body secretions and surfaces. Select lipids, in particular free fatty acids, have been attributed to the antibacterial activity of oral secretions (8), skin (10), human milk (14), and to the protective functions of the vernix caseosa, the waxy coating of newborns (31). In addition, we have shown that cholesteryl esters (CEs), namely cholesteryl linoleate (CL) and cholesteryl arachidonate (CA), contribute to the inherent antimicrobial activity of human nasal fluid (9) and are elevated in maxillary sinus secretions of patients with chronic rhinosinusitis (CRS) compared to non-CRS controls (17). Thus, antimicrobial lipid concentrations in upper airway secretions may impact resistance to upper respiratory tract infections, either acting alone or in synergy with antimicrobial (poly)peptides (9,20,31). However, research examining the relationship between acute exercise and antimicrobial lipids in upper respiratory tract secretions is scarce even though it may serve as an important step in understanding the protective effects of regular exercise.
Airway fluids are complex and are composed of secretions from mucosal epithelial cells and submucosal glands, as well as plasma transudation (35). A respiratory epithelial cell origin for antimicrobial CEs in nasal fluid was substantiated by their identification in the apical secretions of human bronchial epithelial cells (9). Yet, transudation of plasma lipids through endothelial cells of the upper respiratory tract capillaries cannot be excluded as a source of lipids in respiratory secretions in vivo. Albumin, a major serum protein that serves as a primary unesterified free fatty acid carrier in circulation, is a widely accepted marker of transudation from the plasma to the nasal mucosa (3). Transudation of albumin from the plasma across the upper respiratory epithelium is driven by elevated subepithelial hydrostatic pressures, which occur in association with upright dynamic exercise (18,22).
In general, exercise-induced alterations in mucosal secretions appear to be largely influenced by exercise intensity or duration. Higher intensity (> 70% VO2max) or longer duration (> 40 min) exercise appears to elicit greater concentrations and secretion rates of antimicrobial (poly)peptides (1,7,32). In contrast, low intensity or short duration exercise does not appear to significantly alter antimicrobial (poly)peptide responses compared to resting values (1,34). Usui et al. (32) noted transient increases in salivary LL-37 after prolonged strenuous exercise at 75% VO2max for 60 min. Similarly, Davison et al. (7) observed significant increases in salivary LL-37 concentration after 2.5 h of cycling exercise at 60% of VO2max, although antibacterial activity of thawed and clarified saliva against Escherichia coli did not change. Significant increases in salivary lysozyme were also found after ~22 min of incremental exhaustive exercise and high-intensity continuous exercise (>70% VO2max) on a cycle ergometer (1). In contrast, salivary lysozyme concentration did not increase after ~22 min of lower-intensity (50% VO2max) exercise (1). Also, discontinuous, graded, submaximal rowing exercise to a blood lactate level of 4mM was not found to significantly alter salivary lysozyme concentration (33).
Considering the increasing recognition of antimicrobial lipids as essential effector molecules of the innate host defense and the limited research examining the effect of exercise on antimicrobial lipids in airway secretions, we compared the concentrations of antimicrobial CEs in nasal fluid before and after moderate to vigorous aerobic exercise in active and inactive subjects, hypothesizing that regular exercise augments the lipid-mediated arm of innate host defense.
METHODS
Subjects
Fourteen healthy, inactive subjects (inactive) (25 ± 3 yr, mean ± SEM, range 18 – 28 yr, 7 males,7 females) and 14 healthy, recreationally-active subjects (active) (32 ± 11 yr, mean ± SEM, range 20 – 52 yr, 7 males,7 females) participated in the study (see Figure, Supplemental Digital Content 1. Age distribution of study subjects). All participants were non-smokers, free of allergies, respiratory infection or any other respiratory illness for at least two weeks, and not on medication (self-reported in questionnaire). Active exercise status was determined by questionnaire and conferred if subjects performed 20-60 min aerobic exercise 3-5 times per week for at least 3 months. Athletes actively engaged in competition were excluded from the study. Inactive subjects had not engaged in physical activity for at least 3 months, as determined by questionnaire during the initial screening visit. All experimental procedures were performed with full institutional review board approval and written informed consent was obtained from all participants prior to enrollment in the study.
Experimental design
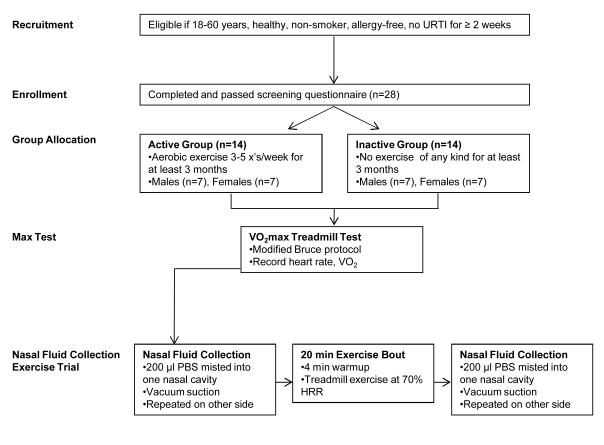
Subjects completed two exercise bouts: a preliminary trial to determine peak oxygen uptake (VO2peak), and a main trial conducted at 70% VO2peak with nasal fluid collection before and after exercise. Fig. 1 outlines the experimental design.
FIGURE 1. Experimental design.
URI: upper respiratory tract infection; R: right nasal cavity; L: left nasal cavity; PBS: phosphate buffered saline.
Preliminary trial
In order to determine the treadmill speed and incline required to elicit 70 % VO2peak in the main trial, a graded, incremental test to exhaustion on the treadmill (Quinton Q55 Series 90, Bothell, WA) was performed. Expired carbon dioxide and oxygen were analyzed using a Moxus metabolic system with pneumotach (AEI Technologies, Naperville, IL), while heart rate was monitored continuously throughout the test using heart rate telemetry (Polar, USA).
Main trial
The main trial was performed at least 24 h after the preliminary trial but not more than seven days after to ensure that the measured immune function would not be impacted by the exhaustive preliminary trial. Submaximal exercise intensity at 70% VO2peak was determined by calculating 70% of heart rate reserve (HRR) (HRR*0.70 + resting heart rate), which was then used as proxy for 70% VO2peak (15). With heart rate monitored continuously by telemetry (Polar, USA), subjects began by walking on the treadmill at 50% of maximal heart rate for two minutes. Grade and treadmill speed gradually increased over a two minute period until intensity corresponding to 70% of HRR was reached, marking the beginning of the 20 min (exercise bout). Nasal fluid samples were collected by vacuum suction before and immediately after completion of the 20 min exercise trial. Prior to vacuum suction, subjects misted 200 μl of sterile phosphate-buffered saline (PBS) warmed to 37°C into one nostril. A sterile pipette tip connected to a vacuum outlet through neoprene tubing was used to suction the nasal fluid into a pre-weighed polypropylene sterile tube during a timed 20 sec interval. Immediately after collection from the first nostril, the procedure was repeated for the second nostril using the same suction device but with a new, sterile collection tube. For the collection of post-exercise samples, a fresh vacuum suction device was used. The samples collected before exercise were stored at room temperature (RT) until the post-exercise set was collected. The tubes were reweighed after collection, flushed and overlaid with nitrogen gas, and kept frozen at −20 °C for later analysis. This treatment did not lead to overt lysis of contaminating cells in the samples as determined by light microscopic pilot studies (data not shown).
Nasal fluid analysis
As previously described (9), samples were quickly thawed in a 37°C water bath and homogenized by sonication 3 times for 10 sec at power setting 2 using a tip sonicator (Fisher Scientific, Sonic Dismembranator, Model 100C, Pittsburgh, PA). After each sample, the sonicator tip was decontaminated using sequential rinses in 10% bleach, de-ionized water, methanol and de-ionized water. Thereafter, samples were centrifuged at 500 × g for 2 min (Eppendorf Centrifuge, Model 5810R, USA) to remove cellular components and the resulting supernatants were transferred to microcentrifuge tubes and further clarified by high speed centrifugation at 13,200 rpm for 30 min at 4°C in a bench top microcentrifuge (Eppendorf Centrifuge, Model 5424, USA). The resulting supernatant from each sample was transferred into a glass centrifuge tube, the volume recorded, and a 25 μl aliquot was reserved for protein analysis. The remainder was used for lipid extraction.
Lipid analysis
Lipid standards for reverse-phase HPLC (rpHPLC) analysis were obtained from Sigma-Aldrich (St. Louis, MO) and included cholesterol, cholesteryl arachidonate, cholesteryl linoleate, cholesteryl oleate, cholesteryl palmitate, cholesteryl stearate, and tri-palmitin, a highly hydrophobic triglyceride. Heptadecanoic acid, a C17 fatty acid found at very low concentrations in humans (less than 1 % of total lipid) was used as an internal standard and added to samples prior to lipid extraction. Stock solutions were prepared at 10 mg/mL in dichloromethane in glass tubes, overlaid with N2 gas, and stored at −20 °C. Lipid extraction was performed as previously described (2,9). HPLC-grade water was added to each sample to reach an aqueous volume of 1.8 mL. To control for extraction efficiency, 20 μg heptadecanoic acid (Sigma-Aldrich, St. Louis, MO, 20 μL of a 1 mg/mL solution) was added to the sample. A volume of 6.75 mL of a premixed 2:1 (v/v) methanol:chloroform solution was added, followed by 2.25 mL of chloroform and 2.25 mL of HPLC-grade water. After the addition of each solvent, the tubes were flushed with N2 gas and vortexed vigorously for 1 min. The tubes were then wrapped in aluminum foil and rotated on a Maxi rotator (Sparta, NJ) for 20 min at level 6, vortexed for 1 min at maximum speed, then centrifuged at 200 × g and 15°C for 10 min. The resulting lipid-containing chloroform (lower) layer was aspirated through a Pasteur pipette and transferred to a new 15 mL glass tube, with samples from the right and left nostrils combined. To maximize lipid recovery, 4.5 mL of chloroform was added to the residual sample, the sample flushed with N2 gas, vortexed for 1 min, and centrifuged as above. The second chloroform lower layer was then pooled with the first layer, dried under a N2 gas stream at 35°C, and stored at −20°C until rpHPLC analysis was conducted.
rpHPLC analysis was performed with a low-pressure quarternary gradient system (Summit HPLC System with a Dionex P680 low-pressure quaternary pump and degasser, Dionex Corporation, Sunyvale, CA with Dionex PCS1Chromeleon software) and a reversed-phase column (Dionex Acclaim PolarAdvantage 2 with embedded amide group column, 150 mm × 2.1 mm ID, 3 μm particle size, Sunnyvale, CA) preceded by a corresponding guard column (Dionex Acclaim PolarAdvantage 2 guard column, 2.1 × 10 mm, 5 μm particle size) in a temperature-controlled compartment (Dionex model TCC-100 column oven). Lipids were dissolved in 10 μL dichloromethane, injected, then eluted with an isocratic gradient of 15 % dichloromethane/ 85 % acetonitrile at a flow rate of 0.5 mL/min, 25 °C column temperature, and a run time of 40 min. For detection and quantification an evaporative light-scattering detector (ELSD, Alltech model 800, Deerfield, IL) operated at 45 °C and 1.9 bar N2 gas was employed. Standard curves for heptadecanoic acid, cholesteryl linoleate and cholesteryl arachidonate were established and the resulting trend lines were used to determine their concentrations in the samples. Recovery of heptadecanoic acid was calculated and sample cholesteryl ester concentrations were adjusted to 100 % heptadecanoic acid recovery prior to statistical analysis.
Lysozyme quantification
To assert that our study design generated data comparable to preceding studies, which have assessed the antimicrobial peptide and polypeptide-mediated arm of innate immunity, we determined the concentrations of lysozyme, a representative antimicrobial protein, in nasal fluid collected before and after exercise. Lysozyme was quantified with the lysoplate assay, which is based on the peptidoglycan hydrolyzing activity of lysozyme (20). Micrococcus lysodeikticus (Sigma-Aldrich) was added to 66 mM sodium phosphate buffer, pH 7.0, containing 1% agarose to yield a concentration of 0.5 mg/mL and poured into 10 mL aliquots in 10 cm square petri dishes. After solidification, consistent holes 3 mm in diameter were punched into the agar and 5 μL of nasal fluid sample or hen egg white lysozyme (Sigma-Aldrich) standard dilutions were applied in duplicates. After 20 h incubation at RT the diameters of the clearing zones reflecting peptidoglycan degradation of M. lysodeikticus were measured in mm and converted into arbitrary units ([clearing zone – 3] ×10). A standard curve was derived from the values obtained from the hen egg white lysozyme dilutions and the sample lysozyme concentrations were calculated based on the resulting trend line.
Albumin quantification
To address plasma transudation as a possible source for lipids in nasal fluid, the concentration of serum albumin was assessed using an ELISA-based test kit (Alpha Diagnostic International, San Antonio, TX) following the manufacturer’s instructions.
Statistical analyses
Four subjects from the inactive group and two subjects from the active group were excluded from the final data analysis due to cholesteryl ester concentrations that were below the detection limit. Results from the remaining subjects (n = 10 for the inactive group and n = 12 for the active group) were subjected to Levene’s test for homogeneity. Because the data were homogenous, a log transformation was not applied. Statistical differences between inactive and active groups were determined using a one-factor (group) ANOVA. Statistical differences of nasal fluid volume between pre/post exercise were determined using a repeated measures analysis. All analyses were conducted first with age as a covariate; however, since none of the terms involving the covariate showed significance, the final analyses reported here were conducted without age as a covariate. Differences were considered significant at p < 0.05. All data are presented as means ± SEM.
RESULTS
Lysozyme concentration
No differences in lysozyme concentration were found between the groups at baseline (117.7 ± 31.1 μg/mL inactive vs.122.9 ± 15.5 μg/mL active). After exercise, the concentrations significantly increased to 197.1 ± 76.5 μg/mL and 234.2 ± 54.7 μg/mL (inactive and active, respectively, p = 0.03), but there was no statistical difference between the groups.
Antimicrobial lipid concentration
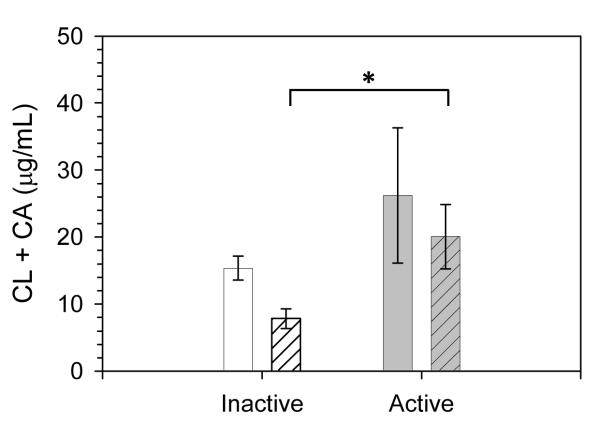
No differences in the concentrations of the antimicrobial lipids CL and CA were found between the inactive and active groups at baseline (pre) (15.3 ± 1.8 μg/mL inactive vs. 26.2 ± 10.05 μg/mL active, means ± SEM). However, a significantly different response to exercise was observed between groups (Fig 2). After exercise (post), CL and CA levels dropped in inactive subjects by nearly 50% to 7.8 ± 1.5 μg/mL, while CL and CA levels were lowered by less than 25% in active subjects to 20.1 ± 4.8 μg/mL. Thus, post exercise CA and CL concentration was significantly greater in the active group compared to the inactive group (p = 0.036).
FIGURE 2. Antimicrobial cholesteryl ester concentrations in nasal fluid aspirate collected Pre (open bars) and post (patterned bars) exercise bout.
CL: cholesteryl linoleate; CA: cholesteryl arachidonate. * p = 0.036 in univariate ANOVA for post inactive versus post active. Shown are means ±SEM, n =10 for inactive and n = 12 for active subjects.
Nasal fluid volume
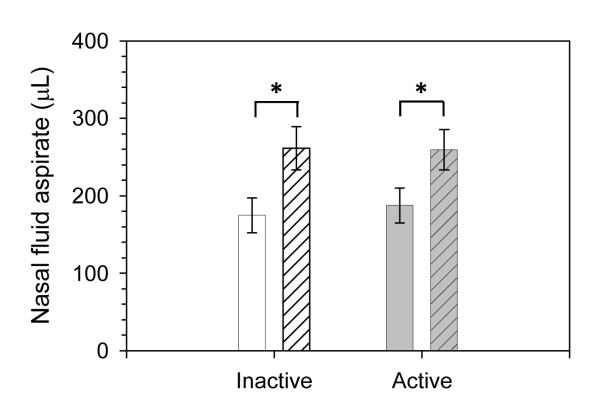
The volume of nasal fluid collected after exercise increased significantly in both groups compared to resting values (Fig 3). Collected nasal fluid volume increased in the inactive group from 174.9 ± 22.7 μL to 261.4 ± 27.9 μL (p = 0.001), and the active group from 187.6 ± 22.5 μL to 259.5 ± 25.9 μL (p = 0.001). However, no significant differences in collected nasal fluid volume were observed between the groups at baseline or after exercise.
FIGURE 3. Change of nasal fluid aspirate volume collected pre (open bars) and post (patterned bars) exercise bout.
* p = 0.001 in repeated measures analysis for pre versus post in both, inactive and active subjects. Shown are means ±SEM, n =10 for inactive and n = 12 for active subjects.
Albumin concentration
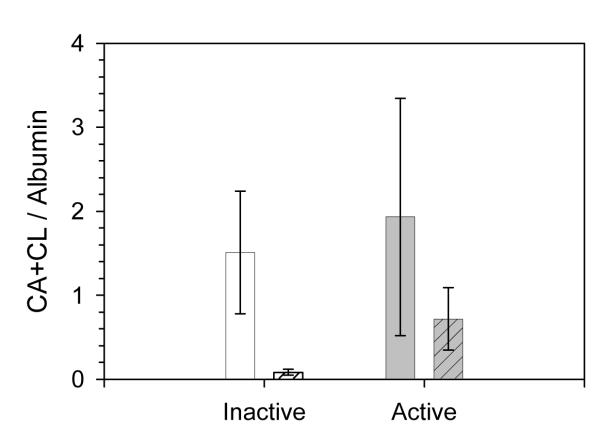
No differences were found in the baseline concentrations of albumin between the inactive and active groups (156.6 ± 54.5 μg/mL vs.126.9 ± 32.8 μg/mL, respectively). Albumin concentration in nasal fluid collected from inactive subjects was unaffected by the exercise bout (156.6 ± 54.5 μg/mL pre to 156.0 ± 31.1 μg/mL post, p > 0.05). For active subjects, the overall mean of albumin concentration increased from pre to post (126.9 ± 32.8 μg/mL pre to 248.2 ± 72.9 μg/mL post), but this was not statistically significant. When CL and CA concentrations were adjusted to albumin concentration in each group, the post-exercise difference between the groups was no longer significant (Fig 4).
FIGURE 4. Antimicrobial cholesteryl esters in nasal fluid aspirate collected pre (open bars) and post (patterned bars) exercise bout after adjustment to albumin.
CL: cholesteryl linoleate; CA: cholesteryl arachidonate. Shown are means ±SEM, n =10 for inactive and n = 12 for active subjects.
DISCUSSION
The goal of this study was to add to the understanding of the molecular basis of the innate mucosal host defense in the upper respiratory tract for the protective effect of moderate-to- vigorous exercise against URI. Antimicrobial lipids, including cholesteryl linoleate (CL) and cholesteryl arachidonate (CA), have emerged as essential effector molecules of innate host defense in the airways, and this study was conducted to test the hypothesis that moderate–to-vigorous exercise augments the lipid mediated arm of innate host defense.
To our knowledge this is the first exercise study on antimicrobial cholesteryl esters (CE). To be able to relate the results from this study to studies on other parameters of the innate immune defense, we initially determined the concentrations of lysozyme, a key effector molecule of the protein mediated arm of innate defense, in nasal fluid. Lysozyme concentrations measured in this study agree with previously published ranges of lysozyme concentrations in nasal secretions (5,12,25). Our measurements reveal a significant increase in lysozyme concentrations in both active and inactive subjects after exercise. The effect of exercise on lysozyme concentrations in body fluids has been primarily studied in saliva. While West et al. (33) observed non-significant increases in salivary lysozyme in response to exercise in elite rowers, Allgrove et al. (1) reported significant increases in salivary lysozyme concentrations after exercise. Notably, the exercise regimen employed in Allgrove’s study was conducted at similar intensity levels (75%VO2max) and duration (22 min) as the present study. Thus, our data on lysozyme concentrations are within the range reported by related studies.
Baseline concentrations of CL and CA in the present study were in accordance with the antimicrobial range reported in previous studies (9), further substantiating the inherent presence of antimicrobial CEs in respiratory secretions and their contribution to mucosal defense. No differences in baseline concentrations of CL and CA were observed between the active and inactive groups pre exercise. However, post exercise, the concentration of antimicrobial CEs was significantly higher in the active group compared to inactive group, with the CE concentration in the inactive group dropping below the antimicrobial effective range. As antimicrobial lipids are essential for the overall inherent antimicrobial activity of nasal secretions, dropping below the antimicrobial effective range may represent a temporary breach in host defense, thus opening a window of opportunity for respiratory pathogens.
Numerous studies have reported that various aspects of immune function are temporarily depressed following heavy exercise such as leukocyte count (26), natural killer cell count (29), and salivary IgA concentration (11).The immunosuppressive effect of exercise has been partially attributed to a milieu of plasma stress hormones, including cortisol, which increases in response to stressful exercise. Administration of glucocorticoids has been shown to cause lymphopenia and suppression of both NK and T cell function (6), corroborating the immunomodulatory effects of exercise. These studies suggest that the decrease in CEs in the inactive subjects may be cortisol-mediated. Though trained subjects are unlikely to respond to 20 min of treadmill exercise at 70% VO2peak with considerable elevation in cortisol levels, exercise at this duration and intensity may elicit a milieu of plasma stress hormones in inactive individuals. Thus, the decrease in CE concentration post-exercise may reflect a stress response to the exercise load in the inactive group. Measurements of serum cortisol levels in future work before, during and immediately post-exercise would clarify this proposed response.
Exercise is known to increase the secretion rate of various body fluids including saliva (1,7,13). Hence, the reduced CA/CL concentrations in the inactive group could have resulted from a relatively increased fluid influx. While there was a significant increase in nasal fluid volume collected after exercise, there was no statistically significant difference between inactive and active subjects. Thus, in active subjects, the fluid in the nasal lumen after exercise contained more CA/CL compared to inactive subjects consistent with either a local production of CA/CL or passage of CE from the plasma to nasal secretions. Subsequently, albumin concentration in the nasal fluid samples was assessed as an indicator of plasma transudation. Previous, non-exercise investigations of albumin reported values similar to resting levels in the present study after considering the wash volumes applied in the nasal fluid collection (3,25). Browning et al. (3) studied approximately the same number of subjects and reported similar variance in the individual concentrations of albumin (± 0.1%) as the present study. This suggests that, at least in part, plasma transudation contributes to CE levels in nasal fluid.
If plasma transudation does indeed contribute to the CE levels in nasal fluid, then higher post-exercise CE levels in active subjects could be attributed to enhanced transudation due to a higher functional capacity of the cardiovascular system. With regular aerobic exercise, an array of structural cardiovascular adaptations is induced, which include the remodeling of the heart and blood vessels to enhance delivery of oxygen and nutrients to skeletal muscle. Circulating levels of atrial natriuretic peptide (ANP) released in response to dynamic exercise influence capillary permeability to albumin and hydrostatic pressure gradients (18). Furthermore, increased transudation of plasma filtrate in active subjects may be driven by elevated subepithelial hydrostatic pressure under increased vagal control (28). The serosa-to-mucosa pressure gradient has been described as disrupting the sites where tight junctions meet and increasing the permeability at apical and basolateral membrane domains (19). As proposed by Serikov et al. (28), the serosa-to-mucosa pressure gradient, and therefore the transvascular flux of plasma components, is mediated by vagal stimulation. After submaximal exercise, the rapid drop in heart rate from elevated to resting values during the first minute of recovery is primarily attributed to the restoration of parasympathetic vagal nerve tone (30). In aerobically-trained men, post-exercise vagal reactivation was observed to accelerate compared to untrained controls (30). Collectively, these studies suggest increased vagal tone in aerobically-trained subjects may induce transudation of plasma constituents across the airway epithelium. In the present study, where nasal fluid collection occurred about 1 min after completion of the exercise, it is conceivable that post-exercise vagal reactivation may stimulate transudation of plasma components, particularly in the case of aerobically-trained active subjects.
CONCLUSIONS
Taken together, these data indicate that moderate aerobic exercise acutely decreases the antimicrobial CE response in inactive subjects, but does not modulate baseline levels of CEs in active and inactive subjects. These findings suggest that compared to active individuals, inactive individuals may be at greater risk for URI immediately post-exercise. Differences between the groups may be attributed to a greater plasma transudation with influx of CEs in the active group or increased immunosuppressive glucocorticoid levels in the inactive group. This study provides first insight into the molecular basis of the lipid-mediated innate immune response in relation to exercise. Future studies need to determine the antimicrobial activity of these secretions against pathogens of URI and address longitudinal measurements of cholesteryl ester and glucocorticoid levels.
Acknowledgments
This study was supported by National Institutes of Health (NIH 1SC1GM096916) and the California State University Los Angeles School of Kinesiology & Nutritional Science General Funds. The authors do not have any conflicts of interest. The results of this study do not constitute endorsement by ACSM.
Footnotes
The authors do not have any conflicts of interest.
REFERENCES
- 1.Allgrove JE, Gomes E, Hough J, Gleeson M. Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J Sports Sci. 2008;26(6):653–61. doi: 10.1080/02640410701716790. [DOI] [PubMed] [Google Scholar]
- 2.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Browning ST, Housley DG, Weeks I. Measurement of albumin in nasal lavage using the technique of chemiluminescent immunoassay. Acta Otolaryngol. 1999;119(4):492–6. doi: 10.1080/00016489950181053. [DOI] [PubMed] [Google Scholar]
- 4.Christie WW, Han X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis. 4 Oily Press; Bridgwater, UK: 2010. pp. 3–19. [Google Scholar]
- 5.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67(7):3267–75. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–55. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 7.Davison G, Allgrove J, Gleeson M. Salivary antimicrobial peptides (LL-37 and alpha-defensins HNP1-3), antimicrobial and IgA responses to prolonged exercise. Eur J Appl Physiol. 2009;106(2):277–84. doi: 10.1007/s00421-009-1020-y. [DOI] [PubMed] [Google Scholar]
- 8.Dawson DV, Drake DR, Hill JR, Brogden KA, Fischer CL, Wertz PW. Organization, barrier function and antimicrobial lipids of the oral mucosa. Int J Cosmet Sci. 2013 Jan 16; doi: 10.1111/ics.12038. doi: 10.1111/ics.12038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do TQ, Moshkani S, Castillo P, Anunta S, Pogosyan A, Cheung A, Marbois B, Faull KF, Ernst W, Chiang SM, Fujii G, Clarke CF, Foster K, Porter E. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol. 2008;181(6):4177–87. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49(1):4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Fahlman MM, Engels HJ, Morgan AL, Kolokouri I. Mucosal IgA response to repeated wingate tests in females. Int J Sports Med. 2001;22(2):127–31. doi: 10.1055/s-2001-11339. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75(1):34–8. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103(2):693–9. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs CE. Human milk inactivates pathogens individually, additively, and synergistically. J Nutr. 2005;135(5):1286–8. doi: 10.1093/jn/135.5.1286. [DOI] [PubMed] [Google Scholar]
- 15.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 1988;5(5):303–11. doi: 10.2165/00007256-198805050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87(2):153–8. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee JT, Jansen M, Yilma AN, Nguyen A, Desharnais R, Porter E. Antimicrobial lipids: novel innate defense molecules are elevated in sinus secretions of patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(2):99–104. doi: 10.2500/ajra.2010.24.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack GW, Yang R, Hargens AR, Nagashima K, Haskell A. Influence of hydrostatic pressure gradients on regulation of plasma volume after exercise. J Appl Physiol. 1998;85(2):667–75. doi: 10.1152/jappl.1998.85.2.667. [DOI] [PubMed] [Google Scholar]
- 19.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–59. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Martinez JG, Waldon M, Huang Q, Alvarez S, Oren A, Sandoval N, Du M, Zhou F, Zenz A, Lohner K, Desharnais R, Porter E. Membrane-targeted synergistic activity of docosahexaenoic acid and lysozyme against Pseudomonas aeruginosa. Biochem J. 2009;419(1):193–200. doi: 10.1042/BJ20081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34(8):1242–8. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Nagashima K, Nose H, Yoshida T, Kawabata T, Oda Y, Yorimoto A, Uemura O, Morimoto T. Relationship between atrial natriuretic peptide and plasma volume during graded exercise with water immersion. J Appl Physiol. 1995;78(1):217–24. doi: 10.1152/jappl.1995.78.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Nieman DC. Exercise, infection, and immunity. Int J Sports Med. 1994;3(15 Suppl):S131–S141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- 24.Nieman DC. Special feature for the Olympics: effects of exercise on the immune system: exercise effects on systemic immunity. Immunol Cell Biol. 2000;78(5):496–501. doi: 10.1111/j.1440-1711.2000.t01-5-.x. [DOI] [PubMed] [Google Scholar]
- 25.Raphael GD, Jeney EV, Baraniuk JN, Kim I, Meredith SD, Kaliner MA. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest. 1989;84(5):1528–35. doi: 10.1172/JCI114329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robson PJ, Blannin AK, Walsh NP, Castell LM, Gleeson M. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med. 1999;20(2):128–35. doi: 10.1055/s-2007-971106. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JH, Kim CH, Yoon JH. Innate immune responses of the airway epithelium. Mol Cells. 2010;30(3):173–83. doi: 10.1007/s10059-010-0146-4. [DOI] [PubMed] [Google Scholar]
- 28.Serikov VB, Jang YJ, Widdicombe JH. Estimate of the subepithelial hydrostatic pressure that drives inflammatory transudate into the airway lumen. J Appl Physiol. 2002;92(4):1702–8. doi: 10.1152/japplphysiol.00645.2001. [DOI] [PubMed] [Google Scholar]
- 29.Shephard RJ, Shek PN. Effects of exercise and training on natural killer cell counts and cytolytic activity: a meta-analysis. Sports Med. 1999;28(3):177–95. doi: 10.2165/00007256-199928030-00003. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur J Appl Physiol. 2001;85(3-4):259–63. doi: 10.1007/s004210100443. [DOI] [PubMed] [Google Scholar]
- 31.Tollin M, Bergsson G, Kai-Larsen Y, Lengqvist J, Sjovall J, Griffiths W, Skuladottir GV, Haraldsson A, Jornvall H, Gudmundsson GH, Agerberth B. Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci. 2005;62(19-20):2390–9. doi: 10.1007/s00018-005-5260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usui T, Yoshikawa T, Orita K, Ueda SY, Katsura Y, Fujimoto S, Yoshimura M. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur J Appl Physiol. 2011;111(9):2005–14. doi: 10.1007/s00421-011-1830-6. [DOI] [PubMed] [Google Scholar]
- 33.West NP, Pyne DB, Kyd JM, Renshaw GM, Fricker PA, Cripps AW. The effect of exercise on innate mucosal immunity. Br J Sports Med. 2010;44(4):227–31. doi: 10.1136/bjsm.2008.046532. [DOI] [PubMed] [Google Scholar]
- 34.West NP, Pyne DB, Renshaw G, Cripps AW. Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol Med Microbiol. 2006;48(3):293–304. doi: 10.1111/j.1574-695X.2006.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widdicombe J. Relationships among the composition of mucus, epithelial lining liquid, and adhesion of microorganisms. Am J Respir Crit Care Med. 1995;151(6):2088–92. doi: 10.1164/ajrccm.151.6.7767562. [DOI] [PubMed] [Google Scholar]