Abstract
This investigation was designed to quantify the coordinative organization of lip muscle activity of 2-year-old children during speech and nonspeech behaviors. Electromyographic (EMG) recordings of right upper and lower lip activity of seven 2-year-old children were obtained during productions of chewing, syllable repetition, lip protrusion, and speech (repeated two-word utterances) tasks. Task comparisons revealed that the coordinative organization of upper and lower lip activity is task specific; different coordinative strategies are employed for different tasks. Lip protrusion and syllable repetition tasks yielded strong coupling of upper and lower lip activity. Lip rounding (sentences containing the lip-rounding vowel /u/) and “nonlabial” speech tasks (sentences free of bilabials and lip-rounding vowels) resulted in low coupling of upper and lower lip activity. Moderate levels of coupling of upper and lower lip activity were evident for chewing and bilabial speech tasks (sentences loaded with bilabial plosion). This finding, that the coordinative elements of the perioral system of 2-year-olds are task specific, extends the results of previous studies of adults and children, where task-specific coordinative strategies were employed by the mandibular and perioral systems (Moore, 1993; Moore & Ruark, 1996; Moore, Smith, & Ringel, 1988; Wohlert & Goffman, 1994). The task-dependent coordination of the perioral system of 2-year-olds supports the notion that developing speech and earlier developing oromotor behaviors (i.e., sucking, chewing) are mediated by different control mechanisms.
Keywords: lips, speech, electromyography, development—children, oral-motor control
Afull description of the normal developmental processes of speech depends on an understanding of the relationship between developing speech motor coordination and the coordination of other emerging oromotor behaviors (Sharkey & Folkins, 1985). This framework is necessary, not only to further our understanding of developmental and mature speech motor processes, but also to formulate efficacious treatment for developmental speech motor disorders (Smith, 1978). A persistent question regarding developing speech and nonspeech behaviors has been the redundancy of control mechanisms for these behaviors. There are two distinct hypotheses we might employ to illuminate this issue, one in which speech is viewed as an emergent behavior from earlier appearing oromotor behaviors, and the second in which speech develops as a new skill, independent of other skills. Support for the former suggestion is compelling, but the evidence for the alternate view is sufficiently strong that it must also be incorporated into consideration of models of speech development.
The prominent conceptualization of developing speech as a successor to earlier appearing oromotor behaviors is an explicit part of some models of speech motor control (e.g., Grillner, 1982; Wolff, 1991). The neurophysiologic infrastructure underlying more basic, primary behaviors (e.g., sucking, chewing, swallowing) may provide at least a rudimentary coordinative organization for developing speech. Grillner (1982), for example, suggested that speech production consists of a combination of centrally generated motor patterns, including those underlying respiration, mastication, and swallowing. Muscle synergies from centrally patterned activities merge to create new muscle synergies for speech that may then be independently controlled by higher order mechanisms.
Part of the appeal of shared mechanisms for developing speech and nonspeech behaviors stems from our understanding of speech motor development as an adaptive process by which extant behaviors are modified to achieve new movement goals (Kent, Mitchell, & Sancier, 1991). Empirical evidence for an organizational hierarchy of earlier and later developing motor patterns is derived from a variety of investigations, including those that demonstrate consistent similarities in the kicking patterns of young infants with later developing locomotor patterns (e.g., Thelen, 1981; Thelen & Cooke, 1987; Thelen & Fisher, 1983). Similarly, the mandibular muscle activation patterns of toddlers exhibit remarkable similarity during early stages of speech development (i.e., reduplicated babbling) to the rhythmic patterns associated with centrally patterned behaviors, such as chewing (Moore & Ruark, 1996).
Additional support for the notion that developing speech evolves from earlier appearing oromotor behaviors can be drawn from the obvious structural and functional overlap of speech and nonspeech behaviors. These behaviors involve the same muscle groups, exhibit similar movement structures (i.e., rhythmic timing of reduplicated babbling, sucking, and chewing), and develop progressively from birth (Ruark & Moore, 1992). The clear and parsimonious suggestion is that these similarities represent variations of a common control mechanism, and that motor development only entails modification of existing patterns (Dellow, 1976, Grillner, 1977, 1982). Finally, support for the functional linkage of speech and nonspeech behaviors has routinely been drawn from clinical practice (e.g., Mueller, 1972; Palmer, 1947; Westlake, 1951). Treatment of infants and toddlers with oromotor deficits is frequently designed to elicit vegetative activities to enhance speech development (Love, Hagerman, & Taimi, 1980; Netsell, 1986). One primary motivation for prespeech/feeding therapy is the belief that muscle activity for developing speech includes elaborations and refinements of motor patterns produced during vegetative movements (Netsell, 1986).
Alternatively, there is the equally compelling argument that the differences between early speech and nonspeech movements are so great that development of speech and nonspeech neural mechanisms must be separate (Netsell, 1986). The developing oromotor system may use distinct coordinative strategies for changing task demands (Moore, 1993). Support for this position is derived from studies of speech by adults in which large and significant differences between the coordinative organizations for speech and nonspeech behaviors were demonstrated (Moore, 1993; Moore & Scudder, 1989; Moore et al., 1988; Ostry & Flanagan, 1989; Wohlert & Goffman, 1994). Investigations of mandibular muscle activity of adults during chewing and speech demonstrated that chewing patterns are characterized by reciprocal activation of mandibular antagonists, whereas coactivation of antagonists is the dominant pattern of activity for speech. This task-specific muscle coordination for speech and nonspeech activities was ascribed to changing task demands and was taken to infer separate control mechanisms (Moore, 1993; Moore et al., 1988). Similarly, lip muscle activation patterns of adults during speech and nonspeech tasks yield varying levels of coupling among the lip quadrants, suggesting that each individual quadrant can be activated independently and receives distinct neural input (Wohlert & Goffman, 1994). For example, Wohlert and Goffman (1994) found in adults that lip protrusion yielded highly coupled EMG activity among lip quadrants, whereas chewing yielded a more intermediate level of coupled activity. The level of coupling during speech was distinct from chewing and protrusion tasks, in that coupling of activity among the quadrants was more dissociated for speech production. This demonstration of task-specific coordination indicates that neural coupling within the perioral system of adults is flexible, yielding “functional independence among perioral quadrants” (Wohlert & Goffman, 1994).
These two hypotheses serve only to elucidate the issue of commonality of control mechanisms for speech and nonspeech behaviors; they do not represent a comprehensive framework for the development of speech. Although speech appears to be distinct and separate from nonspeech behaviors in adults, the infant may assemble novel motor patterns by adapting established movement dynamics to achieve new movement goals. Dynamic pattern theory supports this notion that coordinative patterns and their neural substrates are not preestablished entities, but are “softly assembled” as the result of interactions of intrinsic factors (e.g., timing characteristics from currently established motor patterns), extrinsic constraints (e.g., current size and shape of the oral mechanism), and the psychosocial development of the infant (Thelen, 1995). Thus, the endeavor of description and quantification of developing speech and nonspeech skills must take into account “attractor states” (i.e., stable patterns) and emergent patterns that result from adaptation and modification of these states.
The present investigation was designed to describe, compare, and contrast the coordinative strategies exhibited in lip muscle activation patterns of 2-year-old children during speech and nonspeech tasks. Several potential outcomes were considered. For example, extension of findings of coordination of lip movement in adults to 2-year-olds would predict that toddlers would also exhibit a range of coordinative strategies that vary with task demand. Nonspeech tasks requiring movement of all four lip quadrants (e.g., lip protrusion) would be expected to reveal rigid coupling of upper and lower lip activity. In contrast, speech or speech-like tasks (e.g., syllable repetition), would be expected to exhibit very weak coupling. Chewing by adults involves an intermediate level of coupled activity in the upper and lower lips (Wohlert & Goffman, 1994), and might also be expected to do so in children. Variations from this organizational structure could be attributed to developmental processes.
Examination of lip muscle activation patterns during speech may alternatively demonstrate that coupling of upper and lower lip activity varies systematically across speech tasks. It is likely that tasks involving greater extent of lip displacement, such as that which occurs with some bilabial sounds, will yield greater coupling of activity in the upper and lower lip musculature than tasks in which lip movement is relatively minimal (e.g., speech that contains neither bilabial nor lip-rounded phonemes). This task-dependent activation of lip muscle activity would lend support to the suggestion that early developing lip movements are not merely elaborations, or refinements of movements of extant behaviors. However, unlike adults, the perioral system of 2-year-olds may be characterized by persistent similarities in activation patterns of the upper and lower lips across speech and nonspeech tasks, a finding that would support the suggestion that oromotor tasks, including chewing, sucking, and speech, share common control mechanisms (Dellow, 1976; Grillner, 1982). Speech and nonspeech activities in such a case would be expected to demonstrate similar levels of coupling of upper and lower lip activity, as lip muscle activation patterns would be similar in timing and amplitude.
Two-year-old children were chosen for this study, because, at this age: (a) the CNS (e.g., Hesselmans, Jennekens, van den Oord, Veldman, & Vincent, 1993; Simonds & Scheibel, 1989) and oral and facial structures (Moyers & Carlson, 1990) are not yet fully developed, (b) essential investigations of physiologic development of speech motor control are lacking, and (c) most 2-year-olds are able to follow a minimally constrained experimental protocol. Observation of freely behaving children this young presents several challenges. Aside from the specific difficulty of inferring neural mechanisms from noninvasive observations of the perioral system in children, there is also the more formidable problem of operationally defining coordinative organization. To be useful, a description must include quantitative measures that can be related to known neurophysiologic mechanisms. Prior investigations have addressed these requirements using correlational analyses (e.g., Moore & Scudder, 1989; Moore et al., 1988; Wohlert & Goffman, 1994), although recent investigations have revealed the need to evaluate asynchronous coupling of muscle activity as well (Cooke & Brown, 1990; Moore, 1993; Moore & Ruark, 1996). Accordingly, a crosscorrelational analysis was employed in the present design.
Method
Participants
Participants in this investigation included 7 normally developing Caucasian females between the ages of 24 and 29 months (mean age: 26 months). Inclusion criteria of gender and race were employed to control for potential effects on lip muscle coordination of differences in structure and growth patterns. The children were free of known neurologic deficit or apparent developmental delay, had no evidence of active or recent middle ear pathology, and no known history of chronic pathology (Thelin & Thelin, 1996). Each child demonstrated a hearing sensitivity level of 30 dB HL or better at 0.5, 1, 2, and 4 kHz. An informal oral mechanism examination (e.g., informal observations of facial muscle symmetry, range of movement of the speech articulators during speech and nonspeech movements) performed by an investigator assured that each subject’s oral mechanism was adequate with respect to structure and function. In addition, participants exhibited no apparent delays in expressive language abilities or cognitive and motor functions. The Language Development Survey (LDS) (Rescorla, 1989) was used to screen the children’s expressive language ability. Any potential participant who had an expressive vocabulary less than 50 words, or produced no two-word utterances was excluded (Rescorla, 1989). The Battelle Developmental Screening Test (BDST; Newberg, Stock, & Wnek, 1988) was administered to screen overall development. Screening tasks from the developmental domains, “Cognitive,” “Motor,” and “Communication,” were used to judge each child’s proficiencies in these areas.
Procedures
Each child sat at a small table next to her mother, facing an experimenter. Surface electrodes were applied as the child played with toys or watched a video. Target behaviors included chewing, lip protrusion, syllable repetition, and speech, and all except chewing were elicited via imitation. For chewing, the child was presented with her “snack” (food that was necessarily provided by her parent and part of her normal diet) and was given the verbal direction to eat her snack while sitting quietly (to eliminate possible contamination of muscle activation patterns for chewing). Chewed food included pretzels, Cheerios™, and crackers. Continuous FM recordings were made throughout the 45-minute experimental session. A total of six main tasks were elicited from the children: three nonspeech tasks (lip protrusion, syllable repetition, and chewing) and three speech tasks. Task order, which was determined by the child’s own interest, varied across participants, and resulted in a randomized data set. All tasks were selected for their potential to yield different degrees of mechanical coupling of the lips, to demonstrate the coordinative plasticity of the labial system (Moore, 1993; Wohlert & Goffman, 1994). These tasks were also selected to facilitate comparisons with tasks performed by adults in a previous study of lip muscle coordination (Wohlert & Goffman, 1994). For the lip protrusion and syllable repetition tasks, a series of three successive movements were elicited for each trial (e.g., /pa-pa-pa/). Lip protrusion was modeled by the experimenter as a rounding and protruding gesture. Speech was sampled as each child imitated target phrases containing two-word productions. The investigator elicited each target phrase by presenting the child with a toy along with a verbal model of the phrase, produced at conversational loudness level (Wilson, 1982). After the child imitated the phrase, she was given the toy. Two-word utterances were chosen as children between the ages of 24 and 30 months are generally within Brown’s Stage II, which is characterized by an MLU of 2.00–2.49 (Miller, 1981). Three different speech tasks were chosen for their presumed tendency to elicit various levels of lip muscle activity. The bilabial speech task included five repetitions each of three different speech stimuli intended to promote bilabial plosion: (a) “Papa Bear,” (b) “Baby Bop,” and (c) “Bye-bye baby.” The lip-rounding speech task included five repetitions each of two speech stimuli that promoted lip rounding and excluded bilabials: (a) “Choo-choo” and (b) “Tooti-toot.” The nonlabial speech task was selected for exclusion of bilabial and lip-rounding productions and included five repetitions each of three speech stimuli and included: (a) “Car-car,” (b) “Duck in,” and (c) “Light on.” These nonlabial productions were intended to be control tasks during which little or no activity or modulation was anticipated. Overall, the children were encouraged to produce five trials each of the lip protrusion and syllable repetition tasks, as well as five trials each of the eight speech stimuli (stimuli for the three main speech tasks). In addition, each child produced at least 50 cycles of chewing activity.
Data Selection and Acquisition
Bipolar surface EMG recordings were made from the right upper and right lower lip quadrants. The electrodes within each quadrant were placed 5 mm apart, just lateral to the philtrum, adjacent to the vermilion border, at least 1 cm from the corner of the mouth. This placement procedure minimized the possibility of crosstalk between right upper and lower lip EMG channels and nontarget muscles (e.g., left upper lip quadrant, left lower lip quadrant). Prior to electrode placement, the right upper and lower lip quadrants were lightly scrubbed with an alcohol gauze pad, followed by application of an antiperspirant skin electrode preparation (Prep N’Stay, Pharmaceutical Innovations, Inc.). Recordings were made using Ag/AgCl disk electrodes (In Vivo Metrics, 4 mm outside diameter) filled with electrode gel and attached to the skin surface with adhesive collars. A small strip of adhesive tape was also placed over each electrode to ensure electrode contact. The absence of crosstalk between EMG signals was verified by observing the ability to record segregate EMG activity from the upper and lower lips. Each child’s EMG signals were inspected at the beginning of the experimental session for periods of asynchronous, isolated activity of either upper or lower lip (e.g., high amplitude activity in upper lip that is not seen in the minimally activated lower lip) during spontaneous behaviors. This method was chosen as the children’s ages precluded their ability to perform tasks that aided in the confirmation of segregate activation of upper or lower lip muscles (e.g., participants were unable to produce the /f/ phoneme upon command).
During data collection, EMG signals were amplified using Grass P511 physiologic preamplifiers (frequency response: 30–3000 Hz) and recorded on 1 of 14 tracks on a TEAC XR 510 FM instrumentation recorder (frequency response: DC-2500Hz; S/N > 48 dB) for subsequent offline digitization and analysis. A single audio channel was used to simultaneously record the child’s speech productions and an experimenter’s online gloss of the child’s speech and description of target behaviors. Changes in experimental conditions and preliminary data description were recorded by a second experimenter on a second audio channel. High quality speech signals for the child’s audio channel were recorded using a wireless microphone system (Telex, FMR-25/WT-25; frequency response = 100 Hz–18.75 kHz; S/N = 30 dB). Data channels included:
right upper lip EMG
right lower lip EMG
child’s audio and online gloss of child’s speech and description of target behaviors by Experimenter #1
Experimenter # 2 audio
Data Selection and Parsing
Target behaviors for each task were identified and parsed from the continuously taped 45-minute experimental session. EMG signals and the first audio channel were digitized at a rate of 1,000 samples/s per channel. Though this sample rate undersampled the EMG and audio channels, storage limits and signal characteristics outweighed this problem. EMG signals exhibited very little energy above 500 Hz, and the acoustic signal was only used as a visual cue during the signal analysis routines, not as a source for acoustic analysis (i.e., only a very gross representation of the acoustic signal was required at this stage). For chewing, samples were obtained after the initiation of three or four chewing cycles, and before the final swallowing stage. Samples of lip protrusion and syllable repetition were taken from periods during which the EMG patterns were judged to be regular in amplitude and periodic in at least one channel. Speech samples included those two-word utterances that closely approximated the verbal model provided by the experimenter. These utterances were judged (via listening to the child’s audiotape and viewing EMG signals) to be free of excess loudness, dysfluencies (e.g., sound or syllable repetitions) and nonspeech movement artifacts (e.g., lip muscle activation corresponding to smiling). Utterances that were perceptually judged to be unusually slow or fast or excessive in their prosodic variation were rejected from further analysis. Each digitized file contained five repetitions of a specific task (i.e., the first five acceptable trials). For the chewing task, data from approximately 50 cycles of chewing were digitized and stored in two separate files, each approximately 25 s in length.
Data Analyses
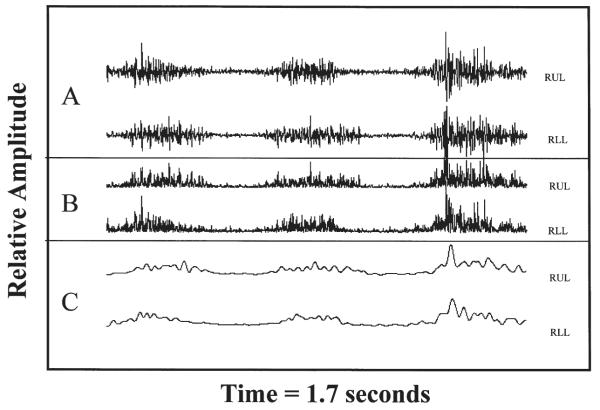
Analyses were completed using algorithms custom designed for MATLAB (v. 4.2c; The Mathworks, Inc., 1993), a commercially available signal processing package. This analysis began with the display of the digitized EMG data from one complete file (e.g., EMG data for five trials of lip protrusion) on a computer screen, and selection of one trial of a target behavior (e.g., one trial of syllable repetition, one complete production of a two-word utterance). Periods of activity for each trial of behavior were selected using a cursor to identify the silent periods just before and after the target EMG signals. This procedure, which has minimal effect on crosscorrelation analysis of EMG signals, omitted unacceptable periods from the analysis (e.g., activity not associated with the target behavior). In the event of artifactual nontarget events, the entire utterance (e.g., “Baby Bop”), or the entire sequence of a target behavior (e.g., /pa-pa-pa/) was rejected. The audio signal was used to verify appropriate digitization and analysis only when signal-to-noise ratio was relatively poor. When the audio signal was used to identify speech events, the entire portion of the signal coincident with energy in the audio trace was digitized, along with approximately 10% of the silent interval prior to and following the speech event (the analyses have been shown to be very robust with respect to the inclusion of varying amounts of inactivity before and after target events; Moore et al., 1988). Next, the selected periods from each trial were full-wave rectified and digitally low-pass filtered (8-pole Butterworth, fc = 30 Hz). Figure 1 illustrates each processing stage of the EMG signals for a single trial of lip protrusion. The activation “envelope” shown in panel C represents a typical synchronous activation pattern, which is characteristic of mature lip protrusion (Wohlert & Goffman, 1994). A 2×2 matrix of crosscorrelation functions were computed for the two rectified and smoothed EMG signals. This analysis has been implemented in previous investigations on mandibular muscle coordination (Moore, 1993; Moore & Ruark, 1996) and was found to be a sufficient and appropriate method for quantifying and describing coordinative organization of muscle activation patterns. From the crosscorrelation function the peak coefficient, lag to the peak coefficient, and the coefficient at zero lag were extracted for subsequent qualitative and statistical analysis. Figure 2 illustrates the crosscorrelation function for upper and lower lip EMG channels from the data in Figure 1. This function yielded a peak coefficient for upper and lower lip EMG of .71, indicating that for this trial of lip protrusion, the activity in right upper lip is highly predicted by right lower lip activity. The synchronous activation of these muscles is indicated by the lag to peak value of zero milliseconds.
Figure 1.
Right upper (RUL) and lower lip (RLL) EMG obtained during one trial of lip protrusion. Signal processing stages: raw (A), rectified (B), and filtered (C) are shown.
Figure 2.

The crosscorrelation function for lip protrusion data shown in Figure 1. The peak coefficient of .71 indicates a moderate-to-high level of coupling of upper and lower lip activity for this task; the lag at zero delineates the synchronous activation of these two muscles.
To allow statistical treatment of the peak coefficients, each coefficient (for each trial within a task) was transformed to a Fisher’s z value (Equation 1) prior to averaging across repetitions within each subject.
For each subject, an average Fisher’s z value was obtained for each task. Prior to averaging task means across subjects, within-subject task effects were eliminated by standardizing each subject’s averaged Fisher’s z value for each task to a mean of zero and a standard deviation of one (Equation 2).
This standardization procedure, using each subject’s overall mean and standard deviation scores (across tasks), allowed across-subject comparisons of task effects. The standardized Fisher’s z values for each task were combined across subjects, and comparisons were made on the group mean standardized Fisher’s z values across six main tasks (i.e., pursing, chewing, syllable repetition, bilabial speech, lip-rounding speech, and nonlabial speech tasks). Statistical comparisons of lags associated with each peak coefficient were performed by transforming each lag to its absolute value (dropping the sign of the lag). This transformation allowed lag scores to be averaged within a task before obtaining the average group lag value for each task (Moore, 1993; Moore & Ruark, 1996). A multivariate analysis of variance for repeated measures and subsequent post-hoc analysis (Student-Newman-Keuls procedure) were used to test for differences across tasks for standardized mean Fisher’s z values and mean absolute lags. These analyses allowed identification of task-specific differences in activation patterns of the upper and lower lips.
Additional Considerations
A primary advantage of the cross-correlational analysis employed was that it permitted reduction of very large samples down to comprehensible and statistically testable values. Application of this technique to EMG signals in young children required careful consideration of its inherent limitations and the decision points in the process. Foremost was the interpretation of the obtained coefficients with respect to known structures and mechanisms. Fortunately, analysis of adult lip muscle activity has provided benchmark values for relating the obtained coefficients with known biomechanical and anatomic relationships (e.g., within-quadrant coefficients, ranging from .89 to .98 in the lips of adults, may be taken as the maxima for this technique; Wohlert & Goffman, 1994). These benchmarks provide an upper limit for what might be expected for tightly coupled muscle activation.
The validity of these analyses was assessed by evaluating several potential sources of systematic error. One potential problem when studying freely behaving children is the susceptibility of correlational measures to cross-channel artifactual effects, including movement artifact, 60 Hz line noise, and abrupt changes in tape transport (i.e., on/off transients). In response to this concern, sampling criteria governing the present data required signals that were free of movement artifact or other cross-channel noise. Another consideration was that signal processing decisions (e.g., digital filter design, including filter type and cutoff frequencies) can significantly affect the correlational results obtained. This effect was addressed by careful design, description, and maintenance of signal processing stages throughout the experiment. The signal processing parameters described were selected empirically as maximizing the contrast among these measures. Finally, a third systematic effect that might have confounded these measures was task-specific variations in signal-to-noise ratio; higher signal-to-noise ratios generally yield slightly higher peak coefficients. This effect, which can yield spurious statistical differences, varies with constantly changing levels activation, but can be evaluated qualitatively.
The potentially systematic effect of task-specific variations in signal-to-noise (S/N) ratio is closely related to variations that may occur in EMG amplitude. This effect was evaluated by modeling variations in S/N ratio over a wide range of values. The extent to which the range of S/N ratios can be estimated and accommodated by these analyses will determine how robust the present methods will be. Comparability of tasks with varying EMG amplitude affords greater generalizability to these results. Although prior investigations have revealed the stability of these analytic techniques and measures over a wide range of signals (Moore, 1993; Moore et al., 1988), the present data set required closer inspection. Accordingly, a synthetic data set was created to model a wide range of S/N ratios. Peak correlations coefficients computed for these paired signals revealed these measures to be remarkably stable, exhibiting a drop of less than .1 in comparing pure signals to those with S/N ratios as poor as 7 dB. With respect to the present data set, a S/N ratio of 7 dB was judged by the experimenters to correspond to signals that were minimally acceptable for inclusion in this analysis. Thus, the present data set, with S/N ratios falling between approximately 7 and 13 dB, was shown to be only weakly susceptible to this effect.
Results
The results of these analyses provided an estimate of the relative strength of coupling and timing of upper and lower lip EMG activity of 2-year-old children over a broad range of oromotor behaviors. The crosscorrelation analysis quantified the coordinative organization of lip muscle activity by yielding a peak coefficient, which indicated the level of coupling, and the lag to the peak, which indicated the timing of activity in the two EMG signals.
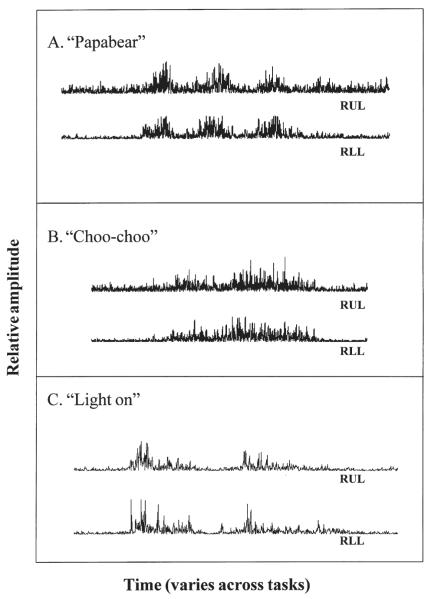
Each participant easily tolerated the surface EMG electrodes, and quickly became accustomed to their presence. Participants successfully performed the six main tasks, although in four instances 3 participants generated only four trials for a specific task. The experimental protocol was successful in eliciting a range of levels of coupling of upper and lower lip activity. As expected, EMG signal-to-noise ratio was relatively poor (i.e., because of the small size of the target muscles and the low levels of activity produced) and varied across tasks. However, for the majority of tasks modulation of EMG of the upper and lower lip was easily observed. For example, Figure 3 shows rectified right upper and lower lip EMG signals for Subject 2 during productions of stimuli for the three main speech tasks. Panel (A) shows EMG activity for the production, “Papa Bear,” a stimulus for the bilabial speech task. Panel (B) shows EMG activity for “Choo-choo,” a stimulus for the lip-rounding speech task, and panel (C) is an example of upper and lower lip EMG for “Light on,” a stimulus for the nonlabial speech task.
Figure 3.
Right upper (RUL) and lower lip (RLL) rectified EMG signals obtained from Subject 2 during three main speech tasks: (A) bilabial speech task, “Papa Bear,” (B) lip-rounding speech task, “Choo-choo,” and (C) nonlabial speech task, “Light on.”
In the case where signal-to-noise ratio was extremely low, the audio signal was used to verify appropriate digitization and analysis. In addition, the online gloss (Audio Channel #2), which included a continuous description of the child’s speech and target behaviors, provided further verification of the target data. Movement artifacts were clearly observable when they occurred under these conditions, and data that were contaminated with such artifacts were excluded from analysis. Less than 7% of the behavioral trials were excluded from analysis on the basis of data selection criteria. Additional reasons for rejecting a trial for analysis included: (a) inadequate audio signal to verify data selection, (b) target behaviors occurred concurrently with other behaviors, and (c) deviation from the verbal model provided by the experimenter (e.g., child’s speech was unclear).
Difference in Coupling of Upper and Lower Lip Muscles Across Tasks
Coupling of upper and lower lip activity of 2-year-old children was found to be task specific. Lip protrusion and syllable repetition tasks yielded strong coupling of upper and lower lip activity, lip rounding and nonlabial speech tasks yielded weak coupling, and chewing and bilabial speech tasks demonstrated moderate coupling. The average Fisher’s z values and corresponding correlation coefficients (shown only for purpose of clarification; all statistical computations were completed on Fisher’s z values) for each subject for all behaviors are shown in Table 1. These values reflected the level of coupling of upper and lower lip activity. Mean task differences were isolated by comparing individually standardized Fisher’s z values across tasks, within each subject. Standardized Fisher’s z values above zero revealed higher than average coupling between two signals, whereas values below zero revealed lower than average coupling. Table 2 shows the average standardized Fisher’s z values for all participants across tasks.
Table 1.
Average Fisher’s z values (and corresponding coefficients) for all subjects across tasks.
| Subjects | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | Standard error |
|---|---|---|---|---|---|---|---|---|---|
| Lip protrusion | .94 (.73) | .84 (.69) | .55 (.50) | .87 (.70) | .73 (.62) | .82 (.68) | .87 (.70) | .70 | .05 |
| Chewing | .95 (.74) | .66 (.58) | .52 (.48) | .41 (.39) | .81 (.67) | .64 (.57) | .60 (.54) | .66 | .07 |
| Syllable repetition | .88 (.71) | .84 (.69) | 1.09 (.80) | .26 (.25) | .78 (.65) | .79 (.66) | .73 (.62) | .77 | .10 |
| Speech containing bilabial consonants | .77 (.65) | .66 (.58) | .57 (.52) | .49 (.46) | .74 (.63) | .72 (.61) | .65 (.57) | .66 | .04 |
| Speech with a lip-rounded vowel | .61 (.55) | .73 (.62) | .76 (.64) | .43 (.42) | .53 (.49) | .46 (.43) | .44 (.41) | .57 | .05 |
| Speech without bilabial or lip-rounding specification |
.46 (.43) | .48 (.45) | .60 (.54) | .44 (.41) | .39 (.37) | .59 (.53) | .53 (.49) | .50 | .01 |
Table 2.
Average normalized Fisher’s z values for all subjects across tasks.
| Subjects | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | Standard error |
|---|---|---|---|---|---|---|---|---|---|
| Lip protrusion | .87 | 1.04 | −.60 | 1.89 | .42 | 1.10 | 1.54 | .89 | .31 |
| Chewing | .91 | −.34 | −.75 | −.34 | .87 | −.24 | −.22 | −.02 | .24 |
| Syllable repetition | .56 | 1.01 | 1.88 | −1.10 | .69 | .91 | .63 | .65 | .34 |
| Speech containing bilabial consonants | .03 | −.31 | −.51 | .02 | .47 | .37 | .07 | .02 | .13 |
| Speech with a lip-rounded vowel | −.80 | .23 | .37 | −.25 | −.80 | −1.55 | −1.28 | −.58 | .28 |
| Speech without bilabial or lip-rounding specification |
−1.57 | −1.62 | −.40 | −.22 | −1.65 | −.59 | −.73 | −.96 | .24 |
Observations of the range of values within each task revealed that across-subject variation was considerable, although task differences were observable. For example, most participants exhibited high standardized Fisher’s z values for pursing and syllable repetition tasks, suggesting strong linkage of upper and lower lip activity for these tasks. By comparison, coupling of upper and lower lip activity for the lip-rounding and nonlabial speech tasks was generally less rigid. Deviations from this trend were seen for Subjects 1 and 5, for whom stronger coupling was exhibited during the chewing task, and for Subject 3, for whom stronger coupling was shown for the lip-rounding speech task. Subject 4 exhibited her weakest coupling of these muscles during the syllable repetition task, during which she frequently exhibited reciprocal organization of upper and lower lip activity. The effects for coupling strength are shown in Figure 4, which shows a bar graph of group means and standard errors of the standardized Fisher’s z values for all tasks. Comparisons across tasks revealed that upper and lower lip EMG was most highly coupled for the lip protrusion task. The syllable repetition task yielded the second highest average value, whereas the chewing and bilabial speech production tasks yielded similar, more moderate group mean standardized Fisher z values. For the remaining conditions, lower group mean values were found for lip-rounding and nonlabial speech tasks, with the lip-rounding speech task demonstrating a slightly higher value than the nonlabial speech task. Statistical analysis, using a multivariate analysis of variance with repeated measures, indicated that effects seen across tasks for the mean standardized Fisher’s z values were significant, p < .01, multivariate F = 4.07 (10, 58). Post hoc analyses revealed that the mean standardized Fisher’s z values of right upper and lower lip activity were not significantly different for most paired comparisons. Significant differences, p < .01; F = 6.03 (5, 30), were found only between: lip-protrusion and lip-rounding tasks, lip-protrusion and nonlabial speech tasks, syllable repetition and lip-rounding tasks, and syllable repetition and nonlabial speech tasks. Activation levels were qualitatively observed to vary substantially within and across tasks, as well as within and across subjects. This wide variation minimized the potential of any systematic effect due simply to the effect of S/N ratio on correlation coefficients. In fact, the speech tasks for which there was the greatest potential for systematic differences in activation levels, bilabial speech versus nonlabial speech tasks, failed to demonstrate significant differences among the correlation coefficients obtained.
Figure 4.

Group standardized Fisher’s z values and standard errors for all tasks. Lip protrusion and syllable repetition tasks differed significantly from the lip-rounding and nonlabial speech tasks. Chewing and bilabial speech tasks demonstrated characteristic coupling patterns that were not significantly different from the other four tasks.
Difference in Timing of Upper and Lower Lip Muscle Activity Across Behaviors
Results of timing of upper and lower lip activity revealed no significant differences across behaviors. Although lip protrusion yielded a marked decrease in absolute lag between upper and lower lip activity, no significant effects were found across tasks, p > .01, F = .77 (5, 30). This result suggests that timing of upper and lower lip activity does not differentiate among coordinative strategies of the target behaviors. Figure 5 shows the group means and standard errors of absolute lags across tasks.
Figure 5.

Group mean and standard errors of absolute lags for all tasks. Although timing differences across tasks were apparent, none were significantly different.
An additional concern in developmental tasks such as these is the potential for learning effects to occur. Though not observed directly in any child or task, a test for a change in peak correlation coefficient or lag with repetition was performed to assess this possibility. Pearson product moment correlation coefficients were computed for trial number by lag and by Fisher’s z values. Coefficients obtained across all trials and subjects for trial number by coefficient and trial number by lag were −.017 and .105, neither of which was significant, supporting the observation that no learning effect occurred.
Discussion
The present results have demonstrated task-specific differences in the level of coupling of right upper and lower lip activity in 2-year-old children. Although the development of speech motor control continues until at least 11 years of age (Kent, 1984), the coordinative organization of the perioral system of 2-year-olds is much like that of the adult perioral system, where different levels of coupling are employed to execute a range of tasks (Wohlert & Goffman, 1994). This task-specific organization supports the suggestion that speech develops separately and distinctly from other developing oromotor behaviors, such as chewing. The present findings are also consistent with other neurophysiologic studies of the development of oromotor control. Sucking and chewing in developing rats (Westneat & Hall, 1992), for example, and infant sucking and adult chewing in humans (Ruark & Moore, 1992), exhibit different coordinative strategies in the mandibular system. Moreover, distinct neural pathways have been found to mediate different oral behaviors (Kubota et al., 1988).
The coordinative flexibility of the perioral system of 2-year-olds probably manifests task-specific differences in demands (e.g., the generation of a sphincter-like activity for pursing versus dissociated coupling for nonlabial speech tasks), as well as differences in coordinative complexity required by each task; for example, rhythmic repetition of syllables versus lip rounding during speech (Moore, 1993). The strong coordinative linkage of upper and lower lip activity for the lip protrusion task was not surprising. Similar to adult lip protrusion, coactivation of lip quadrants, indicated by short lags between upper and lower lip activity, is required for lip protrusion in 2-year-olds. Strong coupling of upper and lower lip activity for the syllable repetition task, however, was less expected because in adults, coupling of the upper and lower lips for speech is significantly less rigid than coupling for lip protrusion. One possible explanation is that the syllable repetition task required tightly coupled activity of the upper and lower lips in order to produce exaggerated patterns of bilabial plosion (e.g., /pa-pa-pa/), for which the upper and lower lips may concurrently move to release intra-oral air pressure.
Speech production tasks (i.e., lip-rounding and nonlabial speech tasks) by these 2-year-olds yielded the weakest coupling of upper and lower lip muscle activity. This finding was similar to that obtained from adult individuals, who exhibited significantly weaker coupling for speech versus lip protrusion or chewing (Wohlert & Goffman, 1994). This finding was supported by the observation that, during the lip rounding and nonlabial speech tasks, EMG of the upper lip frequently exhibited activation that was less modulated than that of the lower lip, indicating that during these activities coupling of the upper and lower lips was weaker. There are at least two processes that might be invoked to address this finding. One possibility is that activation of the lower lip is, in some respect, primary during these speech tasks and is independent of upper lip movement. For lip-rounding productions, however, this explanation seems rather weak. A more likely interpretation of the present finding of lowered coupling during production of words such as “Choo-choo” would be that coarticulatory effects of lip rounding across the utterance yielded more tonic, steady-state patterns of activation, which yield lowered crosscorrelation coefficients.
Although coupling of upper and lower lip activity during chewing and bilabial speech tasks was not significantly different from the other four tasks, these tasks demonstrated specific coordinative characteristics. Stable, rhythmic EMG patterns of upper and lower lip activity, which is characteristic of adult mastication (Wohlert & Goffman, 1994), did not consistently occur. Rather, chewing activity for the majority of children was characterized by periods of rhythmic and nonrhythmic activity, as well as periods of asynchronous activity. This finding is consistent with findings from a previous study, where chewing patterns of 2-year-olds were found to be inconsistently rhythmic, and mouth opening during chewing was frequent (Stolovits & Gisel, 1991). In the present study, the rhythmicity of lip muscle activation patterns of 2-year-olds did not correspond with either opening or closing movements of the mouth (participants generally chewed with their mouth closed). The 2 participants that demonstrated higher correlation coefficients for the chewing task did, however, consume a large bolus per intake (i.e., for each trial of chewing, each of these children took large bites of large-sized pretzels). It is possible that these children produced a stronger lip seal during chewing in order to retain the large bolus within the oral cavity, resulting in a more rhythmic output of lip muscle activation patterns. Although mandibular muscle activity of children (Green et al., in press) and adults (Moore et al., 1988) during chewing has been described as a rhythmic patterned activity associated with the operation of a low-level mechanism, the relatively dissociated patterns of lip muscle activity exhibited by 2-year-olds bring into question the operation of such a mechanism. This finding is consistent with adult lip EMG data where the operation of low-level mechanisms, such as CPGs, in upper and lower lip activity during chewing or other tasks is also questionable (Goffman & Smith, 1994). Goffman and Smith (1994), by recording EMG activity from all four lip quadrants during speech and nonspeech tasks, found that there was no significant coherence between the EMG signals of any two of the four quadrants during chewing, lip protrusion, or syllable repetition tasks. Highly coherent EMG signals from respiratory and mandibular muscles in the frequency ranges of 20–60 Hz and 60–110 Hz have been associated with brain stem-level CPGs (e.g., Goldberg & Chandler, 1983; Smith & Denny, 1990). The lack of coherence in adult lip EMG has been taken to suggest that low-level mechanisms are not involved in generating rhythmic activity of the lips during chewing. Similarly, in 2-year-olds, although coherence analyses have not been applied to the current data set, the coordinative framework of lip muscle activity (i.e., dissociated activity of the upper and lower lips) lends support to the suggestion that, although low-level mechanisms may be involved in mediating mandibular muscle activity during chewing, these mechanisms do not appear to be involved in the generation of lip muscle activity during chewing in this age group.
Although the coordinative strategy for bilabial speech production was distinct from that of the other main tasks, this difference does not necessarily represent underlying control differences. Specifically, the syllable repetition task and the bilabial speech task shared the common goal of bilabial plosion. In contrast to the syllable repetition task, in which the same syllable was repeated rhythmically, the bilabial speech task involved changing contextual effects. These characteristic coarticulatory influences probably affected the level of coupling of upper and lower lip activity, although the extent of this effect could not be revealed by the present design. Moreover, even though the level of coupling of upper and lower lip activity was not significantly different across speech tasks, qualitative observations of the coordinative characteristics of these tasks revealed differences, including the finding that upper and lower lips exhibited deeper modulation of activity for bilabial plosion than for the other two tasks.
An additional objective of this investigation was to evaluate whether the upper and lower lips of 2-year-olds, like adults, receive separate control inputs during various tasks. The present finding, which demonstrated coupling differences across behaviors, supports Wohlert and Goffman’s (1994) finding of independence for upper and lower lip control signals. One generalization to emerge from the present investigation was that the coordinative differences in upper and lower lip activity across tasks were primarily manifest as variations in coupling, rather than in relative timing. This finding may be interpreted as resulting from the anatomic linkage of the upper and lower lips, which promotes synchronous movement of these structures. Thus, although the two structures are activated simultaneously for various activities, the upper and lower lips receive segregate neural inputs (Goffman & Smith, 1994; Wohlert & Goffman, 1994). Recognizing the anatomic constraint of these two structures, previous investigators have focused specifically on the zero lag coefficients, rather than peak cross-correlation coefficients. The very short lags obtained in the present results facilitates comparison of coefficients across studies.
There were clear variations in the coupling exhibited across tasks and across participants. These variations were not tested statistically due to the small number of participants and observations that were obtained. Nevertheless, it may be useful to consider the possible sources of variation with respect to both methodological factors and potential differences in motor control. For example, although Subjects 1 and 5 exhibited moderate-to-high levels of coupling for the pursing and syllable repetition tasks, their highest level of coupling was elicited during chewing. This difference may stem from the observation that the chewing activity of these participants yielded EMG patterns that were more highly modulated and rhythmic than for other subjects, resulting in relatively higher correlation coefficients. Similarly, in contrast to most participants, Subject 4 exhibited very low levels of coupling during the syllable repetition task. This subject frequently generated dissociated activity of the upper and lower lips during rhythmic tasks, as the EMG signals from her lower lip inconsistently lagged behind the EMG signals from her upper lip. This absence of pattern in the context of patterned movement cannot be reconciled by the present analyses, and requires the addition of kinematic observations. An additional possibility that must be considered with respect to within-task variability across participants was the fact that speech samples were collected without regard to whether they were imitative or spontaneous. It is possible that some participants relied on visual observations of the investigator’s lip movements as a model for their own productions, whereas others relied on an acoustic model, or no model at all. The effect of imitation on coordinative organization is, of course, unknown, and will serve as one empirical question among several in an upcoming investigation of younger children. Nevertheless, participants were clearly observed to produce these speech tasks without effort, which might be taken to suggest, as was assumed, that these movements already existed within each child’s repertoire of speech production skills.
Although the present results support the notion that different control mechanisms mediate speech and nonspeech behaviors in 2-year-olds, it remains to be determined whether speech emerges independently of earlier appearing oromotor behaviors. Several of the present tasks yielded comparable levels of coupling and timing, and might still be shown to share common control mechanisms. Chewing and bilabial speech tasks, for example, as well as lip-rounding and nonlabial speech tasks, each yielded similar coupling and timing results, and may be mediated by common mechanisms. The appeal of this suggestion is most severely weakened by the fundamentally different nature of these behaviors (e.g., chewing tasks compared to nonlabial speech tasks).
Another concern arising for developmental studies that rely on a cross-sectional design is the potential for observing behaviors in transition, which may bear slight resemblance to preceding or succeeding conditions. The challenge of differentiating stabilities (“attractor states”; Kelso & Schoner, 1988) from dynamically evolving motor patterns remains. A complete evaluation of the emergence of speech production relative to extant oral motor behaviors entails fine resolution longitudinal sampling of those behaviors. This work is in progress in our laboratories.
Conclusions
The present investigation of 2-year-old children demonstrated task-specific differences in the coordinative organization of lip muscle activity for speech and nonspeech behaviors. This level of coordinative specialization is consistent with earlier work in mandibular coordination by 15-month-olds (Moore & Ruark, 1996) and contributes to the accumulation of findings suggesting that children develop speech-specific coordinative mechanisms very early in life. Although conclusive results are yet to be obtained, the present findings support the suggestion that speech emerges separately from extant oromotor behaviors, and fail to support the existence of redundancy in control mechanisms across tasks. Future efforts, in addition to refining the description of normal coordinative development, will be directed toward developing diagnostic and descriptive criteria relative to developmental speech motor disorders. Such a description of oromotor disruption will provide essential insight into these pathologic processes, as well as into the nature and limits of typical motor control.
Acknowledgments
Both authors are formerly of the Department of Communication Sciences and Disorders, University of Pittsburgh, Pittsburgh, Pennsylvania, which also provided support for this investigation. This work was also supported by research grant number 7R29DC00822 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Contributor Information
Jacki L. Ruark, University of Tennessee Knoxville
Christopher A. Moore, University of Washington Seattle
References
- Cooke JD, Brown SH. Movement-related phasic muscle activation. II. Generation and functional role of the triphasic pattern. Journal of Neurophysiology. 1990;63:465–472. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- Dellow PG. The physiological background of chewing and swallowing. In: Sessle BJ, Hannam A, editors. Mastication and Swallowing. University of Toronto; Toronto, Canada: 1976. pp. 6–21. [Google Scholar]
- Goffman L, Smith A. Motor unit territories in the human perioral musculature. Journal of Speech and Hearing Research. 1994;37:975–984. doi: 10.1044/jshr.3705.975. [DOI] [PubMed] [Google Scholar]
- Goldberg LJ, Chandler SH. Evidence for the presence of a high frequency oscillation in the CPG for rhythmic jaw movement in the guinea pig. Processes in Neuroscience. 1983;9:361–368. [Google Scholar]
- Green JR, Moore CM, Ruark JL, Rodda PR, Morvee WT, Van Witzenburg MJ. Development of chewing in children from 12 to 48 months. Journal of Neurophysiology. doi: 10.1152/jn.1997.77.5.2704. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. On the neural control of movement—a comparison of different basic rhythmic behaviors. In: Stent GS, editor. Function and formation of neural systems. Dahlem Konferenzen; Berlin: 1977. pp. 197–224. [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom P, Lubker J, Persson A, editors. Speech motor control. Pergamon Press; New York: 1982. pp. 217–230. [Google Scholar]
- Hesselmans LFGM, Jennekens FGI, van den Oord CJM, Veldman H, Vincent A. Development of innervation of skeletal muscle fibers in man: Relation to acetylcholine receptors. Anatomical Record. 1993;236:553–562. doi: 10.1002/ar.1092360315. [DOI] [PubMed] [Google Scholar]
- Kelso JA, Schoner G. Self-organization of coordinate movement patterns. Human Movement Science. 1988;7:27–46. [Google Scholar]
- Kent RD. Psychobiology of speech development: Coemergence of language and movement system. American Physiologic Society. 1984:R888–R894. doi: 10.1152/ajpregu.1984.246.6.R888. [DOI] [PubMed] [Google Scholar]
- Kent RD, Mitchell PR, Sancier M. Evidence and role of rhythmic organization in early vocal development in human infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated actions. Elsevier; New York: 1991. pp. 135–139. [Google Scholar]
- Kubota K, Narita N, Ohkubo K, Shibania S, Nagae K, Kubota M, Odagiri N, Kawamoto T. Morphological studies of the neuromusculature shifting from sucking to biting of mice. Acta Anatomica. 1988;133:200–208. doi: 10.1159/000146640. [DOI] [PubMed] [Google Scholar]
- Love RJ, Hagerman EL, Taimi EG. Speech performance, dysphagia, and oral reflexes in cerebral palsy. Journal of Speech and Hearing Disorders. 1980;45:59–75. doi: 10.1044/jshd.4501.59. [DOI] [PubMed] [Google Scholar]
- MathWorks . MATLAB: High performance numeric computation and visualization software [Computer program] The Matworks, Inc; Natick, MA: 1993. [Google Scholar]
- Miller JF. Assessing language production in children. University Park Press; Baltimore, MD: 1981. [Google Scholar]
- Moore CA. Bilateral symmetry of mandibular muscle activation during speech and nonspeech tasks. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from early developing oral motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Scudder RR. Coordination of jaw muscle activity in Parkinsonian movement: Description and response to traditional treatment. In: Yorkston K, Beukelman D, editors. Recent advances in clinical dysarthria. College Hill Press; Boston: 1989. pp. 147–163. [Google Scholar]
- Moore CA, Smith A, Ringel R. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Moyers RE, Carlson DS. Maturation of the orofacial neuromusculature. In: Enlow DH, editor. Facial growth. 3rd ed W. B. Saunders Company; Philadelphia: 1990. pp. 267–279. [Google Scholar]
- Mueller H. Facilitating feeding and prespeech. In: Pearson P, Williams C, editors. Physical therapy for the developmental disabilities. Charles C. Thomas; Springfield, IL: 1972. [Google Scholar]
- Netsell R. The acquisition of speech motor control: A perspective with directions for research. In: Netsell R, editor. Neurobiologic view of speech production and the dysarthrias. College-Hill Press; San Diego: 1986. pp. 133–152. [Google Scholar]
- Newberg J, Stock JR, Wnek L. The Battelle Developmental Inventory. LINC Associates, Inc; Allen, TX: 1988. [Google Scholar]
- Ostry DJ, Flanagan JR. Human jaw movement in mastication and speech. Archives of Oral Biology. 1989;34:685–693. doi: 10.1016/0003-9969(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Palmer M. Studies in clinical techniques: Part II. Normalization of chewing, sucking, and swallowing in cerebral palsy: A home program. Journal of Speech and Hearing Disorders. 1947;12:415–418. [Google Scholar]
- Rescorla L. The Language Development Survey: A screening tool for delayed language in toddlers. Journal of Speech and Hearing Disorders. 1989;54:587–599. doi: 10.1044/jshd.5404.587. [DOI] [PubMed] [Google Scholar]
- Ruark J, Moore CA. Coordination of orofacial muscles during sucking by human infants. Paper presented at the 25th Annual ASHA Convention; San Antonio, TX. 1992. [Google Scholar]
- Sharkey SG, Folkins JW. Variability of lip and jaw movements in children and adults: Implications for the development of speech motor control. Journal of Speech and Hearing Research. 1985;28:8–15. doi: 10.1044/jshr.2801.08. [DOI] [PubMed] [Google Scholar]
- Simonds RJ, Scheibel AB. The postnatal development of the motor speech area: A preliminary study. Brain and Language. 1989;37:42–58. doi: 10.1016/0093-934x(89)90100-4. [DOI] [PubMed] [Google Scholar]
- Smith A, Denny M. High-frequency oscillations as indicators of neural control mechanisms in human respiration, mastication, and speech. Journal of Neurophysiology. 1990;63:745–758. doi: 10.1152/jn.1990.63.4.745. [DOI] [PubMed] [Google Scholar]
- Smith B. Temporal aspects of English speech production: A developmental perspective. Journal of Phonetics. 1978;6:37–67. [Google Scholar]
- Stolovitz P, Gisel EG. Circumoral movements in response to three different food textures in children 6 months to 2 years of age. Dysphagia. 1991;6:17–25. doi: 10.1007/BF02503459. [DOI] [PubMed] [Google Scholar]
- Thelen E. Rhythmical behavior in infancy: An ethological perspective. Developmental Psychology. 1981;17:237–257. [Google Scholar]
- Thelen E. Motor development: A new synthesis. American Psychologist. 1995;50:79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- Thelen E, Cooke DW. Relationship between newborn stepping and later walking: A new interpretation. Developmental Medicine and Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- Thelen E, Fisher DM. From spontaneous to instrumental behavior: Kinematic analysis of movement changes during very early learning. Child Development. 1983;54:129–140. [PubMed] [Google Scholar]
- Thelin SA, Thelin JW. Otitis media: Communication, learning, and medical issues. Paper presented at the annual convention of the Tennessee Association of Audiologists and Speech-Language Pathologists; Murfreesboro, TN. 1996. [Google Scholar]
- Westlake H. Muscle training for cerebral palsied speech cases. Journal of Speech and Hearing Disorders. 1951;16:105–109. doi: 10.1044/jshd.1602.103. [DOI] [PubMed] [Google Scholar]
- Westneat MW, Hall W. Ontogeny of feeding motor patterns in infant rats: An electromyographic analysis of suckling and chewing. Behavioral Neuroscience. 1992;106:539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]
- Wilson DK. Voice problems in children. Williams & Wilkins; Baltimore, MD: 1982. [Google Scholar]
- Wohlert A, Goffman L. Human perioral muscle activation patterns. Journal of Speech and Hearing Research. 1994;37:1032–1040. doi: 10.1044/jshr.3705.1032. [DOI] [PubMed] [Google Scholar]
- Wolff PH. Endogenous motor rhythms in young infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated action. Elsevier; New York: 1991. pp. 93–133. [Google Scholar]