Abstract
AIM: To investigate histological and immunohistochemical differences in hepatitis between autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC) with AIH features.
METHODS: Liver needle biopsies of 41 PBC with AIH features and 43 AIH patients were examined. The activity of periportal and lobular inflammation was scored 0 (none or minimal activity) to 4 (severe), and the degree of hepatitic rosette formation and emperipolesis was semiquantatively scored 0-3. The infiltration of mononuclear cells positive for CD20, CD38, CD3, CD4, and CD8 and positive for immunoglobulins (IgG, IgM, and IgA) at the periportal areas (interface hepatitis) and in the hepatic lobules (lobular hepatitis) were semiquantitatively scored in immunostained liver sections (score 0-6). Serum aspartate aminotransferase (AST), immunoglobulins, and autoantibodies at the time of liver biopsy were correlated with the histological and immunohistochemical scores of individual lesions.
RESULTS: Lobular hepatitis, hepatitic rosette formation, and emperipolesis were more extensive and frequent in AIH than in PBC. CD3+, CD4+, and CD8+ cell infiltration scores were higher in the hepatic lobules and at the interface in AIH but were also found in PBC. The degree of mononuclear cell infiltration correlated well with the degree of interface and lobular hepatitis in PBC, but to a lesser degree in AIH. CD20+ cells were mainly found in the portal tracts and, occasionally, at the interface in both diseases. Elevated AST correlated well with the hepatocyte necroinflammation and mononuclear cell infiltration, specifically CD38+ cells in PBC. No correlation existed between autoantibodies and inflammatory cell infiltration in PBC or AIH. While most AIH cases were IgG-predominant at the interface, PBC cases were divided into IgM-predominant, IgM/IgG-equal, and IgG-predominant types, with the latter sharing several features with AIH.
CONCLUSION: These results suggest that the hepatocellular injuries associated with interface and lobular hepatitis in AIH and PBC with interface hepatitis may not be identical.
Keywords: Primary biliary cirrhosis, Autoimmune hepatitis, Interface hepatitis, Lobular hepatitis, Plasma cell subclass
Core tip: Primary biliary cirrhosis (PBC) can sometimes present with features of autoimmune hepatitis (AIH). Therefore, the clinicopathological features and immunophenotypes of infiltrated mononuclear cells of AIH and PBC with AIH features were examined. Lobular hepatitis, hepatitic rosette formation, and emperipolesis were more frequent in AIH than PBC. The degree of mononuclear cell infiltration correlated well with hepatitis. Furthermore, elevation of aspartate aminotransferase levels correlated well with necroinflammation and infiltration of mononuclear cells in PBC, which tended to be IgM-predominant. These results suggest that hepatocellular injuries in AIH and PBC may not be identical.
INTRODUCTION
Primary biliary cirrhosis (PBC) is characterized by the progressive destruction of interlobular bile ducts (chronic nonsuppurative destructive cholangitis) and frequent anti-mitochondrial antibodies (AMAs) in the serum[1-8]. While some PBC patients may remain in the initial stage with chronic cholangitis, a considerable number exhibit histological progression suggested by interface changes, such as chronic cholestatic changes (biliary piecemeal necrosis) and hepatitic features (lymphocytic piecemeal necrosis or interface hepatitis)[9-11]. Rarely, PBC patients with considerable interface hepatitis will also have circulating antinuclear antibodies (ANAs), a characteristic feature of autoimmune hepatitis (AIH). These cases are referred to as PBC with AIH features or PBC-AIH overlapping syndrome[12-17]. These patients may have a more severe disease course with the earlier development of liver fibrosis and liver failure, though steroid or immunosuppressive therapies may be effective[1,3,11,12,15-18].
Histologically, AIH exhibits the features of chronic hepatitis with interface hepatitis and plasma cells are reportedly predominant in the portal tract and at the interface[19-21]. Entrapment of hepatocytes in the enlarged portal tract, called “hepatitic rosette formation”, is relatively frequent and lobular hepatitis is often severe in AIH. Furthermore, engulfment of lymphocytes by hepatocytes, termed emperipolesis, is often found and has been adopted as a useful histological feature for the diagnosis of AIH[19,22]. The combination of these lesions has been regarded as a typical feature of AIH[22].
Histologically, the hepatitic features of PBC resemble those of AIH. Plasma cells are relatively prominent at the interface of PBC with interface hepatitis, similar to AIH[3,11]. However, several histopathological studies have distinguished PBC from AIH. Several reports have shown that the density and proportion of IgM+ and IgG+ plasma cells in portal tracts can be used to differentiate AIH from PBC by liver biopsies[9,10,23,24]. In addition, Graham et al[25] reported that CD1a infiltration favors a diagnosis of PBC. However, differences and similarities in the hepatitic lesions at the interface and in the hepatic lobules between these two groups have not been studied in detail. Whether the hepatitic features in PBC reflect the overlap of AIH in PBC or an inherent feature of PBC is yet to be determined[3,11,12,14,26].
In this study, we examined similarities and differences in interface hepatitis and the lobular changes in AIH and PBC with interface hepatitis by needle liver biopsies and immunohistochemistry. The subtypes of infiltrating mononuclear cells in interface and lobular hepatitis were also compared. The histological and immunohistochemical features of hepatic necroinflammation were then correlated with the clinical and laboratory features of the two groups.
MATERIALS AND METHODS
Selection of patients with PBC and AIH and tissue preparation
In this study, we tried to compare PBC with interface hepatitis with AIH, and we selected PBC patients with interface hepatitis by adopting Batts and Ludwig’s scoring system[27]. In this system, the activity of periportal (interface) and lobular inflammation was scored semiquantitatively in routinely processed sections: portal/periportal activity score 0 (none or minimal activity), 1 (portal inflammation only), 2 (mild interface hepatitis), 3 (moderate interface hepatitis), and 4 (severe interface hepatitis); and lobular activity score 0 (none), 1 (inflammatory cells, but no hepatocellular death), 2 (focal cell death), 3 (severe focal cell death, confluent necrosis without bridging), and 4 (damage including bridging necrosis). We collected liver specimens of PBC with portal/periportal activity scores ≥ 2 (Figure 1A and B).
Figure 1.
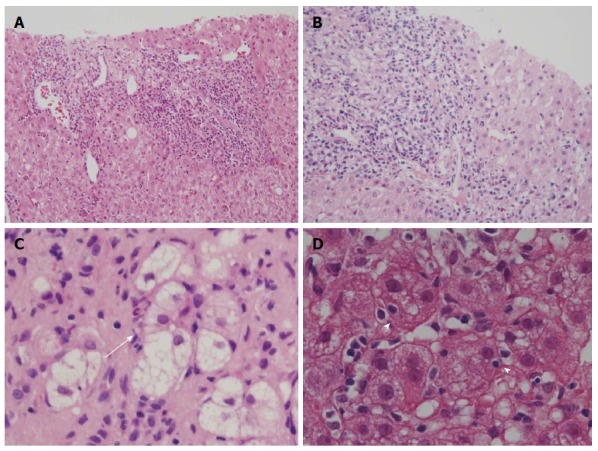
Histological features of primary biliary cirrhosis with interface hepatitis and autoimmune hepatitis. A: Moderate interface hepatitis (score 3) in autoimmune hepatitis (AIH); B: Moderate interface hepatitis (score 3) in primary biliary cirrhosis (PBC); C: Rosette formation; regenerative hepatocytes arranged around a bile canaliculus (arrow), found in AIH; D: Emperipolesis, the engulfment of lymphocytes within hepatocytes (arrowheads), was found in areas of interface hepatitis. Hematoxylin and eosin stain, original magnifications × 200 ( A, B); × 600 (C, D).
Patient collection: A total of 84 liver needle biopsies were used in this study. They consisted of specimens from 41 patients with PBC with more than a 2+ portal/periportal activity score (above-mentioned) and 43 patients with AIH, obtained at Kanazawa University hospital and affiliated hospitals (1998-2012). All AIH cases were associated with a portal/periportal activity score of ≥ 2. All of these patients were negative for hepatitis B and hepatitis C viral screening and had no history of alcohol abuse. All PBC and AIH cases fulfilled the clinical and pathological diagnostic features of PBC[1] and AIH type I[10,12], respectively. Regarding a diagnosis of PBC, when histological changes compatible with PBC such as chronic cholangitis and/or bile duct loss were found in the patients with elevation of biliary enzymes and serological data compatible with PBC, such cases were diagnosed as PBC and included in this study. Other hepatobiliary diseases were excluded in these cases. No patient had a history of treatment with ursodeoxycholic acid (PBC) or steroid or immunosuppressive drugs (AIH) at the time of liver biopsy. The main clinicopathological and laboratory findings of these cases are shown in Table 1. As for AMA positivity, AMA and/or M2 were evaluated in a combination in individual cases.
Table 1.
Main clinicopathological features of primary biliary cirrhosis with interface hepatitis and autoimmune hepatitis
| PBC1 (n = 41) | AIH (n = 43) | |
| Gender (male:female) | 5:36 | 4:39 |
| Age (yr, mean ± SD) | 59.0 ± 13.3 | 61.7 ± 10.8 |
| Laboratory data | ||
| Ratio of AMA(+) or M2(+) and titer of AMA | 75% and 141.2 ± 211.4 | 7% and 0 ± 0 |
| Ratio of ANA(+) and titer2 | 72% and 724.3 ± 1262.4 | 91% and 411.1 ± 550.8 |
| AST (IU/L) | 51.6 ± 29.7 | 219.5 ± 180.5 |
| ALT (IU/L) | 54.0 ± 40.5 | 261.8 ± 253.4 |
| ALP (IU/L) | 615.5 ± 572.8 | 449.9 ± 251.8 |
| IgG (mg/dL) | 1832.9 ± 607.2 | 2246.2 ± 673.0 |
| IgM (mg/dL) | 300.9 ± 220.6 | 193.7 ± 120.0 |
| IgA (mg/dL) | 307.9 ± 122.4 | 387.2 ± 183.1 |
Laboratory data is shown as the mean ± SD; anti-mitochondrial antibodies (AMA)(+) ≥ 40; M2(+) ≥ 5; antinuclear antibodies (ANA)(+) ≥ 40.
With interface hepatitis;
The average titer of ANA was higher in primary biliary cirrhosis (PBC) than in autoimmune hepatitis (AIH) because two PBC cases had a titer of 5120, which influenced the average. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
Tissue preparation: All liver specimens were fixed in 10% formalin and embedded in paraffin. Routinely processed hematoxylin and eosin staining was available in one PBC and 19 AIH cases. Routine histological stains and immunohistochemistry for the remaining cases were perfumed on 4 μm-thick serial sections.
Histological features
The activity of periportal (interface) and lobular inflammation was scored semiquantitatively in routinely processed sections by Batts and Ludwig’s scoring system as described above[27]. Hepatitic rosette formation was defined as the entrapment of several hepatocytes with dilated canaliculi and clear cytoplasm in the enlarged portal tract (Figure 1C), and emperipolesis was defined as engulfed lymphocyte(s) in the cytoplasm of hepatocytes in the periportal regions (Figure 1D). The lesions were surveyed and scored as follows: 0 (no lesion), 1 (one to several lesions), 2 (intermediate), and 3 (many lesions).
Immunohistochemistry
The antibodies used in this study and their sources are shown in Table 2. Deparaffinized sections were pretreated by methods suitable for each antibody, if necessary, followed by pretreatment with 1% H2O2 in methanol for 20 min to block endogenous peroxidase activity, and then with normal goat serum for 20 min to block nonspecific reactions. The sections were then incubated overnight at 4 °C with each primary antibody at optimal dilutions and then at room temperature for 1 h with anti-mouse (except for CD38) or anti-rabbit (for CD38) immunoglobulins conjugated to a peroxidase-labeled dextran polymer (Simple Staining Kit; Nichirei). The reaction products were developed by immersing the sections in a solution of 3,3’-diaminobenzidine tetrahydrochloride solution containing 0.03% hydrogen peroxide. Nuclei were lightly counterstained with hematoxylin.
Table 2.
Primary antibodies used in this study
| Primary antibody | Source | Clone | Animal | Type | Pretreatment | Dilution |
| CD3 | Nichirei | PS1 | Mouse | Mono | MW3 | 1:1 |
| CD4 | Nichirei | 4B12 | Mouse | Mono | MW3 | 1:1 |
| CD8 | Dako | C8/144B | Mouse | Mono | MW1 | 1:20 |
| CD20 | Dako | L26 | Mouse | Mono | - | 1:400 |
| CD38 | Epitomics | EPR4106 | Rabbit | Mono | AC2 | 1:800 |
| CD138 | Dako | ML15 | Mouse | Mono | MW3 | 1:100 |
| IgG | Dako | A57H | Mouse | Mono | - | 1:200 |
| IgM | Nichirei | R1/69 | Mouse | Mono | MW3 | 1:1 |
| IgA | Novocastra | N1CLA | Mouse | Mono | PK | 1:300 |
Citrate, pH = 6.0;
Ethylenediaminetetraacetic acid (EDTA) buffer, pH8.0;
Tris EDTA buffer, pH = 9.0. MW: Microwave; AC: Autoclave; PK: Proteinase K, pH = 7.5 (Dako).
Double immunofluorescence staining
Double-color immunostaining of CD38 and CD138, IgG, or IgM was performed as follows: deparaffinized sections were pretreated and incubated overnight at 4 °C with a mixture of primary antibodies for CD38 and CD138, CD38 and IgG, or CD38 and IgM (Table 2). The sections were firstly incubated with the Alexa Fluor® 488 (Invitrogen, Carlsbad, CA) secondary antibody against rabbit IgG for the detection of CD38 and with Alexa Fluor® 594 (Invitrogen) secondary antibody against mouse IgG for the detection of CD138 and IgM or the secondary antibody against mouse IgM for the detection of IgG.
Semiquantitative immunohistochemical evaluation of inflammatory cells
The infiltration of mononuclear cells positive for CD20, CD38, CD3, CD4, and CD8 and for immunoglobulin classes (IgG, IgM, and IgA) at the periportal areas (interface hepatitis) and in the hepatic lobules (lobular hepatitis) were semiquantitatively scored twice in immunostained liver sections (0, no positive cells; 1, minimal, a few positive cells in several regions; 2, moderate, considerable amount of positive cells in about half of the regions; and 3, many positive cells in almost all regions). The sum of the two evaluations was used as the individual score of individual cases (score 0-6).
CD20 is known to be expressed in immature B cells, CD38 in mature plasma cells and also in other T or cell series, CD3 in T cells, CD4 in helper T cells, and CD8 in cytotoxic T cells[28,29]. IgG, IgM, and IgA are expressed in mature plasma cells. CD138 is more specifically expressed in plasma cells[28], while it is also expressed variably in bile ductules and bile ducts as well as in the hepatocyte membranes in our preliminary study. The expressions of CD38 and CD138, shown by double immunostaining, were similar in the infiltrating mononuclear cells (data not shown). There were no double positive cells when double immunostaining was performed for CD38 and CD3 in AIH and PBC (data not shown); therefore, T cells were not included in the pool of CD38+ cells in the liver tissue specimens in this study. Thus, CD38 was used to survey mature plasma cells.
Comparison of histopathological and immunohistochemical data with laboratory data
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), IgG, IgM, IgA, AMAs, and ANAs at the time of liver biopsy were correlated with the scores of individual lesions (degree of interface hepatitis, lobular hepatitis, emperipolesis, and hepatitic rosette formation) and scores of infiltrating mononuclear cells positive for individual phenotypes (CD20, CD38, CD3, CD4, CD8, IgG, IgM, and IgA).
Statistical analysis
All statistical analyses were performed using JMP software (version 8.0, SAS Institute Japan, Tokyo, Japan). Values were expressed graphically as the mean ± SD and median. Statistical differences between groups were determined using a two tailed Mann-Whitney unpaired test and Fisher’s exact probability test with 95%CI. The correlation coefficient of 2 factors was evaluated using Spearman’s rank correlation test. P values of < 0.05 were considered significant.
RESULTS
Histopathologies of the interfaces and hepatic lobules of PBC and AIH
Interface hepatitis and lobular hepatitis were more or less diffuse in the needle liver biopsies in AIH than in PBC. Lobular hepatitis was more or less irregularly distributed in the liver specimens of PBC. There was no significant difference in the scores of interface hepatitis between AIH and PBC (Figure 2A), which was expected because PBC cases with interface hepatitis were selected in this study. The scores of lobular hepatitis were significantly higher in AIH than in PBC (Figure 2B), and the former frequently showed zonal or even bridging necrosis in addition to focal hepatocellular necrosis. The scores of hepatitic rosette formation (Figure 1C) and emperipolesis (Figure 1D) were significantly higher in AIH than in PBC (Figure 2C and D).
Figure 2.

Comparison of necroinflammation in autoimmune hepatitis and primary biliary cirrhosis with interface hepatitis. A: The scores of interface hepatitis (IFH) were not significantly different between primary biliary cirrhosis (PBC) (2.49 ± 0.64) and AIH (2.74 ± 0.66) (P = 0.0599) because PBC cases with IFH were chosen for this comparative study with autoimmune hepatitis (AIH); B: The scores of lobular hepatitis (LH) were higher in AIH (2.58 ± 0.70) than in PBC (2.22 ± 0.61) (P = 0.0003); C: The scores of hepatitic rosette formation were higher in AIH (0.55 ± 0.83) than in PBC (0.17 ± 0.44) (P = 0.0134); D: The scores of emperipolesis were higher in AIH (1.00 ± 0.96) than in PBC (0.32 ± 0.52) (P = 0.0003). Horizontal bars of the graph show the median scores; aP < 0.05 vs control, bP < 0.01 vs control in the Mann-Whitney test.
Immunophenotypes of infiltrating inflammatory cells at the interfaces and in the hepatic lobules
The degree of infiltration (scores) of mononuclear cells with individual immunophenotypes at the interfaces and in the hepatic lobules are shown in Table 3.
Table 3.
Degree of mononuclear cell infiltration with respect to immunological phenotypes at the interface and in the hepatic lobules
| PBC1 | AIH | P value | |
| At the interface; scores of infiltrating mononuclear cells positive for | |||
| CD3 | 2.8 ± 1.2 (3)2 | 4.1 ± 1.1 (4) | 0.0002 |
| CD4 | 1.5 ± 1.0 (1) | 2.0 ± 1.2 (2) | 0.0387 |
| CD8 | 1.5 ± 0.9 (1) | 2.7 ± 1.7 (3) | 0.0048 |
| CD20 | 0.7 ± 0.6 (1) | 1.0 ± 1.0 (1) | 0.0046 |
| CD38 | 2.7 ± 1.5 (3) | 3.7 ± 1.3 (4) | 0.0046 |
| IgG | 1.3 ± 1.0 (1) | 1.8 ± 1.3 (2) | 0.0301 |
| IgM | 1.0 ± 0.8 (1) | 0.6 ± 0.8 (0.5) | 0.0249 |
| IgA | 0.5 ± 0.6 (0) | 0.6 ± 0.7 (1) | 0.4486 |
| In the hepatic lobules; scores of infiltrating mononuclear cells positive for | |||
| CD3 | 2.2 ± 0.9 (2) | 3.3 ± 1.3 (3) | 0.0016 |
| CD4 | 0.8 ± 0.9 (0.5) | 1.8 ± 1.1 (2) | 0.0002 |
| CD8 | 0.8 ± 0.8 (1) | 2.5 ± 1.5 (2) | < 0.0001 |
| CD20 | 0.3 ± 0.6 (0) | 0.7 ± 0.8 (0.5) | 0.0147 |
| CD38 | 1.1 ± 1.0 (1) | 2.0 ± 1.2 (2) | 0.0025 |
| IgG | 0.6 ± 0.8 (0) | 1.4 ± 1.3 (1) | 0.0007 |
| IgM | 0.4 ± 0.5 (0) | 0.6 ± 0.7 (0.5) | 0.166 |
| IgA | 0.3 ± 0.7 (0) | 0.6 ± 0.7 (0.5) | 0.0239 |
With interface hepatitis;
The scores were graded from 0-6 for each case, and are shown as the mean ± SD and (median). PBC: Primary biliary cirrhosis; AIH: Autoimmune hepatitis.
CD3, CD4, and CD8: CD3+ cells were frequently observed in the portal tracts, at the interface, and also within the hepatic lobules in both diseases (Figures 3A and 4A). Their scores at the interface and within hepatic lobules were higher in AIH than in PBC. The scores of CD3+ cell infiltration at the interface and the hepatic lobules increased with the degree of interface hepatitis and lobular hepatitis in PBC (Figure 5A). The scores of CD3+ cell infiltration at the interface also increased with the degree of interface hepatitis in AIH, but such a correlation was not seen in lobular hepatitis (Figure 5B).
Figure 3.
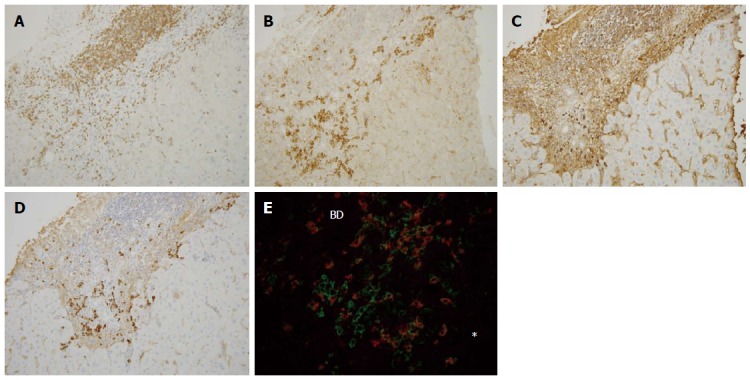
Immunohistochemical findings of primary biliary cirrhosis with interface hepatitis. Many CD3+ cells were found within the portal tract and at the interface (A), whereas CD38+ cells heavily infiltrated the interface (B), IgG+ cells were mainly found in the portal tract and also at the interface (C), and IgM+ cell infiltration was predominant at the interface (D). Double staining for IgM (red) and CD38 (green) (E) showed that IgM-CD38 double positive cells, IgM-producing plasma cells, were frequently seen in the periportal area (star) (original magnifications: × 200). IgM+ plasma cells were also found around the intralobular bile duct. Asterisk: Hepatic lobule, BD: Bile duct.
Figure 4.
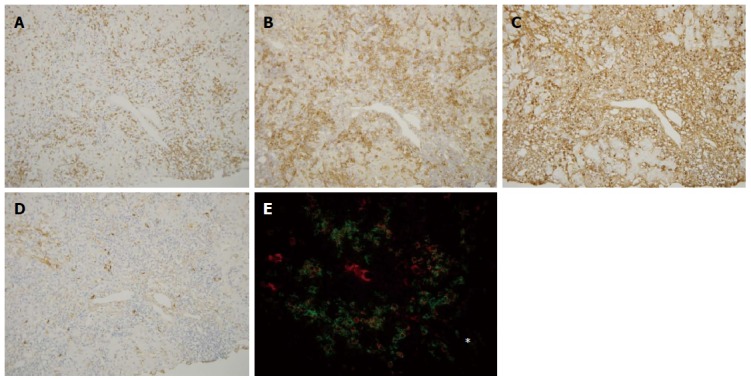
Immunohistochemical findings of autoimmune hepatitis. CD3+ cells were found within the portal tract and in the periportal area (A), whereas CD38+ cells infiltrated prominently in the periportal area (B). IgG+ cells (C) and IgM+ cells (D) were also found in the periportal area, and IgG+ cell infiltration was predominant in the majority of autoimmune hepatitis cases. The distribution of these cells was similar to primary biliary cirrhosis (Figure 3); E: Double staining for IgG (red) and CD38 (green) showed that IgG-CD38 double positive cells, which may be IgG-producing plasma cells, were frequently seen in the periportal area (star). Original magnifications: × 200. Asterisk: Hepatic lobule.
Figure 5.
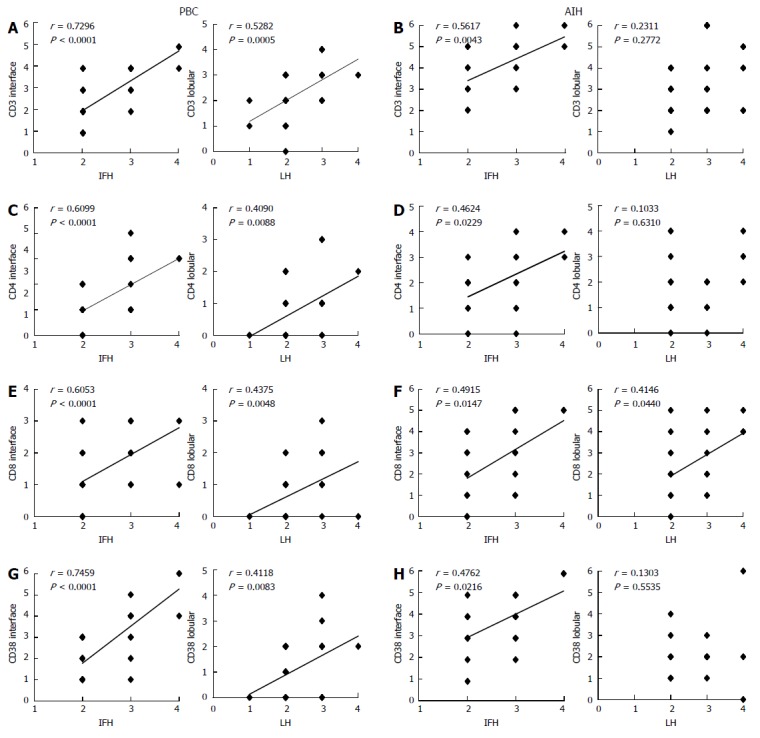
Correlation between scores for necroinflammation and infiltrating mononuclear cells positive for CD3, CD4, CD8, and CD38 in primary biliary cirrhosis with interface hepatitis and autoimmune hepatitis. A: In primary biliary cirrhosis (PBC), the degree of CD3+ mononuclear cell infiltration and the scores of interface hepatitis and lobular hepatitis showed positive correlations; B: In autoimmune hepatitis (AIH), the degree of CD3+ mononuclear cell infiltration and the scores of interface hepatitis showed positive correlation, whereas that of CD3+ mononuclear cell infiltration and lobular hepatitis failed to correlate positively; C: In PBC, the degree of CD4+ mononuclear cell infiltration and the scores of interface hepatitis and lobular hepatitis showed positive correlations; D: In AIH, the degree of CD4+ mononuclear cell infiltration and the scores of interface hepatitis showed a positive correlation, whereas that of CD4+ mononuclear cell infiltration and lobular hepatitis failed to correlate positively; E: In PBC, the degree of CD8+ mononuclear cell infiltration and the scores of interface hepatitis and lobular hepatitis showed positive correlations; F: In AIH, the degree of CD8+ mononuclear cell infiltration and the scores of interface hepatitis and lobular hepatitis showed positive correlations; G: In PBC, the degree of CD38+ mononuclear cell infiltration and the scores of interface hepatitis and lobular hepatitis showed positive correlations; H: In AIH, the degree of CD38+ mononuclear cell infiltration and the scores of interface hepatitis showed positive correlation, whereas that of CD38+ mononuclear cell infiltration and lobular hepatitis failed to correlate positively. Spearman’s rank correlation test in A-H.
The distribution of CD4 and CD8 was similar to that of CD3, though their number was smaller than that of CD3 in both diseases. The scores of CD4+ and CD8+ T cells at the interface and in the hepatic lobules were higher in AIH than in PBC, and their scores increased with the degree of interface hepatitis and lobular hepatitis, respectively, in PBC (Figure 5C and E). The scores of CD4+ and CD8+ cells in AIH increased with the degree of interface hepatitis. CD8+ cells were associated with the scores of lobular hepatitis, whereas CD4+ cells were not (Figure 5D and F).
CD20: CD20+ cells were mainly found in the portal tracts, and occasionally at the interface in both diseases. While the number of CD20+ cells was smaller than that of CD3+, CD4+, and CD8+ cells, they were relatively more numerous at the interface and hepatic lobules in AIH than in PBC.
CD38: CD38+ cells with a plump cytoplasm, corresponding to mature plasma cells, were the most predominant at the interface in both diseases (Figure 3B and 4B). The number of these cells was higher in AIH than in PBC both at the interface and in hepatic lobules. The scores of CD38+ cell infiltration at the interface and within the hepatic lobules increased with the degree of interface hepatitis and lobular hepatitis in PBC (Figure 5G). Their scores at the interface increased with the degree of interface hepatitis in AIH, but such correlations were not seen within the hepatic lobules (Figure 5H).
Analysis of infiltrating plasma cells with respect to immunoglobulin classes
Infiltration of IgG+, IgM+, and IgA+ plasma cells: IgG+ plasma cells were frequently observed at the interfaces in PBC and AIH (Figures 3C and 4C), and their scores were significantly higher in AIH (Table 3). IgM+ plasma cells were found in interface hepatitis in both diseases (Figures 3D and 4D), and the scores were significantly higher in PBC. These cells were not prominent in the hepatic lobules in both diseases. Double immunostaining showed that IgG+ and IgM+ cells were also CD38+ (Figures 3E and 4E).
Clinicopathological features of the IgG- or IgM-predominant type in PBC: By comparing the density of IgG+ and IgM+ plasma cells at the interface, 16 cases of AIH were revealed to be IgG-predominant, 8 cases to be IgM/IgG-equal, and none IgM-predominant. In contrast, 5 cases of PBC were IgM-predominant, 23 were IgM/IgG-equal, and 12 were IgG-predominant (Figure 6). Then each PBC case was classified into one of 2 groups: group A (IgM/IgG-equal and IgM-predominant types) and group B (IgG-predominant type). The laboratory data, histological features, and degree of inflammatory cell infiltration were compared between the two groups (Table 4). Serum IgM levels were significantly higher in group A, and interface hepatitis activities were slightly higher in group B. Interestingly, the degree of CD38+ cell infiltration at the interface and in the hepatic lobules was significantly higher in group B, which suggests that PBC in group B may share more features with AIH. There was no correlation in the positive ratio of AMA or M2 and that of ANA between these two groups.
Figure 6.
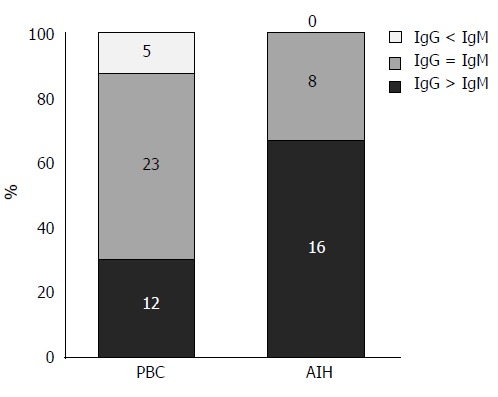
Classification of primary biliary cirrhosis with interface hepatitis and autoimmune hepatitis based on the predominance of the subclass of plasma cells infiltrating the interface. The majority of autoimmune hepatitis (AIH) cases (16 cases) were IgG-predominant, whereas 8 cases were IgG/IgM-equal. There are no IgM-predominant AIH cases. In primary biliary cirrhosis (PBC), 23 cases were IgG/IgM-equal, whereas 12 cases were IgG-predominant and 5 cases were IgM-predominant.
Table 4.
Clinicopathological features of primary biliary cirrhosis based on the predominant immunoglobulin class of the plasma cell
| No. of cases (female:male) | Group A 28 (25:3) | Group B 12 (10:2) | P value |
| Age (yr) | 56.1 ± 14.5 (56) | 66.0 ± 7.2 (66) | 0.0479 |
| Laboratory data | |||
| AST (IU/L) | 50.5 ± 31.2 (43.5) | 55.0 ± 28.3 (42.5) | 0.5839 |
| ALT (IU/L) | 54.2 ± 43.6 (45.5) | 55.0 ± 35.4 (46.5) | 0.7238 |
| ALP (IU/L) | 681.7 ± 655.0 (446) | 389.1 ± 136.3 (358.5) | 0.4166 |
| IgG (mg/dL) | 1737.4 ± 510.4 (1600) | 2081.3 ± 784.4 (2062.5) | 0.2658 |
| IgM (mg/dL) | 340.78 ± 219.0 (280.5) | 197.3 ± 198.7 (133.5) | 0.0077 |
| IgA (mg/dL) | 309.2 ± 129.8 (281) | 304.6 ± 107.0 (268.5) | 0.8184 |
| AMA, M2 (positive ratio) | 80.10% | 55.60% | 0.1916 |
| ANA (positive ratio) | 65.40% | 80.00% | 0.6880 |
| Histological findings (scores) | |||
| Interface hepatitis1 | 2.4 ± 0.6 (2) | 2.8 ± 0.6 (3) | 0.0688 |
| Lobular hepatitis2 | 2.3 ± 0.6 (2) | 2.3 ± 0.6 (2) | 0.8577 |
| Infiltration of mononuclear cells positive for (scores) | |||
| CD3 at the interface3 | 2.7 ± 1.2 (2.5) | 2.8 ± 1.3 (3) | 0.7039 |
| CD38 at the interface3 | 2.4 ± 1.5 (2) | 3.3 ± 1.3 (4) | 0.0205 |
| CD3 in the hepatic lobules3 | 2.1 ± 0.8 (2) | 2.4 ± 1.2 (2) | 0.5341 |
| CD38 in the hepatic lobules3 | 0.9 ± 0.9 (1) | 1.6 ± 1.1 (2) | 0.0497 |
The score was graded from 2 to 4 for each case;
the score was graded from 0 to 4 for each case;
The score was graded from 0 to 6 for each case. Group A, IgM-predominant cases and IgM/IgG-equal cases; group B, IgG-predominant cases. Age, laboratory data expect autoantibodies and scores are shown as the mean ± SD and (median); anti-mitochondrial antibodies (AMA)(+) ≥ 40; M2(+) ≥ 5; antinuclear antibodies (ANA)(+) ≥ 40. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
Laboratory data and immunophenotypic features of interface hepatitis and lobular hepatitis
The laboratory data and immunophenotypes of infiltrating mononuclear cells are summarized in Tables 5 and 6. As a marker of hepatocellular damage, the serum level of AST was evaluated.
Table 5.
Correlation between the elevations in aspartate aminotransferase and the scores of hepatitis and mononuclear cell infiltration of primary biliary cirrhosis and autoimmune hepatitis
| Scores of inflammatory cells at the interface vs elevation of aspartate aminotransferase |
Primary biliary cirrhosis1 |
Autoimmune hepatitis |
||
| r | P value | r | P value | |
| Scores of interface hepatitis | 0.3600 | 0.0286 | 0.1427 | 0.3796 |
| Scores of lobular hepatitis | 0.4056 | 0.0128 | 0.4444 | 0.0041 |
| Scores of CD3+ cells at the interface | 0.4172 | 0.0114 | 0.1540 | 0.4724 |
| Scores of CD3+ in the lobules | 0.3487 | 0.0371 | 0.4306 | 0.0357 |
| Scores of CD4+ at the interface | 0.4410 | 0.0071 | -0.1122 | 0.6017 |
| Scores of CD4+ in the lobules | 0.4478 | 0.0062 | 0.3033 | 0.1496 |
| Scores of CD8+ at the interface | 0.3466 | 0.0384 | 0.3051 | 0.1471 |
| Scores of CD8+ in the lobules | 0.1802 | 0.2929 | 0.7074 | 0.0001 |
| Scores of CD38+ at the interface | 0.6010 | 0.0001 | 0.0969 | 0.6601 |
| Scores of CD38+ in the lobules | 0.3821 | 0.0215 | 0.1541 | 0.4827 |
| Scores of IgG+ at the interface | 0.3909 | 0.0184 | 0.1381 | 0.5200 |
| Scores of IgM+ at the interface | 0.5436 | 0.0006 | -0.1372 | 0.5225 |
With interface hepatitis.
Table 6.
Comparison of the degree of hepatitis and mononuclear cell infiltration in autoantibody positive and negative groups in primary biliary cirrhosis and autoimmune hepatitis
| PBC | AMA(+) or M2(+) | AMA(-) and M2(-) | P value |
| Comparison between AMA , M2 positive and negative group in PBC | |||
| Scores of interface hepatitis1 | 2.41 ± 0.64 (2) | 2.33 ± 0.50 (2) | 0.9120 |
| Scores of CD3+ cells at interface2 | 2.62 ± 1.13 (2) | 2.44 ± 1.24 (3) | 0.7552 |
| Scores of CD38+ cells at interface2 | 2.42 ± 1.39 (2.5) | 2.33 ± 1.22 (2) | 0.9843 |
| Scores of IgG+ cells at interface2 | 1.12 ± 0.59 (1) | 1.22 ± 1.64 (1) | 0.4977 |
| Scores of IgM+ cells at interface2 | 1.00 ± 0.63 (1) | 0.89 ± 0.93 (1) | 0.4196 |
| Comparison between ANA positive and negative group in PBC | |||
| Scores of interface hepatitis1 | 2.42 ± 0.65 (2) | 2.30 ± 0.48 (2) | 0.6241 |
| Scores of CD3+ cells at interface2 | 2.47 ± 1.16 (2) | 2.80 ± 1.23 (2) | 0.4548 |
| Scores of CD38+ cells at interface2 | 2.43 ± 1.16 (2) | 2.50 ± 1.18 (3) | 0.8113 |
| Scores of IgG+ cells at interface2 | 1.09 ± 1.08 (1) | 1.30 ± 0.67 (1) | 0.4193 |
| Scores of IgM+ cells at interface2 | 0.83 ± 0.65 (1) | 1.30 ± 0.82 (1) | 0.0742 |
| Comparison between ANA positive and negative group in AIH | |||
| Scores of interface hepatitis1 | 2.70 ± 0.64 (3) | 2.33 ± 0.58 (2) | 0.3543 |
| Scores of CD3+ cells at interface2 | 4.21 ± 1.13 (4) | 3.33 ± 1.53 (3) | 0.2965 |
| Scores of CD38+ cells at interface2 | 3.89 ± 1.18 (4) | 2.33 ± 1.15 (3) | 0.0692 |
| Scores of IgG+ cells at interface2 | 1.95 ± 1.39 (2) | 1.00 ± 1.00 (1) | 0.2552 |
| Scores of IgM+ cells at interface2 | 0.63 ± 0.76 (1) | 0.00 ± 0.00 (0) | 0.1141 |
The scores were graded from 2 to 4 for each case, and are shown as the mean ± SD and (median);
The scores were graded from 0 to 6 for each case, and are shown as the mean ± SD and (median). Anti-mitochondrial antibodies (AMA)(+) ≥ 40; M2(+) ≥ 5; antinuclear antibodies (ANA)(+) ≥ 40. PBC: Primary biliary cirrhosis; AIH: Autoimmune hepatitis.
Serum level of AST and interface hepatitis, lobular hepatitis, and mononuclear cell infiltration: The degree of scores for CD3+, CD4+, CD38+, IgG+, IgM+, and IgA+ cell infiltration at the interface and within the hepatic lobules and that of CD8+ cell infiltration at the interface correlated well with the elevation in AST (Table 5), suggesting that these mononuclear cells play a role in immune-mediated hepatocellular injuries at the interfaces and within the hepatic lobules. In AIH, only the degree of lobular hepatitis, CD3+ cell and CD8+ cell infiltration within the hepatic lobules was correlated with the elevation in AST, whereas the other correlations found in PBC were not evident.
Correlation between AMA or M2 and ANA positivity and the degree of interface hepatitis and infiltration of mononuclear cells positive for CD3, CD38, IgG, and IgM: There was no difference in the degree of interface hepatitis between AMA or M2 and ANA positive and negative groups in both AIH and PBC (Table 6). There was also no difference in the degree of infiltrated mononuclear cells expressing CD3, CD38, IgG, or IgM at the interface between AMA or M2 positive and negative groups in PBC or between the ANA positive and negative groups in AIH.
DISCUSSION
The findings obtained in this study can be summarized as follows: (1) AIH exhibited more intense lobular hepatitis and more frequent hepatitic rosette formation and emperipolesis than that seen in PBC with interface hepatitis; (2) CD3+ and CD38+ cells were predominant in interface and lobular hepatitis in PBC, and increased with the degree of interface and lobular hepatitis in PBC. CD4+ and CD8+ showed a similar distribution to CD3+ cells, and the degree of infiltration of these cells correlated well with the degree of necroinflammation and elevations in AST. In AIH, the infiltration of these cells was more intense, while the degree of mononuclear cells did not correlate well with the degree of necroinflammation and elevations in AST seen in PBC; and (3) IgG+ plasma cells at the interface were predominant in AIH, while IgM+ predominant and IgG+/IgM+ equal cases were common in PBC. Thus, it seems likely that immunopathological processes appear to be similar at the hepatitic lesions in PBC and AIH, whereas hepatocellular injuries may not occur by the same mechanisms.
PBC and AIH are relatively well-defined clinicopathological entities; nevertheless, the histological distinction between PBC and AIH is sometimes challenging because interface hepatitis, which is characteristic of AIH, may also be encountered in PBC[9]. This study found that lobular hepatitis was more extensive in liver biopsies in AIH than in PBC with interface hepatitis. In the latter, such changes were more or less irregularly distributed in the liver specimens. Hepatitic rosette formation and emperipolesis have reportedly been significantly correlated with autoimmune liver disease[30] and are regarded as the typical features of AIH in the simplified AIH scoring system[22]. In this study, both of these features were also found in PBC, though their incidence and scores were much lower. These hepatitic features may be closely related to the progression of these diseases and may eventually be followed by the development of fibrosis and cirrhosis in PBC and AIH[3,19]. However, it is unclear whether the mechanism(s) of hepatocellular injuries are the same in these two diseases.
CD3+, CD4+, and CD8+ T cells were found in interface and lobular hepatitis in PBC and their scores at the interface and in the hepatic lobules increased with the degree of necroinflammation and correlated well with the elevation in AST. These cells have been suggested to be involved in immune-mediated hepatocellular injuries in PBC, and CD8+ cells particularly may be cytotoxic against hepatocytes.
Interestingly, mononuclear cells with similar phenotypes were also found in AIH and were more prominent in the hepatitic lesions than in PBC. While the infiltration scores of CD3+ and CD8+ cells were also correlated with the degree of lobular hepatitis in AIH, other correlations between the infiltration of these cells and the degree of necroinflammation and elevations in AST shown in PBC were not evident in AIH. Thus, it appears possible that the immunopathological features in interface and lobular hepatitis may be similar or shared in AIH and PBC; however, the precise hepatocellular injury mechanisms may not be the same. More detailed phenotypic analysis of the immunopathologies including antigen recognition and immunomodulation such as Th1 and Th2 cells, regulatory T cells, and NK cells[29] appear to be necessary to evaluate the mechanisms of hepatocellular injuries in PBC and AIH.
As for the B cell series, a small number of CD20+ B cells were only occasionally seen in interface hepatitis in PBC. In contrast, CD38+ cells, the majority being mature plasma cells, were numerous in interface and lobular hepatitis. Their scores increased with the degree of interface hepatitis and lobular hepatitis, suggesting that they are involved in the pathogenesis of necroinflammation at these areas in PBC. In fact, the scores of CD38+ and IgG+ cells at the interface and in the hepatic lobules and those of IgM+ cells at the interface were correlated with the elevations in AST in PBC. While the scores of CD38+, IgG+, and IgM+ cells in interface hepatitis and lobular hepatitis were higher in AIH than in PBC, no correlation existed between these scores and the elevation in serum AST in AIH.
The elevation in serum IgM level is typical of PBC, and serum IgG elevations are common in AIH[23]. Some reports have shown that IgG and IgM immunostaining of the liver helped to differentiate AIH from PBC[9,10,23,24]. Daniels et al[23] reported that PBC showed equal or more IgM staining than IgG staining, and that AIH always showed more IgG staining than IgM staining. Moreira et al[10] reported that an IgM/IgG ratio of ≥ 1 accurately distinguished PBC from AIH or PSC. However, Lee et al[9] reported that although IgM predominance seemed specific for PBC, a significant number of PBC cases also demonstrated IgG predominance. This study found that the average IgG+ cell infiltration score was significantly higher in AIH, whereas the IgM+ cell infiltration score was significantly higher in PBC. The number of IgA+ cells was small at the interface in both diseases. The majority of AIH cases were IgG-predominant, whereas the majority of PBC cases were IgM-predominant or IgM/IgG-equal and some cases were IgG-predominant. When PBC cases were largely divided into IgM-predominant or an equivalent group (group A) and IgG-predominant group (group B), serum IgM levels were significantly higher in group A. Interestingly, the severity of interface hepatitis was slightly higher and the degree of CD38+ cell infiltration at the interface and in the hepatic lobules was significantly higher in group B, suggesting that the PBC in group B may share more features with AIH.
More than 90% of PBC patients are AMA-positive, and more than 90% of AIH patients are ANA-positive[20,31,32]. These antibodies have been considered to be important for the diagnosis of PBC and AIH, though their pathogenic roles in hepatocellular injuries are speculative. This study found no difference in the degree of interface and lobular hepatitis or mononuclear cell infiltration at the interface irrespective of an AMA and ANA positivity in PBC and AIH. Therefore, it appears likely that ANA and AMA have no direct influence on interface and lobular hepatitis. While ANA and AMA may play a role in hepatocyte or cholangiocyte injury[31,33], these autoantibodies are not necessarily linked to the severity and pathogenesis of PBC and AIH[32-37], which was supported by the results of this study.
While PBC with interface hepatitis has been regarded as a hepatitic form of PBC or PBC with AIH features, some reports insist that PBC and AIH overlap in such cases and could preferably be called PBC-AIH syndrome[1,2,13,14,26]. It remains unclear whether the hepatitic features of these cases indicate an overlap of AIH in PBC or an inherent feature of PBC. The present study suggests that the immunopathogenesis of the hepatitic lesions in AIH and in PBC with interface hepatitis appears to be similar, whereas the precise hepatocellular injury mechanisms may not be the same in these two diseases, indicating that these hepatitic features in PBC do not reflect an overlap of AIH in PBC. Coss Adame et al[13] reported that so-called PBC-AIH overlapping cases showed different HLA phenotypes from type I AIH, suggesting that the former does not reflect an overlap of AIH in PBC, but a different disease from AIH, which supports the above-mentioned suggestion.
In conclusion, while the immunophenotypes of infiltrating cells at the interface and lobular hepatitis in AIH and PBC with interface hepatitis appear to be similar, the precise mechanisms of hepatocellular injuries may not be identical. More studies on the mechanisms of hepatocellular injuries in PBC are necessary to distinguish PBC with interface hepatitis from AIH.
COMMENTS
Background
Primary biliary cirrhosis (PBC) infrequently presents with the features of autoimmune hepatitis (AIH), such as interface hepatitis histologically, in addition to the classical features of PBC. Such cases have been called PBC with AIH features, and may respond well to steroid therapy.
Research frontiers
Previous studies have shown that the density and proportion of IgM+ and IgG+ plasma cells in portal tracts can be used to differentiate AIH from PBC by liver biopsies: IgM+ plasma cells tended to be predominant in portal tracts of PBC. However, the differences and similarities in the hepatitic lesions at the interface and in the hepatic lobules between PBC with interface hepatitis and AIH have not been studied pathologically and immunohistochemically in detail till now.
Innovations and breakthroughs
Immunohistochemical analysis showed that AIH exhibited more intense lobular hepatitis and more frequent hepatitic rosette formation and emperipolesis than that seen in PBC with interface hepatitis. In PBC, CD3+ and CD38+ cells were predominant in interface and lobular hepatitis, and the degree of infiltration of these cells correlated well with the degree of necroinflammation and elevations in AST. In AIH, the infiltration of these cells was more intense than in PBC, while the degree of mononuclear cell infiltration did not correlate well with the degree of necroinflammation and elevations in AST seen in PBC. IgG+ plasma cells at the interface were predominant in AIH, while IgM+ predominant and IgG+/IgM+ equal cases were common in PBC.
Applications
This study implicates that while the histological features of interface hepatitis in AIH and PBC with interface hepatitis appear to be similar, the precise pathogenetic mechanisms of hepatocellular injuries may not be identical. These results guarantee further studies on the pathogenesis of PBC with interface hepatitis in comparison with AIH.
Terminology
Interface hepatitis is characterized by dense lymphoplasmacytic infiltration and destruction of hepatocytes at the limiting plates. This lesion is consistently found in chronic progressive diseases such as AIH and chronic viral hepatitis. Hepatitic rosette is entrapped hepatocyte clusters arranged in glandular structures in the enlarged portal tracts and reflects ongoing hepatocellular loss at the limiting plates.
Peer review
The authors discuss clinicopathological differences between interface and lobular hepatitis seen in PBC with interface hepatitis and AIH. They conclude that the immunological mechanism in both disease entities is similar but that of hepatocyte injuries may be different, suggesting that autoimmune features observed in PBC with interface hepatitis might not be derived from the overlap of AIH. This study is an interesting one and worthy enough for possible publication.
Footnotes
Supported by Primary Biliary Cirrhosis Subdivision of Intractable Hepatobiliary Diseases Study Group of Japan (Chairman, Hirohito Tsubouchi; Department of Human and Environmental Sciences, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan)
P- Reviewers: Takahashi T, Umemura T S- Editor: Zhai HH L- Editor: Logan S E- Editor: Zhang DN
References
- 1.Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. doi: 10.1016/j.jhep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Aoyama H, Zen Y, Aishima S, Kitagawa S, Nakanuma Y. Proposal of a new staging and grading system of the liver for primary biliary cirrhosis. Histopathology. 2006;49:466–478. doi: 10.1111/j.1365-2559.2006.02537.x. [DOI] [PubMed] [Google Scholar]
- 3.Portman BC, Nakanuma Y. Diseases of bile ducts. In: Burt AD, Portman BC, Ferrell LD, editors. MacSween's Pathology of the Liver. 5th ed. London: Churchill Livingstone; 2006. pp. 517–581. [Google Scholar]
- 4.Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257–1260. doi: 10.1177/003591576706001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 6.Harada K, Hsu M, Ikeda H, Zeniya M, Nakanuma Y. Application and validation of a new histologic staging and grading system for primary biliary cirrhosis. J Clin Gastroenterol. 2013;47:174–181. doi: 10.1097/MCG.0b013e31827234e4. [DOI] [PubMed] [Google Scholar]
- 7.Kakuda Y, Harada K, Sawada-Kitamura S, Ikeda H, Sato Y, Sasaki M, Okafuji H, Mizukoshi E, Terasaki S, Ohta H, et al. Evaluation of a new histologic staging and grading system for primary biliary cirrhosis in comparison with classical systems. Hum Pathol. 2013;44:1107–1117. doi: 10.1016/j.humpath.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Shi TY, Zhang FC. Role of autoimmunity in primary biliary cirrhosis. World J Gastroenterol. 2012;18:7141–7148. doi: 10.3748/wjg.v18.i48.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Stapp RT, Ormsby AH, Shah VV. The usefulness of IgG and IgM immunostaining of periportal inflammatory cells (plasma cells and lymphocytes) for the distinction of autoimmune hepatitis and primary biliary cirrhosis and their staining pattern in autoimmune hepatitis-primary biliary cirrhosis overlap syndrome. Am J Clin Pathol. 2010;133:430–437. doi: 10.1309/AJCPE93GZSHUNTAI. [DOI] [PubMed] [Google Scholar]
- 10.Moreira RK, Revetta F, Koehler E, Washington MK. Diagnostic utility of IgG and IgM immunohistochemistry in autoimmune liver disease. World J Gastroenterol. 2010;16:453–457. doi: 10.3748/wjg.v16.i4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanuma Y, Saito K, Unoura M. Semiquantitative assessment of cholestasis and lymphocytic piecemeal necrosis in primary biliary cirrhosis: a histologic and immunohistochemical study. J Clin Gastroenterol. 1990;12:357–362. doi: 10.1097/00004836-199006000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Coss Adame E, Granados J, Uribe M, Torre A. Does HLA-DR7 differentiate the overlap syndrome of auto-immune hepatitis-primary biliary cirrhosis (AIH-PBC) from those with auto-immune hepatitis type 1? Ann Hepatol. 2013;10:28–32. [PubMed] [Google Scholar]
- 14.Wang Q, Selmi C, Zhou X, Qiu D, Li Z, Miao Q, Chen X, Wang J, Krawitt EL, Gershwin ME, et al. Epigenetic considerations and the clinical reevaluation of the overlap syndrome between primary biliary cirrhosis and autoimmune hepatitis. J Autoimmun. 2013;41:140–145. doi: 10.1016/j.jaut.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Harada K, Ebinuma H, Komori A, Yokokawa J, Yoshizawa K, Abe M, Miyake Y, Kikuchi K, Ohira H, et al. Primary biliary cirrhosis - Autoimmune hepatitis overlap syndrome: A rationale for corticosteroids use based on a nation-wide retrospective study in Japan. Hepatol Res. 2011;41:877–886. doi: 10.1111/j.1872-034X.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 16.Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14:3368–3373. doi: 10.3748/wjg.14.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 18.Gossard AA, Lindor KD. Development of autoimmune hepatitis in primary biliary cirrhosis. Liver Int. 2007;27:1086–1090. doi: 10.1111/j.1478-3231.2007.01538.x. [DOI] [PubMed] [Google Scholar]
- 19.Washington MK, Manns MP. Autoimmune hepatitis. In: Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s pathology of the liver. London: Churchill Livingstone; 2011. pp. 467–490. [Google Scholar]
- 20.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 21.Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol. 2011;55:171–182. doi: 10.1016/j.jhep.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 23.Daniels JA, Torbenson M, Anders RA, Boitnott JK. Immunostaining of plasma cells in primary biliary cirrhosis. Am J Clin Pathol. 2009;131:243–249. doi: 10.1309/AJCP8WHR0IEVUUOJ. [DOI] [PubMed] [Google Scholar]
- 24.Cabibi D, Tarantino G, Barbaria F, Campione M, Craxì A, Di Marco V. Intrahepatic IgG/IgM plasma cells ratio helps in classifying autoimmune liver diseases. Dig Liver Dis. 2010;42:585–592. doi: 10.1016/j.dld.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Graham RP, Smyrk TC, Zhang L. Evaluation of langerhans cell infiltrate by CD1a immunostain in liver biopsy for the diagnosis of primary biliary cirrhosis. Am J Surg Pathol. 2012;36:732–736. doi: 10.1097/PAS.0b013e31824b1dff. [DOI] [PubMed] [Google Scholar]
- 26.Nakanuma Y, Zen Y, Harada K, Sasaki M, Nonomura A, Uehara T, Sano K, Kondo F, Fukusato T, Tsuneyama K, et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int. 2010;60:167–174. doi: 10.1111/j.1440-1827.2009.02500.x. [DOI] [PubMed] [Google Scholar]
- 27.Theise ND, Bodenheimer Jr HC, Ferrell LD. Acute and chronic viral hepatitis. In: Burt AD, Portman BC, Ferrell LD, editors. MacSween's Pathology of the Liver. 6th ed. Edinburg, London: Churchill Livingstone; 2011. pp. 361–401. [Google Scholar]
- 28.Takahashi T, Miura T, Nakamura J, Yamada S, Miura T, Yanagi M, Matsuda Y, Usuda H, Emura I, Tsuneyama K, et al. Plasma cells and the chronic nonsuppurative destructive cholangitis of primary biliary cirrhosis. Hepatology. 2012;55:846–855. doi: 10.1002/hep.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longhi MS, Ma Y, Mieli-Vergani G, Vergani D. Aetiopathogenesis of autoimmune hepatitis. J Autoimmun. 2010;34:7–14. doi: 10.1016/j.jaut.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Kumari N, Kathuria R, Srivastav A, Krishnani N, Poddar U, Yachha SK. Significance of histopathological features in differentiating autoimmune liver disease from nonautoimmune chronic liver disease in children. Eur J Gastroenterol Hepatol. 2013;25:333–337. doi: 10.1097/MEG.0b013e32835a68a1. [DOI] [PubMed] [Google Scholar]
- 31.Moritoki Y, Lian ZX, Ohsugi Y, Ueno Y, Gershwin ME. B cells and autoimmune liver diseases. Autoimmun Rev. 2006;5:449–457. doi: 10.1016/j.autrev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ. Long-term follow-up of antimitochondrial antibody-positive autoimmune hepatitis. Hepatology. 2008;48:550–556. doi: 10.1002/hep.22380. [DOI] [PubMed] [Google Scholar]
- 33.Czaja AJ. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol. 1999;30:394–401. doi: 10.1016/s0168-8278(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 34.Goodman ZD, McNally PR, Davis DR, Ishak KG. Autoimmune cholangitis: a variant of primary biliary cirrhosis. Clinicopathologic and serologic correlations in 200 cases. Dig Dis Sci. 1995;40:1232–1242. doi: 10.1007/BF02065530. [DOI] [PubMed] [Google Scholar]
- 35.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, Zuin M, Podda M. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 36.Silveira MG, Talwalkar JA, Lindor KD, Wiesner RH. Recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant. 2010;10:720–726. doi: 10.1111/j.1600-6143.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 37.Mehendiratta V, Mitroo P, Bombonati A, Navarro VJ, Rossi S, Rubin R, Herrine SK. Serologic markers do not predict histologic severity or response to treatment in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2009;7:98–103. doi: 10.1016/j.cgh.2008.08.043. [DOI] [PubMed] [Google Scholar]


