Abstract
AIM: To elucidate the potential impact of examined lymph nodes (eLNs) on long-term survival of node-negative gastric cancer patients after curative surgery.
METHODS: A total of 497 node-negative gastric cancer patients who underwent curative gastrectomy between January 2000 and December 2008 in our center were enrolled in this study. Patients were divided into 4 groups according to eLNs through cut-point analysis. Clinicopathological features were compared between ≤ 15 eLNs group and > 15 eLNs group and potential prognostic factors were analyzed. The Log-rank test was used to assess statistical differences between the groups. Independent prognostic factors were identified using the Cox proportional hazards regression model. Stratified analysis was performed to investigate the impact of eLNs on patient survival in each stage. Overall survival was also compared among the four groups. Finally, we explored the recurrent sites associated with eLNs.
RESULTS: Patients with eLNs > 15 had a better survival compared with those with eLNs ≤ 15 for the entire cohort. By the multivariate survival analysis, we found that the depth of invasion and the number of eLNs were the independent predictors of overall survival (OS) of patients with node-negative gastric cancer. According to the cut-point analysis, T2-T4 patients with 11-15 eLNs had a significantly longer mean OS than those with 4-10 eLNs or 1-3 eLNs. Patients with ≤ 15 eLNs were more likely to experience locoregional and peritoneal recurrence than those with > 15eLNs.
CONCLUSION: Number of eLNs could predict the prognosis of node-negative gastric cancer, and dissection of > 15 eLNs is recommended during lymphadenectomy so as to improve the long-term survival.
Keywords: Gastric carcinoma, Examined lymph nodes, Node-negative, Prognosis
Core tip: The number of metastatic lymph nodes has been shown to be associated with prognosis in gastric cancer patients. In this study, we found that the overall survival of node-negative gastric cancer patients was significantly affected by examined lymph nodes (eLNs), and number of eLNs was an independent prognostic factor in multivariate analysis. Dissection of > 15 eLNs could reduce locoregional and peritoneal recurrence. We suggest that over 15 lymph nodes should be examined to improve the long-term outcome of node-negative gastric cancer patients following curative gastrectomy.
INTRODUCTION
Lymph node metastasis is one of the most important prognostic factors for gastric cancer patients following curative resection[1,2]. It is generally accepted that patients could benefit from a standardized approach of lymphadenectomy in terms of a higher overall survival (OS)[3-5]. At present, the category based on the absolute number of metastatic lymph nodes has been adopted by the Union for International Cancer Control (UICC) and American Joint Commission for Cancer as the N stage of the tumour, node, metastasis (TNM) classification since 1997 and the examination of no less than 15 regional lymph nodes was recommended for nodal metastatic status determination according to the latest version[6-8]. The 7th edition of the TNM classification designates node-negative (N0) disease as any gastric cancer with all examined lymph nodes (eLNs) negative, regardless of the total number of eLNs. Several studies have found that the prognosis of node-negative gastric cancer patients was associated with the number of dissected lymph nodes[9,10]. However, there is still controversy over how many nodes should be removed and examined when performing a radical gastrectomy for node-negative gastric cancer patients[9-11]. Therefore, the aim of the present study was to determine the optimal number of eLNs required for curative surgery. Furthermore, we intend to explore an appropriate classification based on the count of eLNs for precise prediction of the prognosis of gastric cancer patients with node-negative metastasis after curative surgery.
MATERIALS AND METHODS
Patients
A total of 2824 gastric cancer patients who underwent curative resection at the Gastric Cancer Surgery Department of Tianjin Medical University Cancer Hospital from January 2000 through December 2008 were selected for this study. The inclusion criteria for this study included: (1) patients with histologically proven primary adenocarcinoma of the stomach; (2) patients who underwent a lymphadenectomy (limited or extended); and (3) patients with all dissected lymph nodes proven to be negative by pathological examination. The exclusion criteria were: (1) patients who underwent palliative surgery; (2) patients with gastroesophageal junction tumor or cardia tumor; (3) patients who had distant metastasis or peritoneal dissemination that was confirmed during the operation; (4) patients who received neoadjuvant chemotherapy or radiotherapy before surgery; (5) patients with remnant gastric carcinoma; (6) patients who died during the initial hospital stay or within 1 mo after surgery; and (7) patients who were lost to follow-up. Based on these inclusion and exclusion criteria, a total of 497 gastric cancer patients were enrolled in this study.
Surgical treatment and pathological examination
All patients were operated on according to the potentially curative gastrectomy and lymphadenectomy. Curative resection was defined as a complete lack of grossly visible tumor tissue and metastatic lymph nodes remaining after resection, with pathologically negative resection margins[12]. Primary tumors were resected en bloc with limited or extended lymphadenectomy according to the Japanese Gastric Cancer Association[13]. The choice of surgical procedure of gastrectomy was made by the attending surgeon,s preference, and based mainly on the gastric cancer treatment guidelines in Japan[14]. Lymph nodes were meticulously dissected from the en bloc specimens by a specialist surgeon. Dissected lymph nodes were fixed in 10% formalin, embedded in paraffin, and stained with hematorylin-eosin. Each lymph node was re-examined microscopically and the status was determined by experienced pathologists.
Adjuvant therapy
Most of the patients received the adjuvant chemotherapy based on fluorouracil and leucovorin calcium after curative gastrectomy. Radiotherapy was not routinely administrated.
Statistical analysis
To determine the appropriate cutoffs for the number of examined negative lymph nodes, the cut-point survival analysis was adopted. The χ2 or Fisher’s exact test and t test were used to test differences in distributions between different eLNs subgroups. OS was calculated from the date of surgery to death from any causes. Survival curves were estimated using the Kaplan-Meier method and compared by the Log-rank test. Factors deemed to be of potential importance on univariate analyses (P < 0.05) were included in the multivariate analyses. Multivariate analysis of OS was performed by means of the Cox proportional hazards model, using the forward stepwise logistic regression procedure for variable selection. Hazard ratios and 95%CIs were generated. In all statistical analyses, significance was defined as P < 0.05 (two-sided). All analyses were performed using the statistical analysis program package SPSS version 17.0 (SPSS, Chicago, IL, United States).
Follow-up
After curative surgery, all patients were followed every 6 mo for 2 years, then every year or until death. The follow-up of all patients who were included in this study was completed in October 2012. The routine examination during follow-up included a physical examination, blood chemistry, B-mode ultrasonography, computed tonography scans, chest X-ray, and endoscopy.
RESULTS
Clinicopathological outcomes
The mean ± SD number of pathologically proved eLNs for the entire cohort of 497 patients was 13.8 ± 10.5 (ranging from 1 to 67). The 5-year survival rate (5-YSR) of all enrolled patients was 67.2%, and 332 patients were alive when the follow-up was completed. The median OS of all patients after surgery was 58.3 mo. The percentage of the number of eLNs for the entire cohort is shown in Figure 1.
Figure 1.
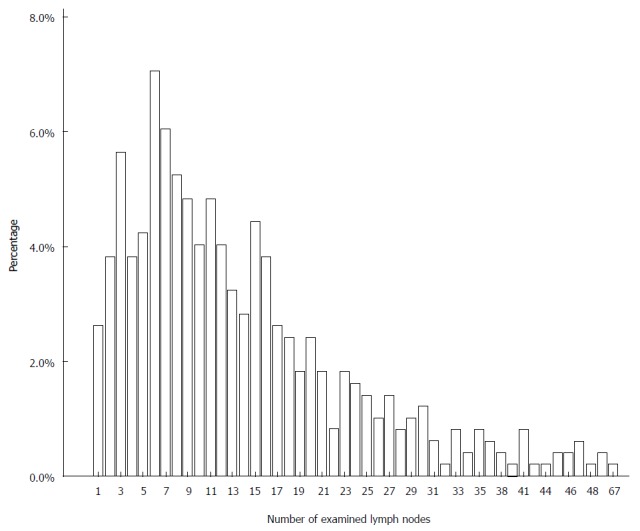
Percentage of the number of examined lymph nodes for the entire cohort.
Cut-point survival analysis for detection of best cutoffs of negative node counts
A cut-point survival analysis was performed to determine the negative lymph node counts that determine the greatest actuarial survival difference among the resulting subgroups. We selected the ability to detect differences among the subgroups on the basis of the magnitude of the log rank test χ2 statistic. Results for all relevant cut-points and stage subgroups are listed in Table 1. The cut-point analysis yielded the greatest survival difference at the level of 15 in pT1-2, pT3-4, and entire cohort. Patients with ≤ 15 eLNs were divided into 3 subgroups: 1-3 eLNs, 4-10 eLNs, and 11-15 eLNs according to the result of cut-point analysis. Comparison of clinicopathologic characteristics in patients of different eLNs groups is shown in Table 2. There were significant differences between the two groups of patients in the distribution of gender, age at surgery, tumor location, type of gastrectomy, histological type, and the extent of eLNs.
Table 1.
Overall survival by total examined lymph nodes and cut-point analysis in each subgroup
| Depth of invasion |
≤ 3 vs ≥ 4 |
≤ 10 vs ≥ 11 |
≤ 15 vs ≥ 16 |
≤ 25 vs ≥ 26 |
||||
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | |
| T1 + T2 | 8.333 | 0.004 | 9.679 | 0.002 | 11.023 | 0.001 | 0.825 | 0.364 |
| T3 + T4 | 5.529 | 0.019 | 7.884 | 0.005 | 13.204 | < 0.001 | 9.476 | 0.002 |
| Total | 8.711 | 0.003 | 16.589 | < 0.001 | 20.612 | < 0.001 | 5.770 | 0.016 |
Table 2.
Comparison of clinicopathologic characteristics between patients with ≤ 15 and >15 examined lymph nodes n (%)
| Variables |
Number of examined nodes |
χ2 | P value | |
| ≤ 15 | > 15 | |||
| Total | 331 (66.6) | 166 (33.4) | ||
| Gender | 11.741 | 0.001 | ||
| Male | 259 (78.2) | 106 (63.9) | ||
| Female | 72 (21.8) | 60 (36.1) | ||
| Age at surgery (yr) | 5.355 | 0.023 | ||
| ≤ 60 | 155 (46.8) | 96 (57.8) | ||
| > 60 | 176 (53.2) | 70 (42.2) | ||
| Tumor size (cm) | 0.845 | 0.392 | ||
| ≤ 4 | 163 (49.2) | 89 (53.6) | ||
| > 4 | 168 (50.8) | 77 (46.4) | ||
| Tumor location | 8.672 | 0.034 | ||
| Lower | 158 (47.7) | 98 (59.0) | ||
| Middle | 31 (9.4) | 16 (9.6) | ||
| Upper | 111 (33.5) | 35 (21.1) | ||
| Diffuse | 31 (9.4) | 17 (7.7) | ||
| Type of gastrectomy | 4.455 | 0.041 | ||
| Subtotal | 284 (85.8) | 130 (78.3) | ||
| Total | 47 (14.2) | 36 (21.7) | ||
| Histological type | 9.694 | 0.002 | ||
| Undifferentiated | 177 (53.5) | 113 (68.1) | ||
| Differentiated | 154 (46.5) | 53 (31.9) | ||
| Extent of lymphadenectomy | 111.652 | < 0.001 | ||
| Limited | 242 (73.6) | 39 (23.6) | ||
| Extended | 87 (26.4) | 126 (76.4) | ||
| Extent of examined lymph nodes | 84.522 | < 0.001 | ||
| Perigastric | 189 (57.1) | 23 (13.9) | ||
| Extragastric | 142 (42.9) | 143 (86.1) | ||
| Depth of invasion | 1.445 | 0.695 | ||
| T1 | 21 (6.3) | 13 (7.8) | ||
| T2 | 59 (17.8) | 35 (21.1) | ||
| T3 | 47 (14.2) | 24 (14.5) | ||
| T4 | 204 (61.6) | 94 (56.7) | ||
| TNM Classification 7th | 1.738 | 0.784 | ||
| Ia | 21 (6.3) | 13 (7.8) | ||
| Ib | 59 (17.8) | 35 (21.1) | ||
| IIa | 47 (14.2) | 24 (14.5) | ||
| IIb | 196 (59.2) | 89 (53.6) | ||
| IIIb | 8 (2.4) | 5 (3.0) | ||
Univariate survival analysis and multivariate survival analysis
By univariate survival analysis, we found that five clinicopathological variables were significantly associated with the OS of node-negative gastric cancer patients after curative resection. They are as follows: extent of eLNs, extent of lymphadenectomy, depth of invasion, number of eLNs, and the 7th UICC TNM classification of gastric caner (Table 3). All of the five variables considered significant on univariate analysis were included in the Cox proportional hazards model (forward stepwise procedure) to calculate the independent prognostic factors. As a result, there were two independent, statistically significant prognostic parameters: depth of invasion (P < 0.001) and number of eLNs (P < 0.001). The hazard ratios and their 95%CIs are listed in Table 3.
Table 3.
Univariate and multivariate survival analyses of 497 node-negative gastric cancer patients
| Variables | 5-YSR |
Univariate analysis |
Multivariate analysis |
||
| χ2 | P value | HR (95%CI) | P value | ||
| Gender | 1.213 | 0.271 | |||
| Male | 66.0 | ||||
| Female | 70.4 | ||||
| Age at surgery (yr) | 2.720 | 0.099 | |||
| ≤ 60 | 70.1 | ||||
| > 60 | 64.2 | ||||
| Tumor size (cm) | 3.548 | 0.060 | |||
| ≤ 4 | 71.0 | ||||
| > 4 | 63.2 | ||||
| Tumor location | 0.167 | 0.682 | |||
| Upper | 69.9 | ||||
| Middle | 53.2 | ||||
| Lower | 69.5 | ||||
| Diffuse | 60.4 | ||||
| Type of gastrectomy | 2.045 | 0.153 | |||
| Subtotal | 68.3 | ||||
| Total | 61.4 | ||||
| Histological type | 0.117 | 0.732 | |||
| Undifferentiated | 67.9 | ||||
| Differentiated | 66.2 | ||||
| Extent of lymphadenectomy | 6.093 | 0.014 | |||
| Limited | 63 | ||||
| Extended | 73.2 | ||||
| Extent of examined lymph nodes (LNs) | 12.112 | 0.001 | |||
| Perigastric | 58.9 | ||||
| Extragastric | 73.3 | ||||
| Depth of invasion | 22.355 | < 0.001 | < 0.001 | ||
| T1 | 88.2 | 1 | |||
| T2 | 79.8 | 1.801 (0.613-5.293) | 0.285 | ||
| T3 | 74.6 | 2.102 (0.703-6.287) | 0.184 | ||
| T4 | 59.1 | 4.066 (1.502-11.007) | 0.006 | ||
| Number of examined LNs | 32.690 | < 0.001 | 0.334 (0.221-0.506) | < 0.001 | |
| ≤ 15 | 58.6 | 1 | |||
| > 15 | 84.3 | 0.327 (0.216-0.495) | < 0.001 | ||
| TNM Classification 7th | 22.541 | < 0.001 | |||
| Ia | 88.2 | ||||
| Ib | 79.8 | ||||
| IIa | 74.6 | ||||
| IIb | 59.3 | ||||
| IIIb | 53.8 | ||||
5-YSR: 5-year survival rate; TNM: Tumour, node, metastasis.
Survival analysis and subgroup analysis
As shown in Figure 2A, the 5-YSR of patients with > 15 eLNs was significantly higher than those with ≤ 15 eLNs (84.3% vs 58.6%, P < 0.001). As shown in Table 4 and Figure 2B, the 5-YSR was 43.3% for patients with 1-3 eLNs compared with 56.6%, 71.8%, and 84.3% for those with 4-10 eLNs, 11-15 eLNs, and > 15 eLNs, respectively. Subgroup analysis was then conducted to evaluate the survival of the patients in different pT categories (Table 5). For patients with stage T2 disease, the 5-YSR differences were statistically significant among 1-3, 4-10, 11-15, and > 15 eLNs groups, and patients with > 15 eLNs had the best OS, so were patients with stage T3 or T4 disease (Figure 2C-E). The OS in patients with T1 tumors showed no statistical difference (Table 5).
Figure 2.
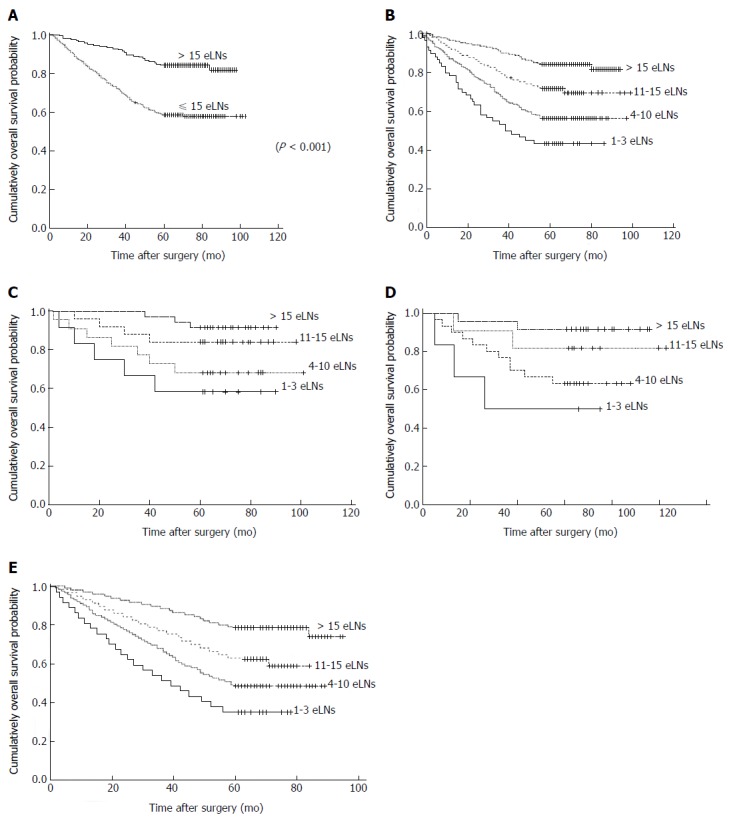
Survival curves. A: For ≤ 15 eLNs group and > 15 eLNs group in total node-negative gastric cancer patients; B: For 4 categories in total node-negative gastric cancer patients according to the number of examined negative lymph nodes (P < 0.001); C: For 4 categories in pathological T2 patients according to the number of examined negative lymph nodes (P = 0.022); D: For 4 categories in pathological T3 patients according to the number of examined negative lymph nodes (P = 0.033); E: For 4 categories in pathological T4 patients according to the number of examined negative lymph nodes (P < 0.001). eLNs: Examined lymph nodes.
Table 4.
Five-year overall survival by T stage subgroups and total number of examined lymph nodes
| Variables |
Number of examined nodes (n) |
χ2 | P value | |||
| 1-3 | 4-10 | 11-15 | ≥ 16 | |||
| Depth of invasion | ||||||
| T1 | 60.0% | 91.7% | - | 92.3% | 4.598 | 0.204 |
| T2 | 58.3% | 68.2% | 84.0% | 91.4% | 9.668 | 0.022 |
| T3 | 50.0% | 63.3% | 81.8% | 91.7% | 8.738 | 0.033 |
| T4 | 35.1% | 48.6% | 62.5% | 78.7% | 31.487 | < 0.001 |
| Total | 43.3% | 56.6% | 71.8% | 84.3% | 51.837 | < 0.001 |
Table 5.
Recurrent sites of node-negative gastric cancer in terms of ≤ 15 and > 15 examined lymph nodes n (%)
| Recurrent sites |
Number of examined nodes |
χ2 | P value | |
| ≤ 15 | > 15 | |||
| Recurrence | 4.491 | 0.043 | ||
| Yes | 65 (19.6) | 20 (12.0) | ||
| No | 266 (80.4) | 146 (88.0) | ||
| Locoregional recurrence | 4.362 | 0.048 | ||
| Yes | 37 (11.2) | 9 (5.4) | ||
| No | 294 (88.8) | 157 (94.6) | ||
| Peritoneal seeding | 4.874 | 0.037 | ||
| Yes | 24 (7.3) | 4 (2.4) | ||
| No | 307 (92.7) | 162 (97.6) | ||
| Hematogenous spread | 1.740 | 0.239 | ||
| Yes | 24 (7.3) | 7 (4.2) | ||
| No | 307 (92.7) | 159 (95.8) | ||
Recurrent sites of node-negative gastric cancer
Among all the 497 patients, 85 (17.1%) had tumor recurrences during follow-up. The recurrence patterns observed in the 85 patients were locoregional recurrence (n = 46), hematogenous spread (n = 31), and peritoneal seeding (n = 28). A single recurrence pattern was noted in 65 patients: 29 had locoregional recurrence, 19 had hematogenous spread, and 17 had peritoneal seeding. More than 1 recurrence pattern was observed in 20 patients. Table 5 shows the distribution of recurrent sites in terms of ≤ 15 eLNs and > 15 eLNs. Patients with ≤ 15 eLNs had a high rate of total recurrence (19.6% vs 12.0%, P = 0.043), locoregional recurrence (11.2% vs 5.4%, P = 0.048) and peritoneal seeding (7.3% vs 2.4%, P = 0.037). There was no statistical difference in hematogenous spread rate between the two groups (7.3% vs 4.2%, P = 0.239).
DISCUSSION
Although several new staging methods have been proposed to evaluate the prognosis of gastric cancer, the TNM staging system based on the count of positive lymph nodes is still widely used, for the number of positive lymph nodes reflects well the prognosis[15-17]. It was reported that gastric cancer patients might be staged incorrectly because of an insufficient number of eLNs[18]. Large population studies from various institutions and countries demonstrated that there was a significant association between the number of eLNs and the OS of patients, so the latest TNM staging system recommends that no less than 15 lymph nodes should be examined for the accuracy of N staging[6-8]. Meanwhile, the same edition staging system designates node-negative disease as any gastric cancer with all eLNs negative, regardless of the total number of eLNs, thus the present study was designed to determine the optimal number of lymph nodes required for curative surgery for node-negative gastric cancer.
Adachi et al[19] pointed out that the clinicopathologic characteristics and prognosis of node-negative gastric cancer patients were similar to those of patients with early gastric cancer. Several studies showed that in patients with node-negative gastric cancer, the 5- and 10-year OS rates varied from 72% to 92% and 88% to 93%, respectively, and these patients had a significantly better survival than the patients with node-positive gastric cancer[20-24]. In this study, the 5-YSR was 67.2%, which was lower than that observed in studies in Japan and Korea due largely to the lower proportion of patients with early stage disease (6.8%) in our study. Our previous study found that the node-positive gastric cancer patients had a better OS with an increased count of negative lymph nodes retrieved[17]. This phenomenon was also observed in the studies about node-negative gastric cancer. There are several possible explanations for this observation. First, there may have been lymph node metastases that were missed during dissection or pathological examination in node-negative patients who had ≤ 15 eLNs, which may cause inappropriate understaging. More eLNs should increase the opportunity to find metastatic lymph nodes, which could reduce the influence of inappropriate understaging[25-27]. Second, micrometastasis was considered to play an important role in prognosis of gastric cancer patients. Patients with micrometastasis were reported to have an especially high risk of recurrence[10]. It was reported that 17%-32% of node-negative gastric cancer patients were identified by routine histologic examination, and micrometastasis was detected by immunohistochemical and molecular examinations. Therefore, dissection of adequate negative lymph nodes could improve the prognosis of node-negative gastric cancer patients by reducing micrometastasis. Third, several studies have confirmed that lymphovascular invasion is associated with poor survival in gastric cancer patients[28,29]. More eLNs means dissection of potential lymphovascular invasion, which may improve prognosis by reducing recurrence. In addition, it is speculated that patients with more lymph nodes in the specimen have better immune response against the tumor, and smaller LNs were correlated with decreased immune response in gastrointestinal cancer[32]. Strong immune status also contributes to good prognosis.
Son et al[33] investigated 10010 patients with gastric cancer, and the results showed that the majority of patients with ≤ 15 eLNs had T1 tumors (64.2%) or pN0 disease (81.3%). Consequently, the patients with no more than 15 eLNs had a significantly worse prognosis after they underwent standard curative lymphadenectomy, compared with patients with > 15 eLNs. Our data revealed that there were significant survival differences between the patients who had > 15 eLNs and ≤ 15 eLNs by cut-point analysis. By the multivariate analyses, we found that the number of eLNs is an independent prognostic factor that was associated with a worse prognosis. In this study, 66.6% of patients had ≤ 15 eLNs, which contributed to the lower OS of the entire cohort. The main reasons for examination of ≤ 15 eLNs after curative gastrectomy were deemed to be the inaccurate lymph node dissection or examination[34-36]. These inaccuracies most likely occur by inexperienced surgeons or pathologists[37]. In addition, the inaccurate lymph node dissection was considered to be closely related to host clinicopathologic status and immunologic response[38,39]. Besides, patient,s comorbidities, the intrinsic number of LNs, and lymphovascular invasion status may play a role in inadequate LN dissection and assessment. Therefore, it is important to evaluate the prognostic value for patients with ≤ 15 eLNs. In this study, we performed cut-point analysis and determined 3 cut-off points to classify all patients into 4 subgroups as 1-3, 4-10, 11-15, and ≥ 16 eLNs, which can not only reflect the distribution of eLNs, but also demonstrate the correlation between the prognosis of patients and the number of eLNs intuitively.
Gastrectomy with lymphadenectomy remains the standard treatment option for gastric cancer, while it is often performed without an accurate knowledge of nodal status. Sentinel lymph node (SLN) is defined as the first LN to receive drainage by a primary tumor. Therefore, the status of SLN could predict the overall status of LN metastasis. As extensive lymphadenectomies are not without complications, limited gastric resections without an extended lymphadenectomy have been applied to the selected patients with early gastric cancer according to the status of SLN[40]. For early N0 gastric cancer, SLN should be used in selected patients for less invasive surgical approaches, while it is not easy to predict accurately the status of LN metastasis through SLN because lymphatic drainage is complex for advanced N0 disease, so the extended lymphadenectomy is still essential for advanced gastric cancer. In addition to the number of eLNs, the depth of tumor invasion was identified as one of the most important prognostic indicators of node-negative gastric cancer in the present study, which was similar to other studies[9,20-23,33]. The number of eLNs was reported to be associated with depth of invasion. Compared with the patients who had less than 6 eLNs, patients who had 6-10 eLNs, 11-15 eLNs, and ≥ 16 eLNs had a significantly lower chance of death in T2-4 disease, while the gastric cancer specific survival among different eLNs groups had no statistical difference in T1 stage, because patients with stage T1 disease have a small number of cases and a higher 5-YSR[9].
Based on our results, patients with > 15 eLNs had better prognosis than those with ≤ 15 eLNs in T2-T4 stages. Patients with ≤ 15 eLNs should be divided into 3 groups as 1-3 eLNs, 4-10 eLNs, and 11-15 eLNs for acquisition of significant statistical differences among the subgroups of patients in T2-T4 stages. Recently, Huang et al[22] suggested that the D2 lymphadenectomy should involve 15 nodes for pT1-T2 disease and 20 nodes for pT3-T4 disease. However, it is difficult to determine accurately the T stage before surgery. From our results, we suggested that patients with node-negative gastric cancer following curative dissection should have more than 15 eLNs. It should be considered as a necessary supplement to the TNM staging system.
COMMENTS
Background
Count of metastatic lymph nodes has been shown to be associated with prognosis of gastric cancer. Several studies have found that the prognosis of node-negative gastric cancer was associated with the number of dissected lymph node. However, there is still controversy over how many nodes should be removed and examined when performing a radical gastrectomy for node-negative gastric cancer patients.
Research frontiers
Radical gastrectomy with regional lymph node dissection is the only possible curative treatment for gastric cancer. A significant portion of patients with gastric cancer have been staged incorrectly because of an insufficient number of examined lymph nodes (eLNs). Research has demonstrated an association between an adequate number of eLNs and improved overall survival. Lymph node stage can be determined appropriately when the number of total eLNs is > 15 according to the latest tumour, node, metastasis (TNM) staging system, while it is still controversial over how many nodes should be removed and examined when performing a radical gastrectomy for node-negative gastric cancer patients. In this study, the authors demonstrated that number of eLNs was an independent prognostic factor for node-negative gastric cancer after curative resection and more than 15 eLNs should be obtained.
Innovations and breakthroughs
This study reported that patients with node-negative gastric cancer following curative dissection should have more than 15 examined lymph nodes in a large sample analysis. It should be considered as a necessary supplement to the TNM staging system. Patients with ≤ 15 examined lymph nodes should be divided into 3 groups as 1-3 eLNs, 4-10 eLNs, and 11-15 eLNs for acquisition of significant statistical differences among the subgroups of patients in T2-T4 stages.
Applications
By understanding the positive association between the number of eLNs and prognosis of gastric cancer, this study may stimulate surgeons and pathologists to acquire more than 15 eLNs during curative gastrectomy.
Terminology
eLNs are the number of dissected lymph nodes during surgery, which is visually estimated by surgeons and pathologists immediately after surgery. Sentinel lymph node is defined as the first lymph node to receive drainage by a primary tumor.
Peer review
The most appropriate number of eLNs required during a radical gastrectomy for node-negative gastric cancer patients is still controversial. This study shows that patients with node-negative gastric cancer following curative dissection should have more than 15 examined lymph nodes in a large sample analysis. This conclusion has some significance for guiding clinical work.
Footnotes
Supported by A grant from the National Basic Research Program of China (973 Program), No. 2010CB529301
P- Reviewers: Stanojevic GZ, Takao S S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Surgical treatment of advanced gastric cancer: Japanese perspective. Dig Surg. 2007;24:101–107. doi: 10.1159/000101896. [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125–133. doi: 10.1007/s101200050006. [DOI] [PubMed] [Google Scholar]
- 4.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasakura Y, Mochizuki F, Wakabayashi K, Kochi M, Fujii M, Takayama T. An evaluation of the effectiveness of extended lymph node dissection in patients with gastric cancer: a retrospective study of 1403 cases at a single institution. J Surg Res. 2002;103:252–259. doi: 10.1006/jsre.2002.6368. [DOI] [PubMed] [Google Scholar]
- 6.Katai H, Yoshimura K, Maruyama K, Sasako M, Sano T. Evaluation of the New International Union Against Cancer TNM staging for gastric carcinoma. Cancer. 2000;88:1796–1800. [PubMed] [Google Scholar]
- 7.Aurello P, D’Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, Ercolani G, De Angelis R, Ramacciato G. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007;73:359–366. [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Xu D, Huang Y, Geng Q, Guan Y, Li Y, Wang W, Yuan S, Sun X, Chen Y, Li W, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS One. 2012;7:e38681. doi: 10.1371/journal.pone.0038681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A, Saragoni L, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–73. doi: 10.1097/SLA.0b013e3181e4585e. [DOI] [PubMed] [Google Scholar]
- 11.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–2866. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 12.Hermanek P, Wittekind C. Residual tumor (R) classification and prognosis. Semin Surg Oncol. 1994;10:12–20. doi: 10.1002/ssu.2980100105. [DOI] [PubMed] [Google Scholar]
- 13.Jaehne J, Meyer HJ, Maschek H, Geerlings H, Burns E, Pichlmayr R. Lymphadenectomy in gastric carcinoma. A prospective and prognostic study. Arch Surg. 1992;127:290–294. doi: 10.1001/archsurg.1992.01420030052010. [DOI] [PubMed] [Google Scholar]
- 14.Shimada Y. JGCA (The Japan Gastric Cancer Association). Gastric cancer treatment guidelines. Jpn J Clin Oncol. 2004;34:58. [PubMed] [Google Scholar]
- 15.Deng J, Sun D, Pan Y, Zhang L, Zhang R, Wang D, Hao X, Liang H. Ratio between negative and positive lymph nodes is suitable for evaluation the prognosis of gastric cancer patients with positive node metastasis. PLoS One. 2012;7:e43925. doi: 10.1371/journal.pone.0043925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J, Liang H, Sun D, Wang D, Pan Y. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol. 2010;17:1259–1266. doi: 10.1245/s10434-010-0939-x. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Liang H, Wang D, Sun D, Ding X, Pan Y, Liu X. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol. 2010;17:1043–1051. doi: 10.1245/s10434-009-0863-0. [DOI] [PubMed] [Google Scholar]
- 18.Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 19.Adachi Y, Mori M, Maehara Y, Kitano S, Sugimachi K. Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg. 1997;184:373–377. [PubMed] [Google Scholar]
- 20.Lee CC, Wu CW, Lo SS, Chen JH, Li AF, Hsieh MC, Shen KH, Lui WY. Survival predictors in patients with node-negative gastric carcinoma. J Gastroenterol Hepatol. 2007;22:1014–1018. doi: 10.1111/j.1440-1746.2006.04488.x. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Takahashi M, Kikuchi S, Yamauchi H. Significant prognostic factors in patients with node-negative gastric cancer. Int Surg. 1999;84:331–336. [PubMed] [Google Scholar]
- 22.Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Lin BJ, Lu HS. Prognostic impact of dissected lymph node count on patients with node-negative gastric cancer. World J Gastroenterol. 2009;15:3926–3930. doi: 10.3748/wjg.15.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng J, Liang H, Sun D, Zhang R, Zhan H, Wang X. Prognosis of gastric cancer patients with node-negative metastasis following curative resection: outcomes of the survival and recurrence. Can J Gastroenterol. 2008;22:835–839. doi: 10.1155/2008/761821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito H, Kuroda H, Matsunaga T, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic indicators in node-negative advanced gastric cancer patients. J Surg Oncol. 2010;101:622–625. doi: 10.1002/jso.21562. [DOI] [PubMed] [Google Scholar]
- 25.Dudeja V, Habermann EB, Abraham A, Zhong W, Parsons HM, Tseng JF, Al-Refaie WB. Is there a role for surgery with adequate nodal evaluation alone in gastric adenocarcinoma? J Gastrointest Surg. 2012;16:238–246; discussion 246-247. doi: 10.1007/s11605-011-1756-7. [DOI] [PubMed] [Google Scholar]
- 26.Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol. 2005;12:981–987. doi: 10.1245/ASO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Park JM, Jung CW, Park SS, Kim SJ, Mok YJ, Kim CS, Chae YS, Bae JW. The significances of lymph node micrometastasis and its correlation with E-cadherin expression in pT1-T3N0 gastric adenocarcinoma. J Surg Oncol. 2008;97:125–130. doi: 10.1002/jso.20937. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–774. doi: 10.1007/BF02574499. [DOI] [PubMed] [Google Scholar]
- 30.Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, Akiyama H, Fukushima T, Nagahori Y, Takahashi M. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204–211. doi: 10.1016/j.surg.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Huynh R, Abraham I, Kim E, Kumar RR. Number of lymph nodes examined and its impact on colorectal cancer staging. Am Surg. 2006;72:902–905. [PubMed] [Google Scholar]
- 32.Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149–2154. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 33.Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH, Noh SH. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687–4693. doi: 10.1002/cncr.27426. [DOI] [PubMed] [Google Scholar]
- 34.Roviello F, Rossi S, Marrelli D, Pedrazzani C, Corso G, Vindigni C, Morgagni P, Saragoni L, de Manzoni G, Tomezzoli A. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol. 2006;94:275–280; discussion 274. doi: 10.1002/jso.20566. [DOI] [PubMed] [Google Scholar]
- 35.Mahar AL, Qureshi AP, Ottensmeyer CA, Pollett A, Wright FC, Coburn NG, Chetty R. A descriptive analysis of gastric cancer specimen processing techniques. J Surg Oncol. 2011;103:248–256. doi: 10.1002/jso.21827. [DOI] [PubMed] [Google Scholar]
- 36.Lemmens VE, van Lijnschoten I, Janssen-Heijnen ML, Rutten HJ, Verheij CD, Coebergh JW. Pathology practice patterns affect lymph node evaluation and outcome of colon cancer: a population-based study. Ann Oncol. 2006;17:1803–1809. doi: 10.1093/annonc/mdl312. [DOI] [PubMed] [Google Scholar]
- 37.Callahan MA, Christos PJ, Gold HT, Mushlin AI, Daly JM. Influence of surgical subspecialty training on in-hospital mortality for gastrectomy and colectomy patients. Ann Surg. 2003;238:629–636; discussion 636-639. doi: 10.1097/01.sla.0000089855.96280.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, Kohno H, Nagasue N. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 39.Dudeja V, Habermann EB, Zhong W, Tuttle TM, Vickers SM, Jensen EH, Al-Refaie WB. Guideline recommended gastric cancer care in the elderly: insights into the applicability of cancer trials to real world. Ann Surg Oncol. 2011;18:26–33. doi: 10.1245/s10434-010-1215-9. [DOI] [PubMed] [Google Scholar]
- 40.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]


