Abstract
Hepatitis C virus (HCV) infection represents a major health issue worldwide due to its burden of chronic liver disease and extrahepatic manifestations including cardiovascular diseases, which are associated with excess mortality. Analysis of published studies supports the view that HCV infection should be considered a risk factor for the development of carotid atherosclerosis, heart failure and stroke. In contrast, findings from studies addressing coronary artery disease and HCV have yielded conflicting results. Therefore, meta-analytic reviews and prospective studies are warranted. The pathogenic mechanisms connecting HCV infection, chronic liver disease, and atherogenesis are not completely understood. However, it has been hypothesized that HCV may promote atherogenesis and its complications through several direct and indirect biological mechanisms involving HCV colonization and replication within arterial walls, liver steatosis and fibrosis, enhanced and imbalanced secretion of inflammatory cytokines, oxidative stress, endotoxemia, mixed cryoglobulinemia, perturbed cellular and humoral immunity, hyperhomocysteinemia, hypo-adiponectinaemia, insulin resistance, type 2 diabetes and other components of the metabolic syndrome. Understanding these complex mechanisms is of fundamental importance for the development of novel therapeutic approaches to prevent and to treat vascular complications in patients with chronic HCV infection. Currently, it seems that HCV clearance by interferon and ribavirin treatment significantly reduces non-liver-related mortality; moreover, interferon-based treatment appears to decrease the risk of ischemic stroke.
Keywords: Hepatitis C virus, Atherosclerosis, Coronary artery disease, Stroke, Inflammation
Core tip: Hepatitis C virus (HCV) infection represents a risk factor for carotid atherosclerosis, heart failure and ischemic stroke. However, findings from studies addressing coronary artery disease and HCV have yielded conflicting results. Moreover, an excess of cardiovascular mortality among anti-HCV positive subjects has been reported. HCV promotes atherogenesis through direct and indirect mechanisms. Inflammation, cytokines activation, cellular and humoral immunity, metabolic derangement, oxidative stress, liver steatosis and fibrosis have been postulated as potential atherogenic mechanisms. Knowledge of such complex mechanisms may be important for understanding disease progression and promoting novel therapeutic approaches. At present, interferon-based treatment of chronic hepatitis C seems to reduce the risk of stroke as well as non-liver-related mortality.
INTRODUCTION
Hepatitis C virus (HCV) infection is endemic worldwide, with an estimated global prevalence of 3%, resulting in approximately 170 million infected people[1]. HCV infection is the major cause of chronic liver diseases leading to a wide range of hepatic diseases, including cirrhosis and hepatocellular carcinoma. In addition, there is a growing body of data concerning the role of HCV in extrahepatic manifestations[2], including metabolic derangements[3], and, more recently, accelerated atherosclerosis eventually triggering cardiovascular events[4].
Atherosclerosis, either subclinical or manifest, is a chronic inflammatory disease. The chief clinical manifestations are coronary artery disease (CAD), stroke and ischemic limbs. In addition to the “traditional” factors of atherosclerosis other “novel” factors have been hypothesized. The possible role of an infectious agent in the development of experimental atherosclerosis in rodents was first reported more than 120 years ago[5], and this concept has gained new life in recent years[6]. In the last few years, a strict association between atherosclerosis and non-alcoholic fatty liver disease (NAFLD) has been reported, challenging the old paradigm that ‘‘chronic liver disease protects from atherosclerosis”[7,8]. Similar to NAFLD, HCV infection is increasingly identified as a potential atherogenic condition. In fact, chronic HCV infection causes hepatic and systemic inflammation[4] via increased levels of pro-atherogenic chemokines and cytokines[4] and hepatic steatosis, a distinguishing feature of this infection[9]. In addition, it has been demonstrated that HCV colonizes and replicates within carotid plaques[10,11] likely causing vascular inflammation. Earlier studies conducted in the general population showed that HCV markers were independently associated with atherosclerosis[12,13]. Subsequent research, however, yielded conflicting results, some studies confirming[14,15] and others denying such an association[16,17]. However, recent data have shown excess cardiovascular mortality during the course of chronic HCV infection[18,19]. Given the high prevalence of HCV infection on a worldwide basis and the primacy of cardiovascular diseases among the causes of mortality, the role of HCV as a cardiovascular risk factor needs to be analysed in-depth.
Accordingly, in this review we evaluated the literature regarding the association between chronic HCV infection and atherosclerosis, the clinical impact on the development of cardiovascular diseases and the possible pathogenic mechanisms underlying increased cardiovascular risk in those with HCV infection.
HCV AND ATHEROSCLEROSIS
Table 1 reports the findings of the studies evaluating the association between HCV and carotid atherosclerosis. Ishizaka et al[12,13], assessing a large number of subjects in two studies conducted in the general population, were first in reporting an association between HCV infection and atherosclerosis. The first study was published in 2002 and showed that HCV infection was associated with an increased risk of carotid atherosclerosis[12]. Similar results were obtained in a second study published in 2003[13]. Irrespective of known atherogenic risk factors, Ishizaka et al[12,13] found that HCV was an independent predictor of carotid atherosclerosis. Another study, which enrolled a large cohort of Japanese subjects from the general population, showed that HCV-infected subjects had increased arterial stiffness compared to HCV-negative controls[20]. Fukui et al[21] confirmed these results in a series of patients with type 2 diabetes in which anti-HCV positivity was demonstrated to be an independent risk factor of both increased carotid intima-media-thickness (IMT) and plaque. Following these pioneer Japanese reports, two Italian studies further confirmed the independent association between HCV infection and atherosclerosis. Boddi et al[10] showed that the prevalence of an IMT greater than 1 mm was significantly higher in anti-HCV positive subjects than in controls. At multivariate analysis, anti-HCV positivity was an independent predictor of IMT. Similarly, Targher et al[15] showed that HCV positivity was an independent predictor of increased carotid IMT.
Table 1.
Main characteristics of the studies which evaluated the association between hepatitis C virus and atherosclerosis
| Ref. | Type of study | Country | HCV+ (n) | HCV- (n) |
| Studies showing association | ||||
| Ishizaka et al[12], 2002 | Population | Japan | 104 | 4784 |
| Ishizaka et al[13], 2003 | Population | Japan | 25 | 1967 |
| Tomiyawa et al[20], 2003 | Cohort | Japan | 87 | 7427 |
| Fukui et al[21], 2003 | Cohort | Japan | 31 | 179 |
| Boddi et al[10], 2007 | Cohort | Italy | 31 | 120 |
| Targher et al[15], 2007 | Cohort | Italy | 60 | 60 |
| Butt et al[35], 2009 | Observational cohort | United States | 82083 | 89562 |
| Mostafa et al[22], 2010 | Cross-sectional | Egypt | 329 | 795 |
| Petta et al[23], 2012 | Cohort | Italy | 174 | 174 |
| Adinolfi et al[9], 2013 | Cohort | Italy | 326 | 477 |
| Studies showing no association | ||||
| Bilora et al[28], 2008 | Cohort | Italy | 40 | 40 |
| Caliskan et al[29], 2009 | Cohort | Turkey | 36 | 36 |
| Tien et al[30], 2009 | Cohort | United States | 273 | 1502 |
| Masiá et al[31], 2011 | Cohort | Spain | 63 | 138 |
HCV: Hepatitis C virus.
A large study in Egyptian individuals, including HCV infected patients, HCV subjects with viral clearance, and subjects never infected (controls), showed that the prevalence of carotid atherosclerosis did not vary when patients with active infection were compared to those with past infection. However, HCV infected patients showed a higher risk of atherosclerosis following adjustment for known cardiovascular risk factors[22].
Petta et al[23] evaluating carotid atherosclerosis in a cohort of biopsy-proven chronic hepatitis C genotype 1 patients, reported that HCV patients had a significantly higher prevalence of atherosclerosis than matched control subjects (41.9% vs 22.9%, respectively). In addition, the severity of hepatic fibrosis was independently associated with a higher risk of carotid plaques.
Recently, our group assessed carotid atherosclerosis in a large cohort of consecutive liver biopsy-proven chronic hepatitis C patients with and without steatosis[24]. HCV patients showed a significantly higher prevalence of atherosclerosis than that observed in the HCV-negative control group (53.7% vs 34.3%, respectively, P < 0.0001). HCV patients without steatosis showed a higher prevalence of atherosclerosis than that observed in the HCV-negative control group without steatosis (26.0% vs 14.8%, P < 0.015). HCV patients with steatosis had a prevalence of atherosclerosis significantly higher than NAFLD patients (77.7% vs 57.8%, P < 0.0001). These results provided definite evidence that HCV was strictly associated with the development of atherosclerosis and that patients with HCV-related steatosis, irrespective of HCV genotype, age, gender and degree of histological liver damage, exhibited the highest prevalence of atherosclerosis. Moreover, at multivariate analysis, HCV-related steatosis was an independent risk factor for carotid atherosclerosis (OR = 32.35; 95%CI: 5.4-230, P < 0.0001). HCV-related steatosis predicted atherosclerosis with an AUC of 0.78 (95%CI: 0.71-0.85, P < 0.0001; with a positive specificity of 81.7% and sensitivity of 74.2%). These data suggest that steatosis is both a good marker for identifying atherosclerosis-prone individuals and an early mediator of atherosclerosis. Evidence for the latter role is based on steatosis being associated with traditional pro-atherogenic factors such as metabolic syndrome, insulin resistance, hyper-homocysteinaemia, liver inflammation and fibrosis scores, hypo-adiponectinaemia and hyper-tumour necrosis factor-alpha (TNF-α)[25] and oxidative stress[26]. In brief, our study[24] suggests that HCV-related steatosis modulates atherogenic factors such as inflammation and the dysmetabolic milieu, therefore, favouring development of atherosclerosis. Furthermore, our study[24] showed that amongst the younger HCV population (e.g., < 50 years old) about 34% showed atherosclerosis and a significant proportion (24.1%) had plaques, whereas in the control group the prevalence of atherosclerosis was significantly lower (16.0%) and the presence of plaques (3.9%) was a relatively uncommon finding. Such data support the view that chronic HCV infection predisposes individuals to the premature development of atherosclerosis and advanced carotid changes despite the more favourable cardiovascular risk profile featuring lower lipid levels and lower prevalence of metabolic syndrome[24].
An increased risk of carotid atherosclerosis was also demonstrated in HIV-infected patients. In this setting, Sosner et al[27] reported an increased prevalence of carotid plaque in HIV/HCV co-infected patients, strengthening the view that HCV infection is an independent risk factor of atherosclerosis.
In contrast to the above studies which highlighted a strict association between chronic HCV infection and carotid atherosclerosis, other studies did not confirm such an association. For example, Bilora et al[28] and Caliskan et al[29], in the setting of hemodialysis patients, did not find any association between HCV infection and atherosclerosis. Similarly, in a cohort of HCV-infected women, with and without HIV co-infection, Tien et al[30], after adjustment for confounding factors, were unable to demonstrate an association between HCV infection and atherosclerosis. Likewise, Masiá et al[31] did not observe any association between HCV infection and atherosclerosis in a cohort of HIV co-infected patients.
It is important to underline that those studies showing a positive association, generally included a very large population of subjects, whereas the studies showing no association were generally conducted in smaller cohorts of patients at high risk for atherosclerosis (e.g., either hemodialysed or HCV/HIV co-infected) (Table 1). Considering that atherosclerosis is a multi-factorial disorder, studies with low power may have generated false negative results of doubtful clinical significance. In addition, inconsistency among studies may be the result of different features in the populations under examination, differences in the assessment of HCV infection and stage of liver disease, technique used to evaluate atherosclerosis and, of primary importance, comparator control groups and adjustment for confounding factors. Overall, we deem that an analysis of available studies supports the view that HCV infection should be considered a risk factor for the development of atherosclerosis. Meta-analytic reviews and adequate prospective studies are eagerly awaited to gauge the impact of HCV on the development of atherosclerosis more precisely.
IMPACT OF HCV-ASSOCIATED ATHEROSCLEROSIS ON CARDIAC AND CEREBRAL VASCULAR DISEASES
A consistent body of evidence indicates that chronic HCV infection should be considered a risk factor for subclinical atherosclerosis. Accordingly, the question is: is there evidence that HCV is a risk factor for the development of cardiac and cerebral vascular diseases? Clearly the answer is not straightforward and involves different levels of assessment. First, it is important to evaluate a hard outcome, such as mortality related to cardiovascular diseases in those with HCV infection, and next, studies evaluating such an association need to be weighted.
Evidence for a significant excess of cardiovascular mortality among anti-HCV positive subjects is provided by a large study which assessed HCV-associated all-cause mortality in the general United States population[32]. Moreover, a study conducted in the general population showed that HCV infection was an independent predictor of cerebrovascular deaths and that such mortality was correlated with serum HCV RNA levels[33]. Of importance, HCV clearance by interferon and ribavirin treatment significantly reduces non-liver-related mortality in these patients[34]. The above data suggest a possible direct impact of HCV infection on cardiovascular diseases.
HCV and cardiac diseases
In the last few years many studies have evaluated the association between HCV infection and atherosclerosis. Table 2 reports the main findings of these published papers.
Table 2.
Main characteristics of the studies which evaluated the association between hepatitis C virus and cardiac diseases
| Ref. | Type of study | Country | HCV+ (n) | HCV- (n) | Comment |
| Studies showing association | |||||
| Vassalle et al[14], 2004 | Cohort | Italy | CAD (491), HCV 6.3% | ||
| vs control (195), 2% | |||||
| Alyan et al[37], 2008 | Cohort | Turkey | 139 | 225 | |
| Butt et al[35], 2009 | Cohort | United States | 82083 | 89582 | CAD patients |
| Yelken et al[38], 2009 | Cohort | Turkey | 26 | 26 | Renal transplanted |
| Maruyama et al[39], 2013 | Cohort | Japan | 217 | Myocardial perfusion defect | |
| Younossi et al[40], 2013 | Population | United States | 173 | 19568 | Congestive heart failure |
| Adinolfi et al[9], 2013 | Retrospective | Italy | 78 | 742 | Hospitalized |
| Studies showing no association | |||||
| Völzke et al[16], 2004 | Cross-sectional | Germany | 21 | 4033 | |
| Arcari et al[17], 2006 | Case-control | United States | 292 | 290 | Myocardial infarction |
| Forde et al[36], 2012 | Population cohort | United Kingdom | 4809 | 71668 | Myocardial infarction |
| Younossi et al[40], 2013 | Population | United States | 173 | 19568 | Myocardial infarction |
CAD: Coronary artery disease; HCV: Hepatitis C virus.
Epidemiological and cohort studies have reported conflicting results. Butt et al[35] conducted the largest epidemiological study (82083 HCV-infected and 89582 HCV-uninfected subjects) in United States veterans over a 5-year period. The data showed that HCV infected subjects had a significantly higher prevalence of cardiac diseases (myocardial infarction, congestive heart failure, coronary artery bypass grafting or coronary angioplasty) despite being younger and having a more favourable cardiometabolic risk profile. In contrast, Forde et al[36] in a retrospective analysis which included HCV-infected and -uninfected subjects in a United Kingdom general practice, failed to show any difference in the incidence of myocardial infarction during a median observational period of 3.2 years. However, as acknowledged by these authors[36], the nature of the study, the outcome evaluated and the relative short follow-up period may have affected the results.
In a case-control study, which included 686 patients, Vassalle et al[14] showed that HCV infection was an independent predictor of angiographically-documented CAD (adjusted OR = 4.2; 95%CI: 1.4-13.0). Similar results were reported in another study conducted in patients with CAD[37] and in a study on hemodialysed patients[38]. Two recent studies have further reinforced the possible role of HCV in the development of cardiac diseases. Maruyama et al[39] showed myocardial perfusion defects in 87% of patients with chronic hepatitis C; of interest, such defects improved after viral eradication with interferon treatment. Younossi et al[40], evaluated 19.741 United States subjects from the National Health and Nutrition Examination Survey (NHANES), and showed that chronic HCV infection was independently associated with the presence of metabolic conditions, such as insulin resistance, type 2 diabetes mellitus and hypertension, and with congestive heart failure.
Recently, we conducted a retrospective study including 78 HCV-positive patients compared to 742 HCV-negative subjects[41]. We observed higher ischemic heart events in the HCV-positive patients than in the HCV-negative patients (22% vs 13%, respectively, P = 0.031).
In contrast to the above studies, several other studies did not show an association between HCV infection and cardiac diseases, particularly myocardial infarction. In this context, in a large study including subjects from the NHANES database, Younossi et al[40] showed that chronic HCV infection was independently associated with congestive heart failure, but not ischaemic heart disease. Similarly, Arcari et al[17] and Maruyama et al[39] did not find any association between HCV infection and myocardial infarction. Völzke et al[16], in their study conducted in the Pomeranian general population, Germany, enrolled 233 HCV-Ab positive cases and 4033 control individuals, and did not show an association between HCV seropositivity and cardiovascular diseases.
In conclusion, the findings from a consistent number of studies which showed a strict association between HCV infection and cardiac diseases are in conflict with negative studies, which mainly addressed myocardial infarction as the outcome. Accordingly, at present, due to the different methodology and parameters evaluated, it is difficult to draw definitive conclusions. Despite such limitations, it seems reasonable to conclude that chronic HCV infection appears to be linked with excess cardiovascular risk, except for myocardial infarction. Again, meta-analytic reviews and prospective studies are warranted.
HCV and stroke
In the last 5 years, six papers, reported in Table 3, were published concerning the association between HCV and stroke. In contrast to those previously reported studies on cardiovascular diseases, 5 out of these 6 reports showed an association between HCV infection and ischemic stroke.
Table 3.
Main characteristics of the studies which evaluated the association between hepatitis C virus and stroke
| Ref. | Type of study | Country | HCV+ (n) | HCV- (n) | Comment |
| Studies showing association | |||||
| Forssen et al[42], 2009 | Population retrospective | United States | 21919 | 67109 | |
| Gutierrez et al[43], 2012 | Population retrospective | United States | - | - | NHANES (2005-2010) |
| Liao et al[44], 2012 | Population cohort | Taiwan | 4094 | 16376 | |
| Hsu et al[45], 2013 | Cohort retrospective | Taiwan | 2875 | 12450 | |
| Adinolfi et al[9], 2013 | Cohort retrospective | Italy | 79 | 741 | Hospitalized |
| Studies showing no association | |||||
| Younossi et al[40], 2013 | Population retrospective | United States | 173 | 19568 | NHANES (1999-2010) |
NHANES: National Health and Nutrition Examination Survey; HCV: Hepatitis C virus.
In a large retrospective population-based study, Forssen et al[42] showed an association between HCV infection and stroke (OR = 1.76; 95%CI: 1.23-2.52). This report, however, was published in abstract form alone. Similarly, another retrospective report published by Gutierrez et al[43] in abstract form, showed a close association between HCV infection and stroke (OR = 9.61; 95%CI: 2.51-35.78) in subjects enrolled from the NHANES cohort during 2005-2010. In contrast, in another retrospective study from the NHANES database, between 1999-2010, Younossi et al[40] were not able to demonstrate an association between HCV infection and stroke. However, many confounding factors, such as gender, race, and hypertension, were significantly different between the HCV population and the control group. Therefore, heterogeneity may have generated unreliable results.
In a large prospective study conducted in Taiwan, utilizing the Taiwan National Health Insurance Research Database, Liao et al[44] demonstrated a strict association between HCV infection and stroke (HR = 1.22; 95%CI: 1.13-1.40). Similarly, Hsu et al[45] showed that HCV infection was a risk factor for stroke (HR = 1.23; 95%CI: 1.06-1.42) in a large retrospective study including Taiwan subjects from the Longitudinal Health Insurance database 2000. Of importance, these authors demonstrated that an interferon-based therapy reduced the risk of stroke in HCV patients[45].
We recently conducted a study with the aims of evaluating the prevalence and role of HCV infection in patients with stroke[41]. The study enrolled 820 consecutive individuals, 123 cases with stroke and 697 age- and gender-matched controls. We found that the prevalence of HCV was higher in cases with stroke than in the control group (26.8% vs 6.6%, P = 0.0001). Moreover, HCV patients with stroke were younger and had lower risk factors and higher inflammation indices than HCV-negative stroke patients. Multivariate analysis showed HCV infection to be an independent risk factor for stroke (OR = 2.04; 95%CI: 1.69-2.46, P = 0.0001).
Taken collectively, these data[41,42,44,45] suggest that HCV infection increases the risk of stroke. Moreover, in HCV patients, stroke occurs at younger age, irrespective of sex and in subjects with lower risk of stroke/atherosclerosis such as lower cholesterol/triglyceride levels, and lower prevalence of hypertension[41]. Finally, HCV viral load appears to be associated with an increased risk of mortality in patients with stroke[33].
A meta-analysis of the studies which evaluated whether HCV infection was a risk factor for stroke has been conducted[46]. The results suggested that HCV infection significantly increased the risk of stroke (OR = 1.97; 95%CI: 1.64-2.30). However, due to the relatively low number of studies performed, the authors suggest caution in the interpretation of the data and advocate further studies to confirm the results[46].
HCV AND ATHEROSCLEROSIS: PATHOGENIC MECHANISMS
The precise pathogenic mechanisms connecting HCV infection, chronic liver disease, and atherogenesis are not completely understood. Figure 1 depicts the potential pathogenic factors involved. It is logical to hypothesize that HCV may promote atherogenesis through several direct and indirect biological mechanisms. Atherosclerotic plaques are widely recognized to develop and to destabilize as a result of persistent inflammatory changes. Chronic HCV infection is associated with both hepatic and systemic inflammation, which is triggered by HCV either directly or indirectly. Such inflammatory pathways involve cytokines release and increased oxidative stress. HCV RNA sequences have been isolated within carotid plaques, supporting the hypothesis that HCV plays a direct pro-atherogenic role by inducing arterial inflammation, likely via the pro-inflammatory cytokine interleukin 1β[47]. In addition, HCV structural and non-structural proteins play a major role in initiating and maintaining chronic inflammation. HCV promotes an imbalanced T helper (Th)1/Th2 cytokines ratio perturbing the equilibrium between cellular immunity, promoted and maintained by interleukin (IL)-2, TNF-α and interferon-γ, and humoral immunity, sustained by IL-4, IL-5, IL-6 and IL-10[48]. Compared to HCV negative individuals, patients with chronic HCV infection have been shown to exhibit higher TNF-α levels which are associated with an increased risk of heart failure and death[49]. Moreover, an intermediate cardiovascular risk, higher levels of pro-inflammatory cytokines (IL-6 and TNF-α), and a higher ratio of pro-inflammatory/anti-inflammatory cytokines (TNF-α/IL-10 and IL-6/IL-10) have been reported in non-obese, non-diabetic, HCV-infected patients than that observed in the control group[50]. HCV-driven secretion of inflammatory cytokines may contribute to the development of cardiovascular disease through several effector mechanisms, including enhanced synthesis of matrix metalloproteinase-9, intracellular adhesion molecules, expression of anti-endothelium antibodies, and generation of oxidative stress and insulin resistance.
Figure 1.
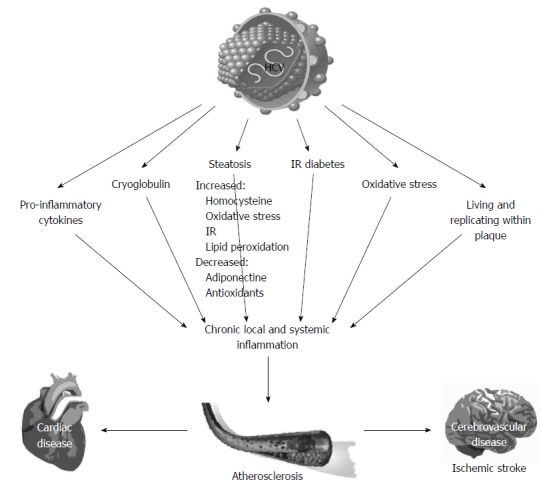
Pathogenic mechanisms associated with the development of atherosclerosis in chronic hepatitis C infection. HCV: Hepatitis C virus; IR: Insulin resistance.
The higher prevalence of atherosclerosis observed in chronic HCV infection is correlated with liver steatosis[24], a key feature of HCV infection which is associated with several pro-atherogenic factors including inflammatory cytokines, hyper-homocysteinaemia, hypo-adiponectinaemia, insulin resistance, and components of the metabolic syndrome[24].
Atherosclerosis in HCV patients is also associated with liver fibrosis[23] which is an expected finding given the tight association between steatosis and fibrosis in those with chronic hepatitis C[9]. Histological hepatic changes play a pivotal role in regulating local and systemic inflammation through proteins secreted by the diseased liver[4].
HCV is considered a metabolic virus and is associated with metabolic disorders, in particular insulin resistance and type 2, which are diabetes pro-atherogenic conditions.
HCV infection is associated with increased levels of endotoxaemia, which promotes a strong inflammatory reaction via TNF-α and toll-like receptors, which are both able to induce a marked inflammatory reaction. Of interest, HCV is also the agent causing mixed cryoglobulinaemia a paradigmatic vasculitic condition[4].
Based on the above considerations it is clear that chronic HCV infection represents a condition directly and indirectly promoting a milieu of pro-atherogenic factors, mainly pro-inflammatory molecules, which trigger atherogenesis and favour its complications eventually leading to an increased prevalence of cardiovascular diseases. A more detailed understanding of the complex mechanisms underlying HCV-related inflammation and atherogenesis is of key importance for the development of novel therapeutic approaches targeting the different steps of the inflammatory response to prevent and treat atherosclerosis in patients with chronic HCV infection.
CONCLUSION
The literature seems to support the view that chronic HCV infection is a risk factor for atherosclerosis, the development of cardiovascular diseases and significant cardiovascular mortality. However, further well-conducted studies are necessary to better assess the impact of HCV on the different cardiovascular conditions, in particular on myocardial infarction. The pathogenic mechanisms should be further elucidated due to their potential impact on the development of novel therapeutic approaches to prevent and to treat cardiovascular complications in patients with chronic HCV infection.
Based on the evidence discussed in the present article, we feel it reasonable to recommend to screen noninvasively for atherosclerosis all patients with chronic HCV infection. Knowledge of the whole pathologic burden will be of help in making a reasoned decision for the management of chronic HCV infection. In this respect, it is important to underline that existing data seem to indicate that interferon-based treatment reduces the risk of stroke and of cardiovascular-related mortality. Future studies should clarify the full impact and the right timing of antiviral treatment in preventing, improving or reversing HCV-related atherosclerosis.
Footnotes
Supported by A grant by Regione Campania, Italy
P- Reviewers: Dang SS, Fusco DN S- Editor: Gou SX L- Editor: Webster JR E- Editor: Ma S
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Zignego AL, Craxì A. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 2008;12:611–636, ix. doi: 10.1016/j.cld.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011;12:2215–2234. doi: 10.1517/14656566.2011.597742. [DOI] [PubMed] [Google Scholar]
- 4.Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–540. doi: 10.4254/wjh.v5.i10.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert A, Lion G. Arterites infectieuses experimentales. C R Hebd Seances Soc Biol Fil. 1889;41:583–584. [Google Scholar]
- 6.Stassen FR, Vainas T, Bruggeman CA. Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep. 2008;60:85–92. [PubMed] [Google Scholar]
- 7.Loria P, Lonardo A, Targher G. Is liver fat detrimental to vessels?: intersections in the pathogenesis of NAFLD and atherosclerosis. Clin Sci (Lond) 2008;115:1–12. doi: 10.1042/CS20070311. [DOI] [PubMed] [Google Scholar]
- 8.Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595–599. doi: 10.1016/j.jhep.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Adinolfi LE, Restivo L, Marrone A. The predictive value of steatosis in hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2013;7:205–213. doi: 10.1586/egh.13.7. [DOI] [PubMed] [Google Scholar]
- 10.Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, Pratesi G, Pratesi C, Gensini G, Zignego AL. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 Suppl 1:S55–S60. doi: 10.1016/s1590-8658(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 11.Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L, et al. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72–75. doi: 10.1016/j.jcv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaka N, Ishizaka Y, Takahashi E, Tooda Ei, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26–30. doi: 10.1253/circj.67.26. [DOI] [PubMed] [Google Scholar]
- 14.Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–566. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Völzke H, Schwahn C, Wolff B, Mentel R, Robinson DM, Kleine V, Felix SB, John U. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99–103. doi: 10.1016/j.atherosclerosis.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43:e53–e56. doi: 10.1086/507031. [DOI] [PubMed] [Google Scholar]
- 18.Guiltinan AM, Kaidarova Z, Custer B, Orland J, Strollo A, Cyrus S, Busch MP, Murphy EL. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743–750. doi: 10.1093/aje/kwm370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomiyama H, Arai T, Hirose K, Hori S, Yamamoto Y, Yamashina A. Hepatitis C virus seropositivity, but not hepatitis B virus carrier or seropositivity, associated with increased pulse wave velocity. Atherosclerosis. 2003;166:401–403. doi: 10.1016/s0021-9150(02)00388-x. [DOI] [PubMed] [Google Scholar]
- 21.Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA. 2003;289:1245–1246. doi: 10.1001/jama.289.10.1245-b. [DOI] [PubMed] [Google Scholar]
- 22.Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M, et al. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
- 23.Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G, et al. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317–1323. doi: 10.1002/hep.25508. [DOI] [PubMed] [Google Scholar]
- 24.Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349–1357. doi: 10.1111/j.1365-2036.2006.03114.x. [DOI] [PubMed] [Google Scholar]
- 26.Vidali M, Tripodi MF, Ivaldi A, Zampino R, Occhino G, Restivo L, Sutti S, Marrone A, Ruggiero G, Albano E, et al. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J Hepatol. 2008;48:399–406. doi: 10.1016/j.jhep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Sosner P, Wangermez M, Chagneau-Derrode C, Le Moal G, Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis. 2012;222:274–277. doi: 10.1016/j.atherosclerosis.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Bilora F, Campagnolo E, Rinaldi R, Rossato A, Arzenton M, Petrobelli F. Carotid and femoral atherosclerosis in chronic hepatitis C: a 5-year follow-up. Angiology. 2008;59:717–720. doi: 10.1177/0003319707311536. [DOI] [PubMed] [Google Scholar]
- 29.Caliskan Y, Oflaz H, Pusuroglu H, Boz H, Yazici H, Tamer S, Karsidag K, Yildiz A. Hepatitis C virus infection in hemodialysis patients is not associated with insulin resistance, inflammation and atherosclerosis. Clin Nephrol. 2009;71:147–157. doi: 10.5414/cnp71147. [DOI] [PubMed] [Google Scholar]
- 30.Tien PC, Schneider MF, Cole SR, Cohen MH, Glesby MJ, Lazar J, Young M, Mack W, Hodis HN, Kaplan RC. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women’s interagency HIV study. AIDS. 2009;23:1781–1784. doi: 10.1097/QAD.0b013e32832d7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masiá M, Padilla S, Robledano C, Ramos JM, Gutiérrez F. Evaluation of endothelial function and subclinical atherosclerosis in association with hepatitis C virus in HIV-infected patients: a cross-sectional study. BMC Infect Dis. 2011;11:265. doi: 10.1186/1471-2334-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]
- 34.Berenguer J, Rodríguez E, Miralles P, Von Wichmann MA, López-Aldeguer J, Mallolas J, Galindo MJ, Van Den Eynde E, Téllez MJ, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55:728–736. doi: 10.1093/cid/cis500. [DOI] [PubMed] [Google Scholar]
- 35.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forde KA, Haynes K, Troxel AB, Trooskin S, Osterman MT, Kimmel SE, Lewis JD, Lo Re V. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19:271–277. doi: 10.1111/j.1365-2893.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, Celebi AS, Ilkay E. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960–1965. doi: 10.1253/circj.cj-08-0459. [DOI] [PubMed] [Google Scholar]
- 38.Yelken B, Gorgulu N, Caliskan Y, Elitok A, Cimen AO, Yazici H, Oflaz H, Turkmen A, Sever MS. Association between chronic hepatitis C infection and coronary flow reserve in dialysis patients with failed renal allografts. Transplant Proc. 2009;41:1519–1523. doi: 10.1016/j.transproceed.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama S, Koda M, Oyake N, Sato H, Fujii Y, Horie Y, Murawaki Y. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol. 2013;58:11–15. doi: 10.1016/j.jhep.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–652. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 41.Adinolfi LE, Restivo L, Guerrera B, Sellitto A, Ciervo A, Iuliano N, Rinaldi L, Santoro A, Li Vigni G, Marrone A. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22–26. doi: 10.1016/j.atherosclerosis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Forssen U, McAfee A, Enger C, Bennett D, Shantakumar S. Risk of thromboembolic events (TEs) among patients infected with hepatitis C. Hepatology. 2009;50:672A. [Google Scholar]
- 43.Gutierrez J, Elkind MSV. Chronic inflammatory diseases and stroke: Evidence for heterogeneous mechanisms. Ann Neurol. 2012;72:S6–S7. [Google Scholar]
- 44.Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One. 2012;7:e31527. doi: 10.1371/journal.pone.0031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu CS, Kao JH, Chao YC, Lin HH, Fan YC, Huang CJ, Tsai PS. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 46.He Huang R, Zhao Z. Hepatitis C virus infection and risk of stroke: a systematic review and meta-analysis. PLoS One. 2013;8:e81305. doi: 10.1371/journal.pone.0081305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costantini S, Capone F, Guerriero E, Maio P, Colonna G, Castello G. Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur Cytokine Netw. 2010;21:251–256. doi: 10.1684/ecn.2010.0214. [DOI] [PubMed] [Google Scholar]
- 48.Abbas Z, Moatter T. Interleukin (IL) 1beta and IL-10 gene polymorphism in chronic hepatitis C patients with normal or elevated alanine aminotransferase levels. J Pak Med Assoc. 2003;53:59–62. [PubMed] [Google Scholar]
- 49.Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451–456. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira CP, Kappel CR, Siqueira ER, Lima VM, Stefano JT, Michalczuk MT, Marini SS, Barbeiro HV, Soriano FG, Carrilho FJ, et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013;164:221–226. doi: 10.1016/j.ijcard.2011.07.016. [DOI] [PubMed] [Google Scholar]


