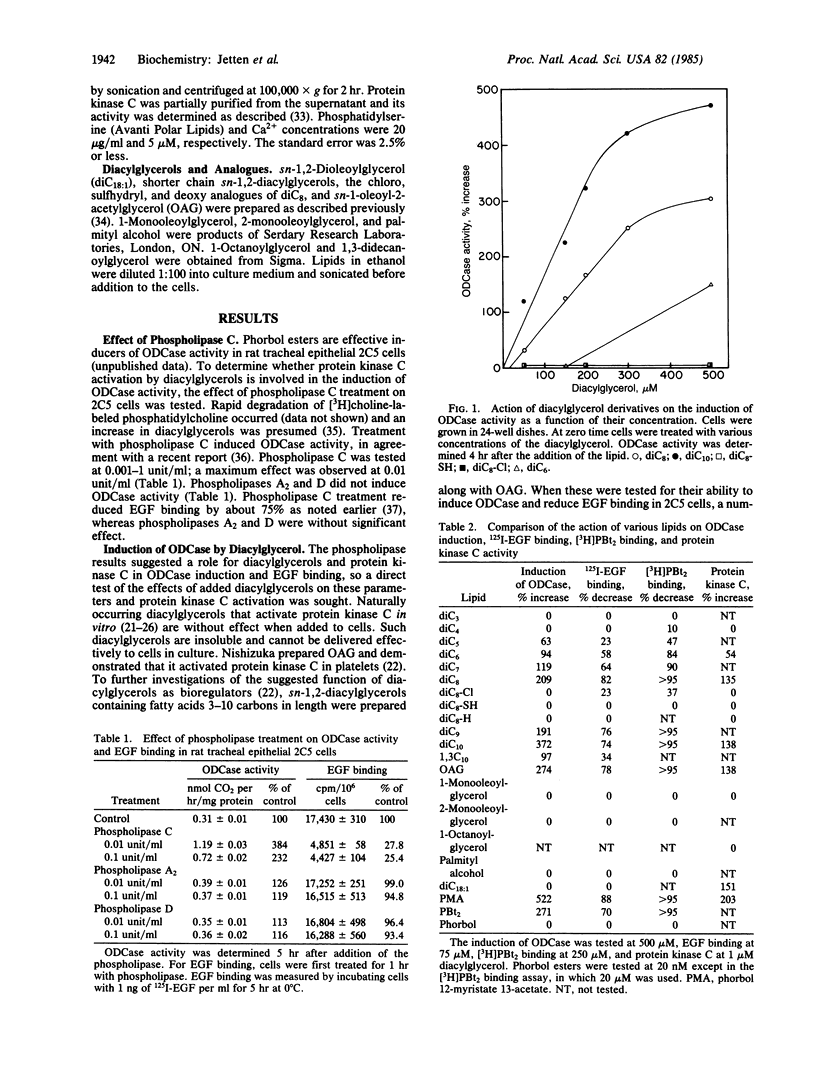
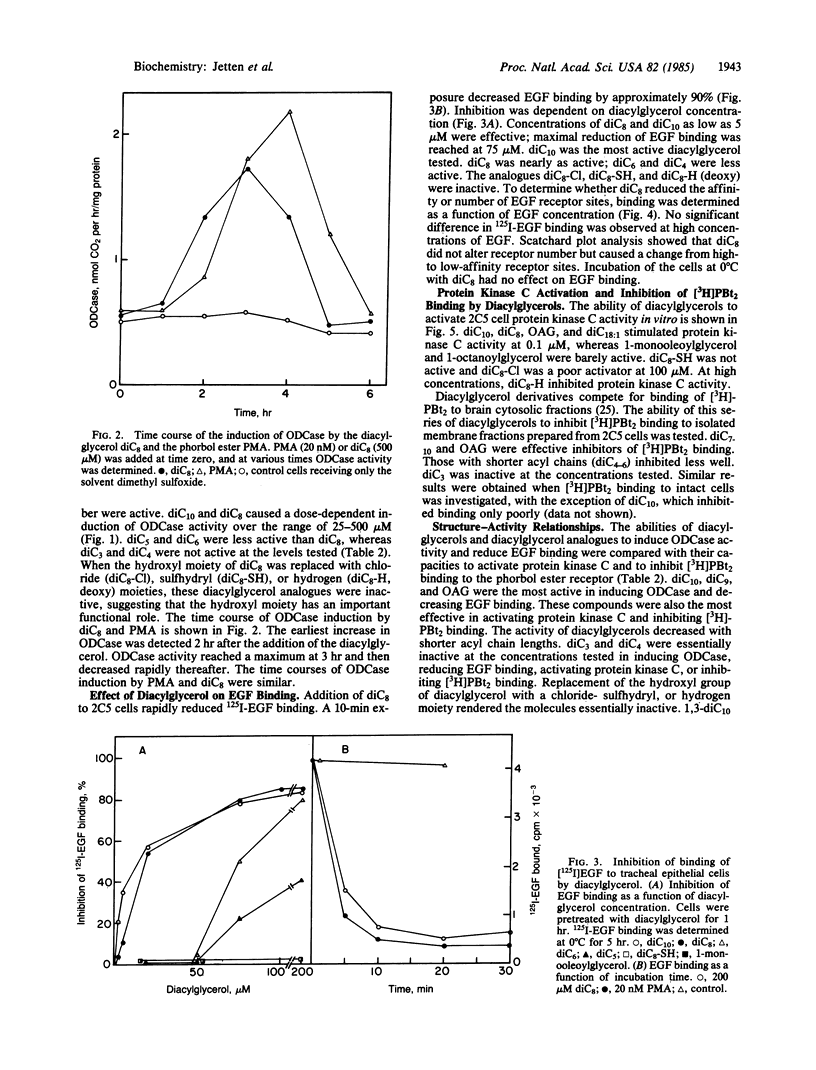
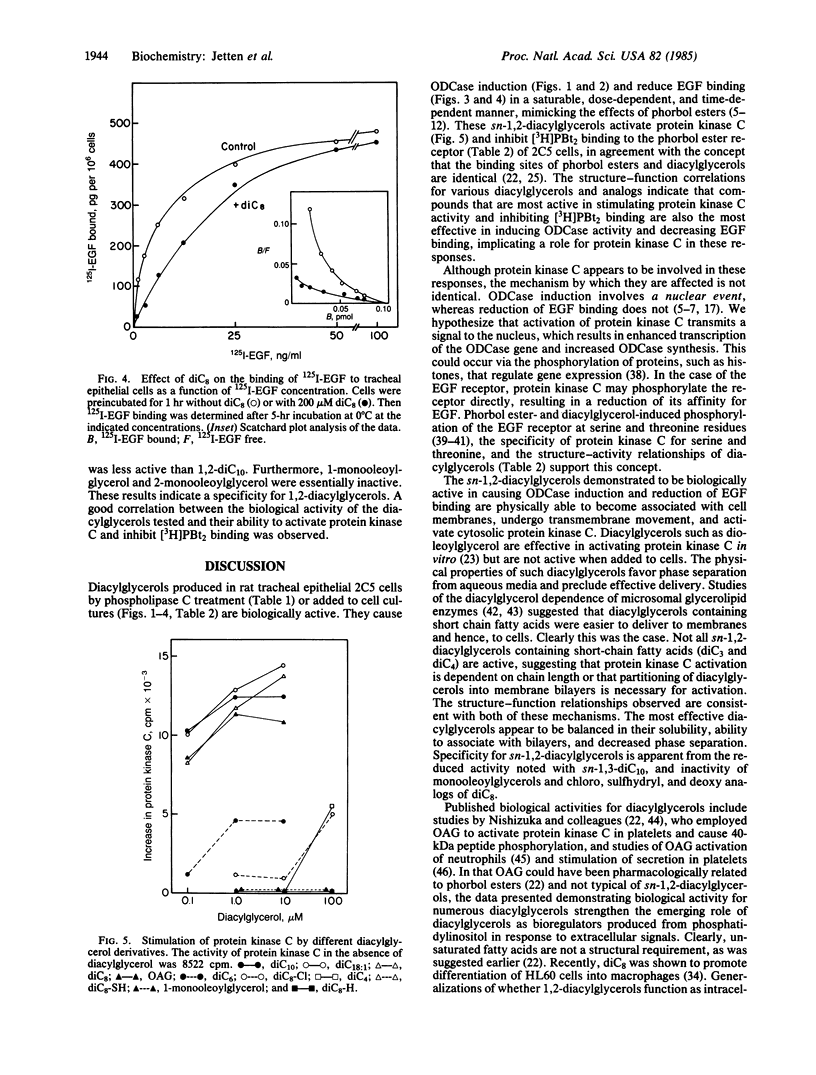
Abstract
Tumor-promoting phorbol esters induce ornithine decarboxylase (ODCase) activity and reduce epidermal growth factor (EGF) binding in rat tracheal epithelial 2C5 cells. Phorbol esters activate protein kinase C by interacting at the same site as sn-1,2-diacylglycerols, the presumed physiological regulators. The effects of added sn-1,2-diacylglycerols and those generated by phospholipase C treatment of 2C5 cells on ODCase induction and EGF binding were investigated to establish a role for protein kinase C in these cellular responses. Treatment of 2C5 cells with phospholipase C induced ODCase activity and reduced EGF binding, whereas phospholipases A2 and D were inactive. When sn-1,2-diacylglycerols containing fatty acids 3-10 carbons in length were added to 2C5 cells, those diacylglycerols containing fatty acids 5-10 carbons in length caused ODCase induction and reduction in EGF binding. sn-1,2-Dioctanoylglycerol was one of the most active compounds tested. It induced ODCase in a dose- (50-500 microM) and time-dependent manner. The reduction of binding of 125I-labeled EGF by sn-1,2-dioctanoylglycerol was also time and dose dependent and appeared to result from a change in EGF affinity and not the number of receptor sites. This series of sn-1,2-diacylglycerols showed similar structure-function relationships in their ability to induce ODCase activity, to decrease EGF binding, to stimulate protein kinase C, and to inhibit [3H]phorbol dibutyrate binding to the phorbol ester receptor. These data demonstrate biological activities for a number of diacylglycerols and indicate that protein kinase C activation is implicated in ODCase induction and decreased EGF binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L., Staller J. M., Boutwell R. K. Identification of a calcium- and phospholipid- dependent phorbol ester binding activity in the soluble fraction of mouse tissues. Biochem Biophys Res Commun. 1983 Feb 28;111(1):340–345. doi: 10.1016/s0006-291x(83)80157-0. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters, part 2. Crit Rev Toxicol. 1981 Jun;8(3):199–234. doi: 10.3109/10408448109109658. [DOI] [PubMed] [Google Scholar]
- Bolmer S. D., Wolf G. Retinoids and phorbol esters alter release of fibronectin from enucleated cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6541–6545. doi: 10.1073/pnas.79.21.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Phospholipid synthesis in isolated fat cells. Studies of microsomal diacylglycerol cholinephosphotransferase and diacylglycerol ethanolaminephosphotransferase activities. J Biol Chem. 1977 May 10;252(9):3050–3056. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- Crutchley D. J., Conanan L. B., Maynard J. R. Induction of plasminogen activator and prostaglandin biosynthesis in HeLa cells by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Mar;40(3):849–852. [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Jetten A. M. Action of retinoids and phorbol esters on cell growth and the binding of epidermal growth factor. Ann N Y Acad Sci. 1981 Feb 27;359:200–217. doi: 10.1111/j.1749-6632.1981.tb12748.x. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Retinoids specifically enhance the number of epidermal growth factor receptors. Nature. 1980 Apr 17;284(5757):626–629. doi: 10.1038/284626a0. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kuramoto A., Otani S., Matsui I., Morisawa S. Induction of ornithine decarboxylase by treatment of guinea pig lymphocytes with phospholipase C. FEBS Lett. 1983 Jan 24;151(2):233–236. doi: 10.1016/0014-5793(83)80076-3. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Lichti U., Gottesman M. M. Genetic evidence that a phorbol ester tumor promoter stimulates ornithine decarboxylase activity by a pathway that is independent of cyclic AMP-dependent protein kinases in CHO cells. J Cell Physiol. 1982 Dec;113(3):433–439. doi: 10.1002/jcp.1041130312. [DOI] [PubMed] [Google Scholar]
- Lichti U., Patterson E., Hennings H., Yuspa S. H. Differential retinoic acid inhibition of ornithine decarboxylase induction by 12-O-tetradecanoylphorbol-13-acetate and by germicidal ultraviolet light. Cancer Res. 1981 Jan;41(1):49–54. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Perchellet J. P., Boutwell R. K. Enhancement by 3-isobutyl-1-methylxanthine and cholera toxin of 12-O-tetradecanoylphorbol-13-acetate-stimulated cyclic nucleotide levels and ornithine decarboxylase activity in isolated epidermal cells. Cancer Res. 1980 Aug;40(8 Pt 1):2653–2660. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, McConnell R., Bronson D., Cuatrecasas P. A specific phosphoprotein phosphatase acts on histone H1 phosphorylated by protein kinase C. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6760–6764. doi: 10.1073/pnas.80.22.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando J. J., Young M. C. Identification of high-affinity phorbol ester receptor in cytosol of EL4 thymoma cells: requirement for calcium, magnesium, and phospholipids. Proc Natl Acad Sci U S A. 1983 May;80(9):2642–2646. doi: 10.1073/pnas.80.9.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Perturbation of membrane phospholipids alters the interaction between epidermal growth factor and its membrane receptors. Arch Biochem Biophys. 1981 Jan;206(1):222–226. doi: 10.1016/0003-9861(81)90084-9. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. I. Effects of phospholipase C treatment on phosphatidylcholine metabolism in Chinese hamster ovary cells and LM mouse fibroblasts. J Biol Chem. 1983 Jan 25;258(2):824–830. [PubMed] [Google Scholar]
- Strickland J. E., Jetten A. M., Kawamura H., Yuspa S. H. Interaction of epidermal growth factor with basal and differentiating epidermal cells of mice resistant and sensitive to carcinogenesis. Carcinogenesis. 1984 Jun;5(6):735–740. doi: 10.1093/carcin/5.6.735. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kawahara Y., Minakuchi R., Sano K., Kikkawa U., Mori T., Yu B., Kaibuchi K., Nishizuka Y. Calcium and phosphatidylinositol turnover as signalling for transmembrane control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:301–313. [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K., Ashendel C. L., Boutwell R. K. Inhibition by prostaglandin synthesis inhibitors of the induction of epidermal ornithine decarboxylase activity, the accumulation of prostaglandins, and tumor promotion caused by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Feb;40(2):308–315. [PubMed] [Google Scholar]
- Verma A. K., Shapas B. G., Rice H. M., Boutwell R. K. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979 Feb;39(2 Pt 1):419–425. [PubMed] [Google Scholar]
- Weeks C. E., Herrmann A. L., Nelson F. R., Slaga T. J. alpha-Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits tumor promoter-induced polyamine accumulation and carcinogenesis in mouse skin. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6028–6032. doi: 10.1073/pnas.79.19.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]



