Abstract
Despite the advent of biological products, such as anti-tumor necrosis factor-α monoclonal antibodies (infliximab and adalimumab), for treatment of moderate to severe cases of inflammatory bowel disease (IBD), most patients depend upon aminosalicylates as the conventional treatment option. In recent years, the increased knowledge of complex pathophysiological processes underlying IBD has resulted in development of a number of newer pharmaceutical agents like low-molecular-weight heparin, omega-3 fatty acids, probiotics and innovative formulations such as high-dose, once-daily multi-matrix mesalamine, which are designed to minimize the inflammatory process through inhibition of different targets. Optimization of delivery of existing drugs to the colon using the prodrug approach is another attractive alternative that has been utilized and commercialized for 5-aminosalicylic acid (ASA) in the form of sulfasalazine, balsalazide, olsalazine and ipsalazine, but rarely for its positional isomer 4-ASA - a well-established antitubercular drug that is twice as potent as 5-ASA against IBD, and more specifically, ulcerative colitis. The present review focuses on the complete profile of 4-ASA and its advantages over 5-ASA and colon-targeting prodrugs reported so far for the management of IBD. The review also emphasizes the need for reappraisal of this promising but unexplored entity as a potential treatment option for IBD.
Keywords: 4-Aminosalicylic acid; 5-Aminosalicylic acid; Sulfasalazine; Colon-specific prodrug; Inflammatory bowel disease; Ulcerative colitis; 2,4,6-trinitrobenzene sulphonic acid; Experimental colitis
Core tip: Anti-inflammatory activity of antitubercular drug 4-aminosalicylic acid (ASA) was first described by Lover in 1984. Since then, numerous clinical trials were carried out to establish its efficacy in left-sided and active/quiescent ulcerative colitis. It is 50% more potent than 5-ASA against inflammation and does not produce 5-ASA-induced immunoallergic acute pancreatitis. 4-ASA is a stable and inexpensive alternative to 5-ASA in patients with acute pancreatitis. Despite all these positive findings, an extensive literature review surprisingly revealed few colon-targeting delivery systems for 4-ASA. The present review presents a complete profile of 4-ASA and its colon-specific prodrugs for inflammatory bowel disease.
INTRODUCTION
Anti-inflammatory activity of antitubercular drug 4-aminosalicylic acid (ASA) was first described by Lover in 1984. Since then, numerous clinical trials were carried out to establish its efficacy in left-sided and active/quiescent ulcerative colitis. It is 50% more potent than 5-aminosalicylic acid (5-ASA) against inflammation and does not produce 5-ASA-induced immunoallergic acute pancreatitis. 4-ASA is a stable and inexpensive alternative to 5-ASA in patients with acute pancreatitis. Despite all these positive findings, an extensive literature review surprisingly revealed few colon-targeting delivery systems for 4-ASA. The present review presents a complete profile of 4-ASA and its colon-specific prodrugs for inflammatory bowel disease (IBD).
IBD
Ulcerative colitis (UC) and Crohn’s disease (CD) are two distinct idiopathic, chronic and relapsing inflammatory disorders of the gastrointestinal tract (GIT) that are grouped under the term IBD. Dysregulation of immune responses and upregulation of proinflammatory mediators are the two hallmarks of IBD that make it recurrent and nearly incurable[1]. UC is characterized by distinct episodes of active and inactive disease in 80%-90% of patients. Repetitive cycles of active and quiescent disease with varying clinical patterns are also observed in CD[2].
UC is one of the most common chronic inflammatory forms of IBD and is characterized by diffused mucosal inflammation, mainly limited to the large intestine and rectum. UC is characterized by diarrhea, rectal bleeding and abdominal pain. Inflammation of the rectum is common and generally extends proximally in a continuous fashion[3]. CD is a patchy transmural granulomatous inflammation that affects any part of the GIT and has a predilection for the terminal ileum and colon[4].
Despite the differences between UC and CD, both forms of IBD cause similar symptoms. UC is characterized by mild symptoms like progressive loosening of the stools, abdominal cramping, and diarrhea. In the severe form of the disease, patients may also experience weight loss, fatigue, and loss of appetite that may result in nutrient deficiencies, mucus in the stools, severe rectal bleeding, fever, and anemia. In CD, abdominal pain, diarrhea and weight loss are often the earliest signs; constitutional symptoms include malaise, lethargy, anorexia, nausea, vomiting and low-grade fever. CD can be complicated by the development of intestinal obstruction, fistulae and perianal disease. Fistulae develop in about one-third of patients. Perianal disease is a frequent complication of colonic and ileocolonic disease and is characterized by fissures, fistulae and abscesses[5,6].
The pathogenesis of IBD is obscure and is considered to involve multifactorial interactions amongst genetic, immunological and environmental triggers. The key factor differentiating individuals with IBD from normal ones is their inability to downregulate the uncontrolled and chronic inflammatory state. The pathological findings associated with IBD are: an increase in certain inflammatory mediators, signs of oxidative stress, a deranged colonic milieu, abnormal glycosaminoglycan content of the mucosa, decreased oxidation of short chain fatty acids, increased intestinal permeability, increased sulfide production, and decreased methylation. Although no single factor has been identified as the initial trigger for IBD, the etiological factors have been elucidated[7]. However, no guaranteed curative therapeutic regimen has been developed so far.
IBD THERAPY
Therapy for IBD has not seen major changes or breakthroughs during the past 4-5 decades and still revolves around the single nonsteroidal anti-inflammatory drug 5-ASA (Figure 1A) and its colon-targeting prodrug sulfasalazine (Figure 1B), followed by second-line treatment with steroids and immunomodulators, because reducing the extent and severity of colonic inflammation remains the primary focus of treatment. Antibiotics, probiotics, and nutritional supplements are used as supportive therapy. The use of anti-tumor necrosis factor monoclonal antibody (infliximab), recombinant anti-inflammatory cytokines, and related gene therapy are recent advances in the field[8,9].
Figure 1.
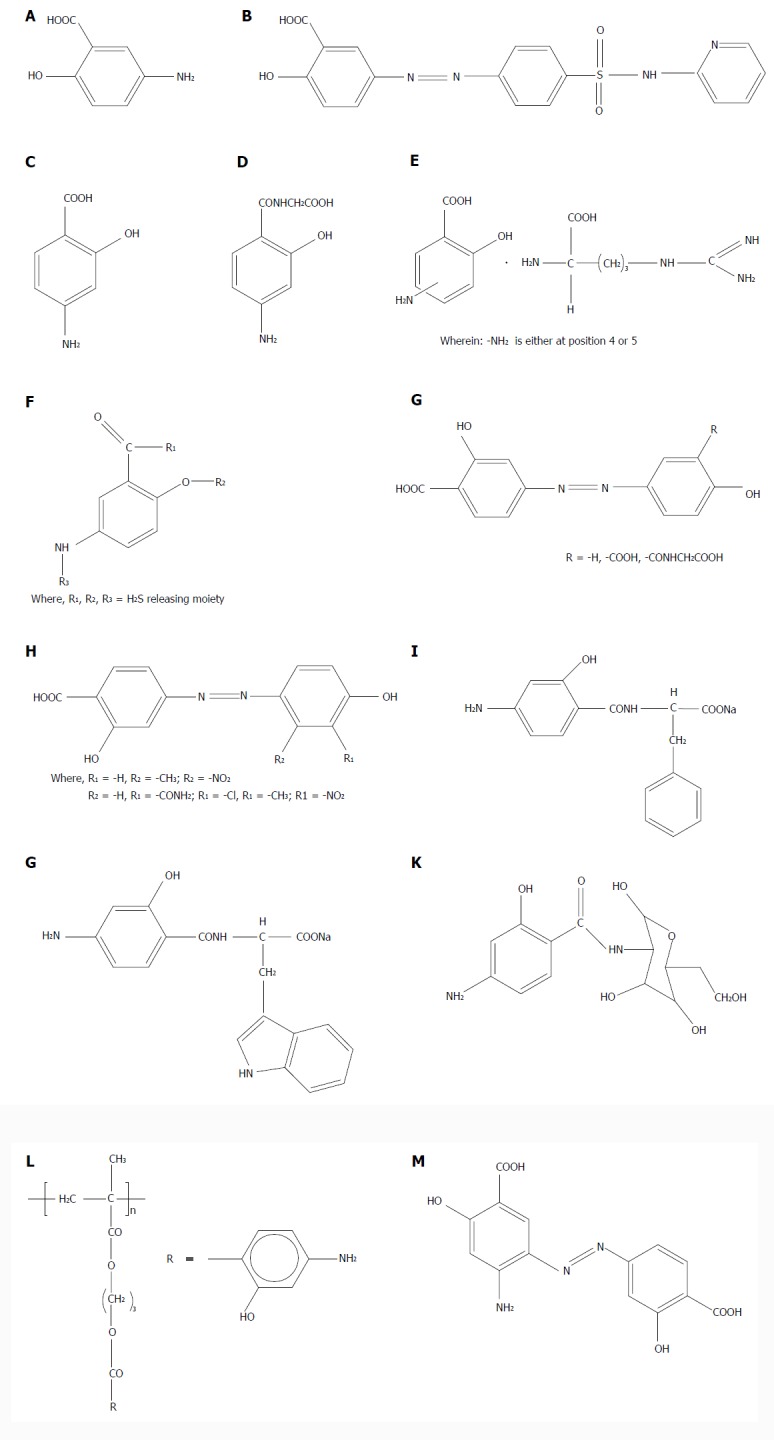
Chemical structures of aminosalicylates and their colon-targeting prodrugs. A: 5-ASA; B: Sulfasalazine; colon-targeting prodrug of 5-ASA with sulfapyridine; C: 4-ASA; D: Amide prodrug of 4-ASA with glycine; E: L-arginine salts of 4-ASA and 5-ASA; F: H2S-releasing prodrugs of 5-ASA and 4-ASA; G: Prodrugs of 4-ASA with salicylic acid, hydroxybenzene and N-salicyloyl glycine; H: Prodrugs of 4-ASA with substituted phenols; I: amide conjugate of 4-ASA with D-phenylalanine; J: Amide conjugate of 4-ASA with L-tryptophan; K: Amide prodrug of 4-ASA with D-glucosamine; L: Polymeric prodrug of 4-ASA; M: Prodrug of 4-ASA with 4-ASA (4A-4AAz).
High and repeated doses of 5-ASA are necessary for maintenance and preventive therapy of IBD relapse. The majority of orally administered 5-ASA is readily and extensively absorbed from the stomach and small intestine, leaving a small amount that is transported to the colon. 5-ASA is absorbed from the upper GIT, causes many systemic side effects, and at the same time, results in poor bioavailability at the site of action, namely, the colon. Bypassing absorption in the upper GIT and targeting the delivery of 5-ASA to the colon by designing colon-specific prodrugs is the rational approach to improve the bioavailability at the site of action. The earliest reported colon-specific prodrug of 5-ASA is sulfasalazine, which is extensively prescribed for the treatment of UC. It is an azo conjugate of 5-ASA with sulfapyridine. However, its sulfapyridine part is used as a carrier and is completely absorbed through the colon and produces adverse effects such as hypersensitivity, impotency, blood dyscrasia, hepatitis, abnormal liver function tests and hepatic failure, agranulocytosis, leukopenia, thrombocytopenia, hemolytic anemia, and cyanosis[10-12]. Although 5-ASA derivatives have an excellent safety profile, some undesirable effects may occur; the most prominent being immunoallergic acute pancreatitis[13,14]. These side effects contraindicate the further use of all 5-ASA-containing drugs and pose a problem, because they limit treatment of acute phases of the disease to corticosteroids and relapse to immunosuppressants. 4-ASA has also been shown to be effective in IBD; in enemas or in oral formulations[15-18].
4-ASA
4-ASA (p-aminosalicylic acid, 4-amino-2-hydroxybenzoic acid) (Figure 1C), is a comparatively safe antitubercular agent that has been used for many years in resorbable high oral doses (ranging from 8 to 12 g/d) in multidrug-resistant tuberculosis. It has a bacteriostatic effect on Mycobacterium tuberculosis and inhibits resistance against streptomycin and isoniazid. Chemically, it is distinguished from its position isomer 5-ASA by the position of the NH2 group, although they share the salicylic acid backbone. Therefore, their method of synthesis is also different. 4-ASA is prepared by heating 3-aminophenol with potassium bicarbonate or ammonium carbonate under pressure, while 5-ASA is prepared by zinc/hydrochloric acid-assisted reduction of m-nitrosalicylic acid[19]. 5-ASA is unstable and is degraded rapidly into a dark purple quinone containing tar but does not decarboxylate in the presence of moisture. However, 4-ASA is readily decarboxylated in the presence of moisture, but unlike 5-ASA, is slowly degraded into brown to purple material[20,21]. In view of the well-recognized chemical distinction between 4-ASA and 5-ASA, it is surprising to discover that not only does 4-ASA have anti-inflammatory activity, but it is also about 50% more potent than 5-ASA.
Lover reported in animal models that 4-ASA may have anti-inflammatory properties comparable with those of 5-ASA[22]. Numerous clinical studies have shown that 4-ASA is highly effective in the topical treatment of active ulcerative proctitis or active left-sided UC[23]. Moreover, slow release tablets of 4-ASA are effective in active UC[17]. 4-ASA has been suggested as an effective treatment for both active and quiescent UC. 5-ASA is well established for maintenance treatment of inactive UC. Moreover, recent studies suggest that 5-ASA may also be effective in maintaining remission in Crohn’s colitis. The efficacy of 1 year maintenance treatment with oral 4-ASA (1.5 g/d, slow release tablets, n = 19) and oral 5-ASA (1.5 g/d, slow release tablets, n = 21) was compared in a randomized double-blind trial in patients with quiescent Crohn’s ileocolitis. That study concluded that 4-ASA may be as effective as 5-ASA for maintenance treatment of quiescent CD and there were no differences in the severity of relapse between both treatment groups[18]. In contrast to 5-ASA, no nephrotoxicity related to 4-ASA has been reported. The only other study that compared these two compounds examined the response to topical treatment in left-sided colitis and showed that 4-ASA and 5-ASA were equally effective[24].
Mechanism of action of 4-ASA
The anti-inflammatory mechanism by which the aminosalicylates exert their therapeutic action is not well established but several hypotheses have been documented. 5-ASA is a potent inhibitor of arachidonic acid metabolism, decreasing the synthesis of both leukotrienes and prostaglandins. Moreover, 5-ASA is a potent scavenger of free radicals[25-27].
In contrast, 4-ASA does not seem to have an inhibitory effect on the lipoxygenation of arachidonic acid and is ineffective as a radical scavenger, but it is thought to act via nuclear factor (NF)-κB inhibition[28]. Both drugs, however, inhibit in vitro activation of T and B lymphocytes by pokeweed mitogen in a dose-dependent manner[29,30]. These findings suggest that the use of either drug in IBD may decrease the heightened state of lamina propria lymphocyte activation as a part of their therapeutic action[31]. Moreover, mechanisms not affecting arachidonic acid metabolism and superoxide release may contribute to the therapeutic potential of both drugs in IBD[32].
The most common side effects of 4-ASA include nausea, vomiting, and epigastric pain. Less frequent side effects are fever, joint pains, skin eruptions, and hepatitis; all of which may be attributed to hypersensitivity reactions[33]. More serious side effects are seen only rarely, such as leukopenia, thrombocytopenia, and agranulocytosis[33]. Because of the lower incidence of serious side effects, it was thought that delayed release formulation of 4-ASA at higher doses could be used in IBD with higher therapeutic efficacy than seen in previous studies. Schreiber et al[18] concluded that oral 4-ASA may be as effective as 5-ASA for maintenance treatment of remission in CD. Several other studies have suggested that 4-ASA is effective in the treatment of active CD or UC; both as a topical formulation (enema) or as an oral slow-release tablet[18].
The risk of cross intolerance reaction between 5-ASA and 4-ASA was evaluated by Gisbert et al[34]. They reported three cases of 5-ASA-induced pancreatitis, with no recurrence of pancreatitis during subsequent treatment with 4-ASA enemas. They concluded that 4-ASA enemas are a safe and well-tolerated therapeutic alternative whenever 5-ASA-induced pancreatitis occurs. 4-ASA has been used in the treatment of mild to moderate UC in patients intolerant of sulfasalazine, and in the treatment of CD[35]. It has been designated an orphan drug by the United States FDA for use in these conditions[36].
Pharmacokinetics of 4-ASA
4-ASA is readily absorbed from the GIT. It is distributed into various tissues and fluids including peritoneal fluid, pleural fluid and synovial fluid in concentrations approximately equal to plasma concentrations of the drug. Cerebrospinal fluid concentrations of 4-ASA are reported to be 10%-50% of concurrent plasma concentrations of the drug in patients with inflamed meninges. 4-ASA is 50%-73% bound to plasma proteins. The plasma half-life of 4-ASA is about 1 h. Plasma concentrations of the drug are not substantially affected by renal or hepatic insufficiency; however, the half-lives of the inactive metabolites may be prolonged in patients with impaired renal function. 4-ASA is inactivated in the intestinal mucosa and liver primarily by acetylation. The major metabolites are N-acetyl-p-aminosalicylic acid and p-aminosalicyluric acid. 4-ASA and its metabolites are excreted in urine by glomerular filtration and tubular secretion. After 2 h in simulated gastric acid, 10% of the dose of unprotected (nonenteric coated) aminosalicylic acid is decarboxylated to form m-aminophenol, a known hepatotoxin[37].
Dose of 4-ASA
4-ASA is about 50% more potent than 5-ASA, therefore, its therapeutic dose is about 60% of the effective dose of sulfasalazine or 5-ASA on a molar basis. The recommended daily dosage of sulfasalazine is 3-4 g (6-8 tablets) for adults and 40-60 mg/kg/d (divided into 3-4 doses) for children (aged > 6 years). On this basis, a dosage regimen of 0.5-0.75 g of 4-ASA divided into two or three doses can be used[38].
Contraindications of 4-ASA
4-ASA is contraindicated in patients who are hypersensitive to ASA or who have severe renal diseases, hepatic disease or gastric ulcers[39].
Interactions of 4-ASA
4-ASA impairs GI absorption of rifampin, reduces rate of acetylation of isoniazid, decreases the GI absorption of digoxin, and enhances the hypoprothrombinemic effect of oral anticoagulants. Concomitant probenecid increases the serum concentration of 4-ASA, ammonium chloride increases probability of crystalluria during therapy, and diphenhydramine impairs its absorption.
Pregnancy
4-ASA is a FDA pregnancy category C drug.
Adverse effects of 4-ASA
The most frequent adverse effect of 4-ASA is GI disturbance including nausea, vomiting, abdominal pain and diarrhea. The acidic carboxylic group of 4-ASA is responsible for the gastrointestinal (GI) irritation. Occurrence of peptic ulcers and gastric hemorrhage is rare. Adverse GI effect can be minimized in patients by administration of the drug with food or discontinuation of the drug when symptoms are severe. Malabsorption of vitamin B12, folic acid, iron and lipids is also observed. Reported hypersensitive reactions include fever, skin eruptions of various types, pruritis, vasculitis, exfoliative dermatitis, joint pain, eosinophilia, leucopenia, agranulocytosis and thrombocytopenia[39,40].
COLON-SPECIFIC PRODRUGS OF 4-ASA
Extensive clinical studies have shown the effectiveness and safety of 4-ASA for the topical treatment of active ulcerative proctitis or left-sided UC[23]. It presents many advantages over 5-ASA, such as: more stability, more potency, and absence of incidences of pancreatitis, but shares the same drawback of fast and extensive absorption in the upper GIT, before it reaches the colon. This is due to its weakly acidic nature (pKa 3-4), which results in its ready absorption in the upper GIT[16]. Studies by Selby suggest that 4-ASA is a stable, inexpensive alternative to 5-ASA for the topical treatment of UC or for linking to carrier molecules for release in the colon[24].
Many colon-specific prodrugs of 5-ASA have been cited in the literature, while the prodrug approach has been only sporadically applied for colonic delivery of 4-ASA, for reasons that are not known. The possible reason could be the steric problem of 4-ASA, due to which it cannot be linked with same polymers (e.g., dextran, chitin, hydroxymethyl cellulose or synthetic polymers) as 5-ASA or to other polymers (e.g., cyclodextrins) using the same methods that have been used for 5-ASA[20].
Zhao et al[41] were the first to design a colon-targeting amide prodrug of 4-ASA (Figure 1D) with the nonessential amino acid glycine for the treatment of IBD. The amide prodrug was synthesized by reacting amino and hydroxyl protected 4-ASA acyl chloride with glycine, followed by deprotection. In vivo experiments on rats suggested that 4-aminosalicylglycine showed more curative effect than 4-ASA.
Wallace et al[42] have patented L-arginine salts of 4-ASA and 5-ASA (Figure 1E) for inflammatory conditions of the GIT, and for irritable bowel syndrome (IBS). They claim that the salt is more effective in reducing the inflammation in the colon compared to individual drugs or L-arginine, as demonstrated from overall greater reduction in the colitis-associated edema, granulocyte infiltration, and body weight loss with enhanced free-radical scavenging and more potent antioxidant activity, thus providing a synergistic effect. They ascribe the synergistic effect to the ability of arginine to act as a source of nitrogen for NO, which is a potent vasodilator that attenuates intestinal tissue damage[43,44].
A US Patent describes 5-ASA and 4-ASA prodrugs as having an H2S-releasing moiety linked via an azo, ester, anhydride, thioester or amide linkage (Figure 1F) for the treatment of IBD and IBS and chemoprevention of colon cancer[45]. The basis for using H2S-releasing carriers in this design is their encouraging and beneficial pharmacological profile. There is documented evidence for antinociceptive effect of H2S in the GIT by activating KATP channels and smooth muscle relaxant activity in intestinal tissue[46].
Based on the design of sulfasalazine and using azo bond chemistry, Zhao et al[47] have reported the synthesis of prodrugs of 4-ASA with salicylic acid, hydroxybenzene and N-salicyloyl glycine (Figure 1G), aiming to target 4-ASA to the colon for management of IBD with enhanced efficacy and fewer side effects. However, we could not find any reports of activity of these prodrugs in any of the experimental models of IBD.
Oxidative stress is one of the key players in the pathogenesis of IBD causing cellular injury and colonic inflammation[48]. Keeping this in mind, Sheng et al[49] designed azo-linked colon-specific prodrugs of 4-ASA (Figure 1H) with several substituted phenols such as m-nitrophenol, salicylamide, o-chlorophenol, m-methylphenol, o-methylphenol, o-nitrophenol and o-dihydroxybenzene, possessing antioxidant properties. Their main aim was to achieve co-antioxidant effects of 4-ASA and substituted phenol that would result in higher reducibility and synergistic free-radical scavenging. These phenols were chosen on the basis of their reducibility, electronic and steric factors. 4-(3’,4’-dihydroxyphenyl) azobenzene-2-acetylsalicylic acid, 4-(2’-methyl-4’-hydroxyphenyl) azobenzene salicylic acid and 4-(3’-methyl-4’-hydroxyphenyl) azobenzene salicylic acid were reported to be the compounds with maximum anti-inflammatory and antioxidant activities.
Dhaneshwar et al[12] have explored mutual amide prodrug strategy for 5-ASA with several amino acids. Applying the same concept further to 4-ASA, they have reported its amide conjugates with D-phenylalanine (Figure 1I) and L-tryptophan (Figure 1G). The proposed mechanism of activation was enzymatic by the action of N-acyl amidases secreted by colonic bacteria. The amino acids used in the design were nontoxic carriers like D-phenylalanine and L-tryptophan, possessing wound healing and anti-inflammatory activities. The prodrugs furnished 86%-91% release of 4-ASA in rat fecal matter in 20 h. Their curative effect was investigated in a 2,4,6-trinitrobenzene-sulfonic-acid-induced experimental colitis model in rats. Pancreas, liver and stomach of rats were studied by histopathology for the assessment of any adverse reactions. The prodrugs were found to possess significantly superior safety profile than sulfasalazine, oral 4-ASA and 5-ASA, with comparable efficacy to sulfasalazine.
In an innovative attempt, colon-specific 4-ASA-D-glucosamine amide prodrug (Figure 1K) was developed by Dhaneshwar et al[50] out of the urgent need to search for such alternate options that would not produce 5-ASA-induced pancreatitis and sulfapyridine-induced hepatitis. The hypothesis was to offer supplementation of D-glucosamine that would suppress activation of intestinal epithelial cells, thus helping to maintain the integrity of the colonic architecture. The protective effect of this prodrug was compared with 5-ASA-D-glucosamine conjugate as well as standard drugs like sulfasalazine, 4-ASA and 5-ASA on the course of 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. The mitigating effect of 5-ASA prodrug was comparable to that of sulfasalazine, while that for 4-ASA prodrug was moderate. However, hepatitis or pancreatitis was not seen with 4-ASA prodrug[50].
A polymeric prodrug of 4-ASA (Figure 1L) with an acrylic polymeric system and degradable ester bonds, such as hydroxy propyl methacrylate, was synthesized and evaluated for colon-targeted drug delivery by Yadav et al[51]. In the first 2 h, 40.01% release was observed in rat fecal matter at pH 7.4, which was followed by sustained release of 93% over a period of 12 h. The authors propose that the developed delivery system could be useful for controlled release, prolonged transit time, and colon-targeted delivery of 4-ASA.
A macromolecular colon-targeting ester prodrug of 4-ASA with β-cyclodextrin as a promoiety was reported very recently by Vadnerkar et al[52]. Interestingly, 20%-23% release of 4-ASA was observed in the stomach and small intestinal homogenates, while the prodrug was found to be resistant to pH-dependent hydrolysis at pH = 1.2 and 7.4. Almost 68% and 92% release was obtained in rat cecal and fecal matter, respectively. The prodrug demonstrated a moderate ameliorating effect on TNBS-induced colitis as compared to sulfasalazine or 4/5-ASA administered rectally, but it was similar to that with orally administered aminosalicylates. Partial activation of the prodrug in the upper GIT homogenates causing incomplete transport of 4-ASA to the site of action could be responsible for its moderate effect. However, the prodrug was reported to be safe, producing no harmful effects on the stomach, liver or pancreas of rats[52].
In another innovation, Suneela et al[53] designed four azo prodrugs of 4- and 5-ASA using the same aminosalicylates as carriers. As known already, olsalazine is a dimer of 5-ASA, which is synthesized by coupling diazo salt of 5-ASA with salicylic acid, which upon reduction by azo reductase, generates two molecules of 5-ASA. However, the azo prodrugs reported by Suneela et al[53] are different in the sense that they are prepared by coupling diazo salt of either 4-ASA or 5-ASA with 4-ASA or 5-ASA as carriers in all possible permutations and combinations. This results in prodrugs that, upon activation by azo reductase reduction, generate only one molecule of either 4-ASA or 5-ASA, while the other molecule released is a diaminosalicylic acid and not another molecule of aminosalicylate. These prodrugs were designed to target the colon affected with IBD. Due to their improved hydrophilic nature, the prodrugs had minimum absorption in the upper GIT. The prodrugs were considerably stable when incubated in the upper GIT. Approximately 68%-91% release was obtained on incubation with rat cecal matter. Amongst the series of four prodrugs, the prodrug of 4-ASA with 4-ASA (4A-4AAz) (Figure 1M) at a dose of 53 mg/kg was found to alleviate all the quantifying markers of TNBS-induced experimental colitis in Wistar rats. Due to absence of adverse effects in the stomach, liver and pancreas, it could be a promising alternative for IBD patients intolerant to 5-ASA-induced pancreatitis and sulfapyridine-induced adverse effects observed with sulfasalazine[53].
CONCLUSION
Despite possessing more stability and potency than 5-ASA in treating UC, without the evident risk of 5-ASA-induced pancreatitis, 4-ASA or its colon-targeted prodrugs remain unexplored and neglected when it comes to the management of IBD patients intolerant to 5-ASA. There is a need to investigate this promising compound, explore its untapped potential, and develop safe but effective systems for its targeted delivery to the colon, for better management of IBD as well as other local diseases of colon like IBS and colorectal cancer.
Footnotes
P- Reviewers: Hernandez-Breijo B, Kiela PR S- Editor: Wen LL L- Editor: Kerr C E- Editor: Wu HL
References
- 1.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol. 1997;273:G769–G775. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 2.Blonski W, Buchner AM, Lichtenstein GR. Inflammatory bowel disease therapy: current state-of-the-art. Curr Opin Gastroenterol. 2011;27:346–357. doi: 10.1097/MOG.0b013e328347aef3. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010;(1):CD004115. doi: 10.1002/14651858.CD004115.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Van Bodegraven AA, Wijmenga C. Inflammatory bowel disease. Genomic and personalized medicine. In: Willard GH, Ginsburg W, editors. Bethesda: Elsevier; 2009. pp. 1040–1055. [Google Scholar]
- 5.Crohn’s and colitis foundation of America, Inc. 1996-2002. Available from: http://www.ccfa.org/physician/colitisb.html.
- 6.National digestive diseases information clearinghouse, 2 information way Bethesda, MD: 2893-3570. Available from: http://www.niddk.nih.gov/health/digest/pubs/colitis/colitis.htm. Accessed on 08/10/2008.
- 7.Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16–25. doi: 10.1007/s00535-006-1995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–1096. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 10.Di Paolo MC, Paoluzi OA, Pica R, Iacopini F, Crispino P, Rivera M, Spera G, Paoluzi P. Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects. Dig Liver Dis. 2001;33:563–569. doi: 10.1016/s1590-8658(01)80108-0. [DOI] [PubMed] [Google Scholar]
- 11.Sulfasalazine. Available from: http://www.drugs.com/mtm/sulfasalazine.html. Accessed on 9th Aug 2013.
- 12.Dhaneshwar SS, Chail M, Patil M, Naqvi S, Vadnerkar G. Colon-specific mutual amide prodrugs of 4-aminosalicylic acid for their mitigating effect on experimental colitis in rats. Eur J Med Chem. 2009;44:131–142. doi: 10.1016/j.ejmech.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536–539. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decocq G, Gras-Champel V, Vrolant-Mille C, Delcenserie R, Sauvé L, Masson H, Andréjak M. Acute pancreatitis induced by drugs derived from 5-aminosalicylic acid: case report and review of the literature. Therapie. 1999;54:41–48. [PubMed] [Google Scholar]
- 15.Marteau P, Halphen M. [Comparative randomized open study of the efficacy and tolerance of enemas with 2 gr of 4-amino-salicylic acid (4-ASA) and 1 gr of 5-amino-salicylic acid (5-ASA) in distal forms of hemorrhagic rectocolitis] Gastroenterol Clin Biol. 1995;19:31–35. [PubMed] [Google Scholar]
- 16.Beeken W, Howard D, Bigelow J, Trainer T, Roy M, Thayer W, Wild G. Controlled trial of 4-ASA in ulcerative colitis. Dig Dis Sci. 1997;42:354–358. doi: 10.1023/a:1018874120749. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg AL, Davis ND, Nochomovitz LE. Placebo-controlled trial of ulcerative colitis with oral 4-aminosalicylic acid. Gastroenterology. 1992;102:448–452. doi: 10.1016/0016-5085(92)90089-h. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber S, Howaldt S, Raedler A. Oral 4-aminosalicylic acid versus 5-aminosalicylic acid slow release tablets. Double blind, controlled pilot study in the maintenance treatment of Crohn’s ileocolitis. Gut. 1994;35:1081–1085. doi: 10.1136/gut.35.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell SC, Waring RH. Aminophenols. In: Ullmann’s Encyclopedia of Industrial Chemistry., editor. Wiley-VCH Verlag GmbH & Co, KGaA. Weinheim: Wiley-VCH; 2000. pp. 5–29. [Google Scholar]
- 20.Lover MJ, inventor. Block Drug Company, Inc, Jersey City, NJ, assignee. Use of 4-aminosalicylic acid as an anti-inflammatory agent. 44 40 763. US patent. 1984 Apr 3;
- 21.Campieri M, Lanfranchi GA, Bertoni F, Brignola C, Bazzocchi G, Minguzzi MR, Labò G. A double-blind clinical trial to compare the effects of 4-aminosalicylic acid to 5-aminosalicylic acid in topical treatment of ulcerative colitis. Digestion. 1984;29:204–208. doi: 10.1159/000199034. [DOI] [PubMed] [Google Scholar]
- 22.Daniel F, Seksik P, Cacheux W, Jian R, Marteau P. Tolerance of 4-aminosalicylic acid enemas in patients with inflammatory bowel disease and 5-aminosalicylic-induced acute pancreatitis. Inflamm Bowel Dis. 2004;10:258–260. doi: 10.1097/00054725-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell LJ, Arvind AS, Hoang P, Cameron D, Talbot IC, Jewell DP, Lennard-Jones JE, Farthing MJ. Double blind, controlled trial of 4-aminosalicylic acid and prednisolone enemas in distal ulcerative colitis. Gut. 1992;33:947–949. doi: 10.1136/gut.33.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selby WS, Bennett MK, Jewell DP. Topical treatment of distal ulcerative colitis with 4-amino-salicylic acid enemas. Digestion. 1984;29:231–234. doi: 10.1159/000199038. [DOI] [PubMed] [Google Scholar]
- 25.Ahnfelt-Rønne I, Nielsen OH, Christensen A, Langholz E, Binder V, Riis P. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology. 1990;98:1162–1169. doi: 10.1016/0016-5085(90)90329-y. [DOI] [PubMed] [Google Scholar]
- 26.Miyachi Y, Yoshioka A, Imamura S, Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987;28:190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JG, Hallett MB. Effect of sulphasalazine and its active metabolite, 5-amino-salicylic acid, on toxic oxygen metabolite production by neutrophils. Gut. 1989;30:1581–1587. doi: 10.1136/gut.30.11.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen OH, Ahnfelt-Rønne I. 4-Aminosalicylic acid, in contrast to 5-aminosalicylic acid, has no effect on arachidonic acid metabolism in human neutrophils, or on the free radical 1,1-diphenyl-2-picrylhydrazyl. Pharmacol Toxicol. 1988;62:223–226. doi: 10.1111/j.1600-0773.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber S, Raedler A, Stenson WF, MacDermott RP. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992;21:451–502. [PubMed] [Google Scholar]
- 31.von Ritter C, Grisham MB, Granger DN. Sulfasalazine metabolites and dapsone attenuate formyl-methionyl-leucyl-phenylalanine-induced mucosal injury in rat ileum. Gastroenterology. 1989;96:811–816. [PubMed] [Google Scholar]
- 32.Schless JM, Inglis RM, Hammond JH, Hale JM. Comparative evaluation of blood levels and tolerance of sodium PAS and conjugated PAS and ascorbic acid. Dis Chest. 1966;50:595–600. doi: 10.1378/chest.50.6.595. [DOI] [PubMed] [Google Scholar]
- 33.Cannemeyer W, Thompson JR, Lichtenstein MR. Severe paraaminosalicylic acid hypersensitivity; blood and lymph node studies. Blood. 1955;10:62–75. [PubMed] [Google Scholar]
- 34.Gisbert JP, Gomollón F, Maté J, Pajares JM. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci. 2002;47:471–488. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 35.McEvoy GK. Aminosalicylic acid. AHFS Drug Information 2007. US: American Society of Health-System Pharmacists; 2007. pp. 544–546. [Google Scholar]
- 36.Food and Drug Administration. Orphan designations pursuant to Section 526 of the Federal Food and Cosmetic Act as amended by the Orphan Drug Act (P.L. 97-414), to June 28, 1996. MD: Rockville; 1996. [Google Scholar]
- 37.Aminosalicylic Acid. Available from: http://www.drugs.com/monograph/aminosalicylic-acid.html. Accessed on 23rd Sep 2013.
- 38.Drug information. Available from: http://www.ahfsdruginformation.com. Accessed on 28th July 2013.
- 39.Nagy F, Karacsony G, Varro V. Experience with topical administration of 4-aminosalicylic acid in ulcerative colitis. Dis Colon Rectum. 1989;32:134–137. doi: 10.1007/BF02553826. [DOI] [PubMed] [Google Scholar]
- 40.Mital OP, Singh G, Raj B. Case report of fatal reaction to para-aminosalicylic acid and streptomycin. Ind J Tub. 1991;2:72–73. [Google Scholar]
- 41.Zhao ZB, Guo J, Wei YG, Liang TD. Synthesis of 4-aminosalicylglycine. Chinese Chem Lett. 2005;16:889–892. [Google Scholar]
- 42.Wallace JL, Cirino G, Sparatore A, inventors. Antibe Therapeutics Inc, Calgary, CA, assignee. Salts of 4- or 5-aminosalicylic acid. 20060003972A1. US patent. 2006 Jan 5;
- 43.Akisu M, Ozmen D, Baka M, Habif S, Yalaz M, Arslanoglu S, Kultursay N, Bayindir O. Protective effect of dietary supplementation with L-arginine and L-carnitine on hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. Biol Neonate. 2002;81:260–265. doi: 10.1159/000056757. [DOI] [PubMed] [Google Scholar]
- 44.Shah PS, Shah VS. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Sys Rev. 2007;(3):CD004339. doi: 10.1002/14651858.CD004339.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Wallace JL, Cirino G, Caheno G, Sparatore A, Santagada V, Fiorucci S, inventors. Derivatives of 4- and 5-aminosalicylic acids. 20060270635A1. US patent. 2006 Nov 30;
- 46.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 47.Zhao ZB, Zheng HX, Wei YG, Liu J. Synthesis of azo derivatives of 4-aminosalicylic acid. Chinese Chem Lett. 2007;18:639–642. [Google Scholar]
- 48.Almenier HA, Al Menshawy HH, Maher MM, Al Gamal S. Oxidative stress and inflammatory bowel disease. Front Biosci (Elite Ed) 2012;4:1335–1344. doi: 10.2741/463. [DOI] [PubMed] [Google Scholar]
- 49.Sheng SF, Zheng HX, Liu J, Zhao ZB. Synthesis of phenol-class azo derivatives of 4-aminosalicylic acid. Chinese Chem Lett. 2008;19:419–422. [Google Scholar]
- 50.Dhaneshwar SS, Sharma M, Vadnerkar G. Co-drugs of aminosalicylates and nutraceutical amino sugar for ulcerative colitis. J Drug Del Sci Tech. 2011;21:527–533. [Google Scholar]
- 51.Yadav R, Mahatma OP, Singhvi I. Preparation and evaluation of polymeric prodrug of 4-aminoslicylic acid: colonic drug delivery in inflammatory bowel disease. IJPI’s J Pharmaceut Cosmetol. 2011;1:1–9. [Google Scholar]
- 52.Vadnerkar G, Dhaneshwar S. Macromolecular prodrug of 4-aminosalicylic acid for targeted delivery to inflamed colon. Curr Drug Discov Technol. 2013;10:16–24. [PubMed] [Google Scholar]
- 53.Suneela D, Gaurav V, Himanshu R. Design and development of novel azo prodrugs using various permutations and combinations of 5- and 4-aminosalicylic acids for inflammatory bowel disease: a colon-targeted approach. Inflamm Allergy Drug Targets. 2013;12:328–340. doi: 10.2174/18715281113129990059. [DOI] [PubMed] [Google Scholar]


