Abstract
Background
Recently we found that fever (part of HIV-related wasting) is induced by the action of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein (gp120) in the preoptic anterior hypothalamus (POAH). As the opioid system plays a role in the pathogenesis of HIV-1, in the present study we sought to examine the capacity of the opioid system to regulate the febrile response induced by gp120.
Methods
Stainless steel cannulas were stereotactically into the POAH, and a biotelemetry system was used to monitor the body temperature (Tb changes). We examined the in vivo effects of naloxone as well as highly opioid-selective receptor antagonists, on gp120-induced fever.
Results
Pretreatment with naloxone or the mu-opioid receptor-selective antagonist, cyclic D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH(2) (CTAP), significantly delayed the febrile response induced by gp120. In contrast, naltriben (NTB), a selective antagonist for the delta-2 opioid receptor, did not cause any effect on gp120-induced fever.
Conclustion
These results (1) provide pharmacologic evidence of a functional in vivo interaction between the opioid system and this viral protein in the POAH and (2) show that mu-opioid receptors can regulate gp120-induced fever.
Keywords: HIV, fever, opioid system and gp120
1. INTRODUCTION
Wasting syndrome is a common complication of HIV infection and is marked by progressive weight loss and weakness, often associated with fever (Weinroth et al., 1995). Fever is a highly complex process initiated by the action on the brain thermosensitive cells of a number of endogenous pyrogens, which are produced by the host in response to infectious as well as non-infectious inflammatory insults (Blatteis and Sehic, 1997). It is believed to be caused by the synthesis and release from monocytes and macrophages of a number of well characterized endogenous pyrogenic factors (Blatteis, 2006). The participation of the opioid system in the pathogenesis of fever has been previously reported (Benamar et al., 2000, 2002a). Specifically, we have shown that mu-opioid receptors in the POAH are implicated in the inflammatory response to bacterial LPS and endogenous pyrogens (Benamar et al., 2000, 2002a). Mu opioid receptors within the POAH have been found to be involved in the fever induced by interleukin-6 (IL-6) (Benamar et al., 2002a), as pretreatment with the selective mu-opioid antagonist (CTAP) significantly blocked the IL-6-induced fever. Moreover, LPS failed to produce fever in mice lacking the mu opioid receptor (Benamar et al., 2005).
HIV-1 enters cells through interaction of glycoprotein (gp120) with host CD4 and specific chemokine receptors (Feng et al., 1996). Gp120 has been detected in the brains of HIV-1-infected individuals (Jones et al., 2000). It is released by infected cells (Schneider et al., 1986) and may be toxic to certain uninfected cells (Lipton, 1991). It is implicated in the pathogenesis of neurological disorders associated with HIV. In rodents, the presence of gp120 in the brain has been associated with several neurobehavioral alterations. In particular, it has been shown that intracerebroventricular injection of gp120 in rats produced a marked sickness behavior syndrome, consisting of reduced exploratory behavior, suppressed consumption of food and saccharin solution, and reduced body weight and fever (Barak et al., 2002).
Recently, we have shown that gp120 given directly into the preoptic anterior hypothalamus (POAH), the main brain area for the control of body temperature (Tb) was able to induce a significant increase in Tb in a dose-related manner (Benamar et al., 2010). The aim of the present study was to investigate whether pharmacological manipulation of the opioid system at level of this brain area interferes with febrile response induced by gp120.
2. METHODS
2.1 Animals
All animal use procedures were conducted in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (250–300 g; Harlan, Indianapolis, IN). They were housed two per cage for at least one week before surgery and were fed laboratory chow and water ad libitum. Ambient temperature of 21 ± 0.3 °C, and a 12 h light/12 h dark cycle was used.
2.2 Surgery procedures
Rats were anesthetized with an intraperitoneal injection of a mixture of ketamine hydrochloride and acepromazine maleate. An incision 2 cm in length was made along the linea alba, and the underlying tissue was dissected and retracted. A calibrated transmitter (E-mitters, series 4000; Mini Mitter, Sunriver, OR) was then inserted in the intraperitoneal space. After the transmitter was passed through the incision, the abdominal musculature and dermis were sutured independently. For cannula implantation, each animal was placed in a stereotaxic instrument. A sterilized stainless steel C313G cannula guide (22 gauge, Plastics One Inc., Roanoke, VA) was implanted into the POAH. The stereotaxic coordinates were as follows: 0.3 mm posterior to bregma, 0.5 mm from the midline and 7 mm ventral to the dura mater (Paxinos and Watson, 2007). A C313DC cannula dummy (Plastic One Inc., Roanoke, VA) of identical length was inserted into the guide tube to prevent its occlusion.
2.3 Microinjection
The animals were habituated to the handling procedure necessary for microinjections during the recovery period. The rats were gently restrained while the dummy stylets were removed and replaced with a C313I injector cannula (28-gauge, Plastics One Inc., Roanoke) extending 1 mm below the cannula tips. After the recovery period, rats were allowed to habituate to test chambers for 1 h before testing. Either vehicle or drug was microinjected into the POAH in a volume of 0.5 μl. The C313I injector cannula was connected by polyethylene tubing to a 10-μl Hamilton syringe. A volume of 0.5 μl of drug or vehicle was delivered at a rate of 0.5 μl per min (manually) and the internal cannula left in place an additional 90 sec to allow diffusion. Immediately thereafter, a dummy cannula (C313DC) was inserted into the cannula guide to prevent any contamination.
2.4 Body temperature measurement (Tb)
Tb was measured by a biotelemetry system (Mini-Mitter, Sunriver, OR) using calibrated transmitters implanted intraperitoneally (i.p.). Signals from the transmitter were delivered through a computer-linked receiver. This method minimizes stress to animals during the Tb reading. Thus, the Tb could be monitored continuously and recorded without restraint or any disturbance to the animal. Rats were tested in an environmental room (Hotpack), maintained at 21 ± 0.3 °C ambient temperature and 50 ± 2 % relative humidity. After one hour of adaptation, two readings at 15-min intervals were averaged to determine the baseline.
2.5 Drugs
HIV-1MN gp120 recombinant viral protein (Advanced Biotechnologies, Columbia) was used. The protein, produced using the Baculovirus expression system and purified by immunoaffinity chromatography, has a molecular weight of 120 Kd. Naloxone, CTAP and NTB were obtained from the National Institute on Drug Abuse, and were dissolved in sterile pyrogen-free saline. Naloxone is a general opioid antagonist, CTAP is a mu-selective antagonist, and NTB is a selective antagonist for the delta-2 opioid receptor.
2.6 Statistical and histological analysis
All data are reported as means ± SEM, and the variations in Tb were compared across treatments and time points and analyzed by two-way ANOVA followed by the Bonferroni’s test. The data were analyzed by Prism software (Graph-Pad, San Diego, CA). Significance was set at P < 0.05.
At the conclusion of the experiments, each rat was injected with 0.5 μl of cresyl violet, anesthetized and perfused transcardially with 0.9% isotonic saline, followed by phosphate-buffered saline (PBS) 4% paraformaldehyde (ph 7.4). The brain was removed, stored in the same fixative for 4 h, kept in 20% sucrose overnight, and cut into 20-μm sections on a freezing microtome. Each coronal section was mounted according to standard histological procedures (Benamar et al., 2004), and the site of injection was verified by locating the dye. Only data from animals in which the site of injection was clearly located within the POAH were included in the studies.
2.7 Experimental Protocol
Tb experiments were started between 9:00 and 10:00 AM. On the morning of the experiment, rats were placed in an environmental room which was maintained at a constant temperature of 21 ± 0.3°C and relative humidity of 52 ± 2%. After a 1-h acclimation interval, baseline temperature measurements were taken. Tb was taken every 15 min during a 60-min baseline interval. After the baseline interval, either gp120 (133 ng) or vehicle was injected directly into the POAH and a biotelemetry system was used to monitor the changes in Tb for 270 min. To assess whether the gp120-induced fever was mediated via the opioid system, either naloxone, CTAP or NTB, respectively, was administered before gp120. After a 60-min baseline interval, naloxone (1–10 mg/kg, sc), CTAP (1 μg, POAH) or NTB (1 μg, POAH) was injected. Gp120 (133 ng, POAH) was injected 30 min later.
3. RESULTS
To determine the role of the opioid system in the gp120-induced fever, first we used a general opioid antagonist, naloxone. While a dose of 1 mg/kg had no effect on gp120-induced fever, the pretreatment with naloxone at doses of 5 and 10 mg/kg significantly attenuated the fever induced by gp120 (Fig 1A). The administration of naloxone (10 mg/kg s.c.) had no significant effect by itself on Tb (Fig 1A).
Fig. 1.
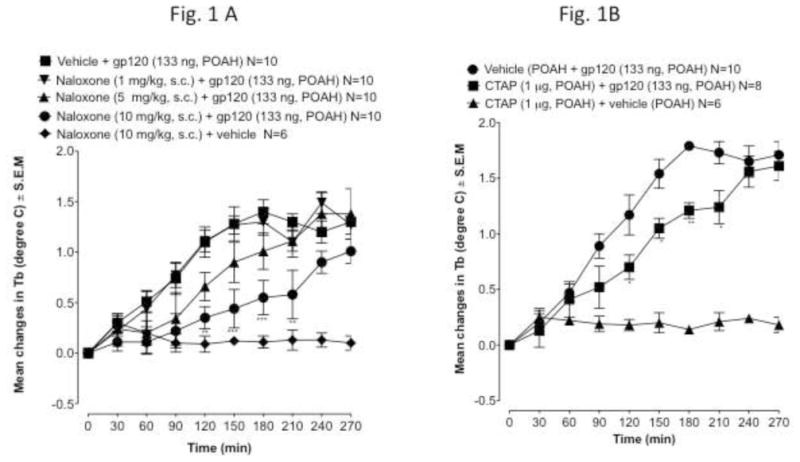
Fig. 1A. The effect of naloxone (1–10 mg/kg, −30 min) given subcutaneously on gp120-induced fever. Gp120 is given at time 0. Values represent mean ± S.E.M from baseline body temperature. ΔTb, is the mean change in body temperature. N, is the number of rats. Basal body temperatures before treatment of each group was as follows: ▲= 37.47 ± 0.15 °C; ■ = 37.54 ± 0.17°C, ● = 37.63 ± 0.18 °C, ▼ = 37.55 ± 0.15 °C and ◆ = 37.70 ± 0.11 °C ** p<0.01, *** p<0.001
Fig. 1B. Effect of intrahypothalamic pretreatment with CTAP (1 μg, −30 min) or saline (1 μl, −30 min) on the fever response induced by gp120 (133 ng), given at time 0. Values represent mean ± S.E.M from baseline body temperature. ΔTb, is the mean change in body temperature. N, is the number of rats. Basal body temperatures before treatment of each group was as follows: ▲= 37.62 ± 0.18 °C; ■ = 37.49 ± 0.19 °C and ● = 37.53 ± 0.21 °C. ** p<0.001. * p<0.05.
To identify which specific opioid receptor is responsible for the effect of naloxone on gp120-induced fever, we used selective opioid receptor antagonists. Previously, it has been shown that kappa opioid receptor activation produces hypothermia and the activation of mu or delta-2 opioid receptors induces hyperthermia (Geller et al., 1983; Benamar et al., 2002b). Therefore, we examined specific mu and delta receptor antagonists. We have also previously found that the mu-selective antagonist CTAP was effective at reducing the febrile response of LPS and IL-6 (Benamar et al., 2000, 2002a). As seen in Fig 1B, CTAP attenuated gp120-induced fever (F2.28=5,45 P < 0.001). The dose of CTAP (1 μg) used had no effect by itself on Tb (Fig. 1B). To determine whether delta opioid receptors play a role in the pathogenesis of the fever induced by gp120, we pretreated the rats with NTB, a potent and selective antagonist for the delta-2 opioid receptor. Previously, we have examined the effect of the intra-POAH injection of NTB, by itself, on Tb (Benamar et al., 2004). NTB at a dose that has no effect on Tb (1 μg) was used to determine whether delta-2 receptors are implicated in the febrile response induced by gp120. NTB was injected directly into the POAH 30 min prior to gp120 (133 ng). In contrast to CTAP, NTB did not cause any effect on gp120-induced fever.
4. DISCUSSION
The principal finding of this study is that opioid system and gp120 interact in the POAH, and pharmacological manipulation of mu-opioid receptors can regulate gp120-induced fever.
Naloxone and CTAP were able to alter the febrile response induced by gp120. This effect was particularly evident during the initial hours following gp120 administration (90–210 min). We also investigated the potential role of the delta-2 opioid receptor in gp120-induced fever. The delta-2 opioid receptors do not seem to be involved, as pretreatment with NTB did not show any effect on the febrile response. From these data, it appears that mu-opioid receptors participate in the initiation of the development of gp120-induced fever, and confirm the earlier data that the opioid system is involved in initiating the pathogenesis of fever (Benamar et al., 2000; Blatteis and Romanovsky, 1994).
Gp120 is implicated in the pathogenesis of neurological disorders associated with HIV and is capable of initiating neurotoxic cascades via an interaction with the CXCR4 and/or CCR5 chemokine receptors. Our recent data demonstrated that CXCR4 receptors in the POAH contribute to the pathogenesis of fever induced by gp120 (Benamar et al., 2010). In situ hybridization and immunocytochemistry showed that CXCR4 neuronal expression is mainly found in several brain areas, including the POAH (Banisadr et al., 2002; Guyon et al., 2005). A functional interaction between the opioid system and chemokine receptors (particularly those involved in HIV life-cycle, CCR5 and CXCR4) is well documented (Szabo et al., 2002, Adler et al., 2006, Chen et al., 2007). The mu-opioid and chemokine receptors are both members of the GPCR superfamily, expressed in the POAH and implicated in the pathogenesis of fever. Together, these data raise the possibility that a functional interaction is likely to occur between gp120 (via CXCR4 or/and CCR5) and mu-opioid receptors at the level of GPCR in the POAH during the development of the febrile response.
HIV and drug abuse are not isolated problems, but they interact and influence each other. Current available data indicating that opioid can exacerbate the central nervous system complications of HIV-1 infection. Wasting syndrome is a common complication of HIV infection and is marked by progressive weight loss and weakness, often associated with fever. Recently, we found that direct infusion of gp120 into POAH induces fever, indicating that this viral protein contributes to generation of fever associated with HIV-wasting syndrome (Benamar et al., 2010). Previously we have shown that mu-opioid receptors are the target for morphine-induced hyperthermia (Benamar et al., 2007). These data together with the present results suggest that the use or abuse of morphine may potentiate the fever associated with HIV-wasting syndrome.
Fig. 2.
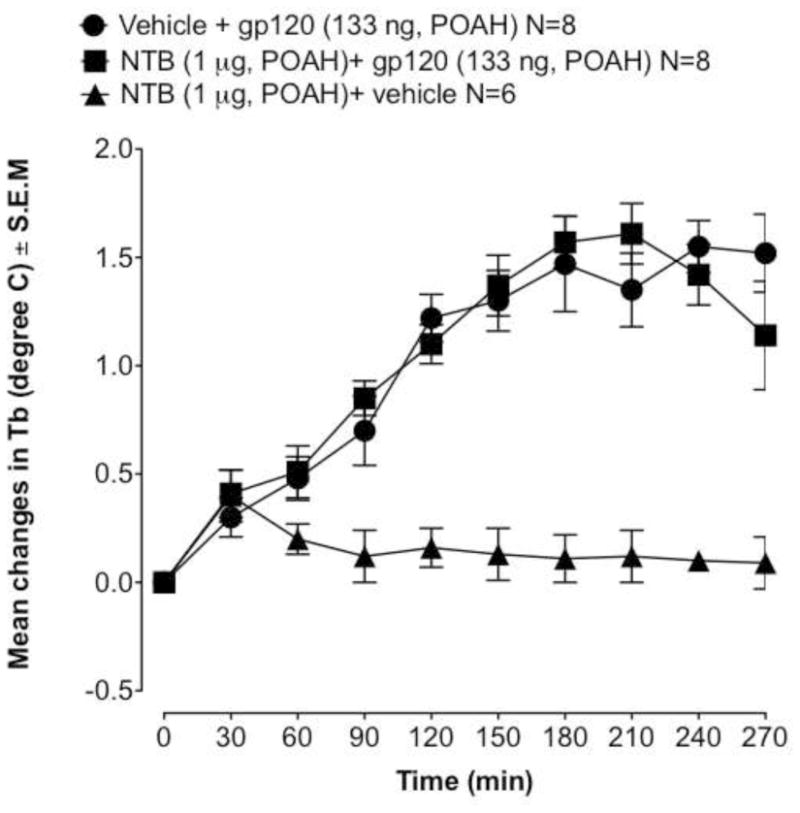
Effect of intrahypothalamic pretreatment with NTB (1 μg, −30 min) or saline (0.5 μl, −30 min) on the fever response induced by gp120 (133 ng), given at time 0. Values represent mean ± S.E.M from baseline body temperature. ΔTb, is the mean change in body temperature. N, is the number of rats. Basal body temperatures before treatment of each group was as follows: ▲= 37.36 ± 0.11°C; ■ = 37.41 ± 0.14°C and ● = 37.47 ± 0.16 °C.
Acknowledgments
Role of funding source
This work was supported by grants DA031605 (to KB) and R21DA029414 (to KB) from the National Institutes of Health.
Footnotes
Contributors
K Benamar designed the study and wrote the manuscript.
J. Palma implementation of experiments.
ME Abood revised the draft.
All the authors have been contributed to and approved the final version of the manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7:E865–70. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Barak O, Weidenfeld J, Goshen I, Ben-Hur T, Taylor AN, Yirmiya R. Intracerebral HIV-1 glycoprotein 120 produces sickness behavior and pituitary-adrenal activation in rats: role of prostaglandins. Brain Behav Immun. 2002;16:720–735. doi: 10.1016/s0889-1591(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Benamar K, Addou S, Yondorf M, Geller EB, Eisenstein TK, Adler MW. Intrahypothalamic injection of the HIV-1 envelope glycoprotein induces fever via interaction with the chemokine system. J Pharmacol Exp Ther. 2010;332:549–553. doi: 10.1124/jpet.109.160309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamar K, Geller EB, Adler MW. Effect of a μ-opioid receptor-selective antagonist on interleukin-6 fever. Life Sci. 2002a;70:2139–2145. doi: 10.1016/s0024-3205(01)01535-1. [DOI] [PubMed] [Google Scholar]
- Benamar K, Geller EB, Adler MW. Role of the nitric oxide pathway in kappa-opioid-induced hypothermia in rats. J Pharmacol Exp Ther. 2002b;303:375–378. doi: 10.1124/jpet.102.036269. [DOI] [PubMed] [Google Scholar]
- Benamar K, McMenamin M, Geller EB, Chung YG, Pintar JE, Adler MW. Unresponsiveness of mu-opioid receptor knockout mice to lipopolysaccharide-induced fever. Br J Pharmacol. 2005;144:1029–1031. doi: 10.1038/sj.bjp.0706145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamar K, Rawls SM, Geller EB, Adler MW. Intrahypothalamic injection of deltorphin-II alters body temperature in rats. Brain Res. 2004;1019:22–27. doi: 10.1016/j.brainres.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Benamar K, Xin L, Geller EB, Adler MW. Blockade of lipopolysaccharide-induced fever by a μ-opioid receptor-selective antagonist in rats. Eur J Pharmacol. 2000;401:161–165. doi: 10.1016/s0014-2999(00)00424-6. [DOI] [PubMed] [Google Scholar]
- Benamar K, Yondorf M, Barreto VT, Geller EB, Adler MW. Deletion of mu-opioid receptor in mice alters the development of acute neuroinflammation. J Pharmacol Exp Ther. 2007;323:990–994. doi: 10.1124/jpet.107.129973. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther. 2006;111:194–223. doi: 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Romanovsky AA. Endogenous opioids and fever. In: Zeisberger E, Schonbaum E, Lomax P, editors. Thermal Balance in Health and Disease. Birkhuser Verlag; Basel: 1994. pp. 435–441. [Google Scholar]
- Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997;12:1–9. [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88:36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Geller EB, Hawk C, Keinath SH, Tallarida RJ, Adler MW. Subclasses of opioids based on body temperature change in rats: acute subcutaneous administration. J Pharmacol Exp Ther. 1983;225:391–398. [PubMed] [Google Scholar]
- Guyon A, Banisadr G, Rovere C, Cervantes A, Kitabgi P, Melik-Parsadaniantz S, Nahon JL. Complex effects of stromal cell-derived factor-1 alpha on melanin-concentrating hormone neuron excitability. Eur J Neurosci. 2005;21:701–710. doi: 10.1111/j.1460-9568.2005.03890.x. [DOI] [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Lipton SA. HIV-related neurotoxicity. Brain Pathol. 1991;1:193–199. doi: 10.1111/j.1750-3639.1991.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2007. [Google Scholar]
- Schneider J, Kaaden O, Copeland TD, Oroszlan S, Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986;67:2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OMZ, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinroth SE, Parenti DM, Simon GL. Wasting syndrome in AIDS: pathophysiologic mechanisms and therapeutic approaches. Infect Agents Dis. 1995;4:76–94. [PubMed] [Google Scholar]


