Abstract
The objectives of this study were to develop an animal model to study Listeria monocytogenes infection during the peri-parturient period and identify sources of maternal shedding of the pathogen. Peri-parturient mice were infected intragastrically with L. monocytogenes that expressed bacterial luciferase. Mice were then imaged in vivo over time. Secreted breast milk samples from mice infected after parturition were enriched and plated for culture and imaging. Bioluminescence imaging technology was able to detect luciferase emitting L. monocytogenes in vaginal secretions and maternal and fetal organs at 72 and 96 h post infection in mice infected prior to, or just after, parturition. The results from this study clearly show that L. monocytogenes is shed in vaginal secretions and disseminates to the mammary chain, from which it can be shed in the milk of peri-parturient mice.
Keywords: Listeria monocytogenes, breast milk, bioluminescence
Introduction
Listeria monocytogenes is an important foodborne pathogen, with a predilection to infect the fetus and neonate in both human and veterinary species. Twenty-two percent of human perinatal infections result in stillbirth or neonatal death (1). L. monocytogenes are also one of the three major worldwide causes of human neonatal meningitis. Neonatal listeriosis manifests in two forms: early or late onset (2). Infants with early onset neonatal listeriosis are thought to be infected either in utero during the bacteremic phase of the mother, who may present with a flu-like illness, or by ascending infection from the birth canal (3). Average time for onset of clinical illness is 1.5 days of age. Presenting symptoms are consistent with sepsis including acute respiratory distress, fever, and lethargy. In addition, infants can develop pneumonia, and rarely meningitis or myocarditis (2). Late onset neonatal listeriosis is less common and occurs more frequently in infants who are healthy at birth from uncomplicated pregnancies and normal maternal health (4). Transmission in late onset listeriosis is assumed to occur during passage through the birth canal, or via nosocomial infection of the infant (5). Average age for onset of clinical disease is 14.3 days and is more commonly associated with meningitis than sepsis. The late onset form of neonatal listeriosis can also present with mild to moderate clinical signs including fever, anorexia, diarrhea, and lethargy or irritability (6).
Although infants infected with L. monocytogenes are at high risk for severe disease, infected adults are often asymptomatic or present with mild flu-like symptoms. However, in people with any degree immunosuppression, which is seen in neonates, the elderly, pregnant women, patients suffering immune compromising infections, and people taking immunosuppressive drugs, listeriosis can result in severe septic disease syndromes (7, 8).
The mouse has been used to study L. monocytogenes infection because similar syndromes of sepsis are seen experimental mouse infection models (9, 10). Specific to pregnant women, L. monocytogenes infection carries a high risk of fetal infection and abortion. This is true in pregnant mice as well and the mouse has emerged as a model to study pathogenesis of listeriosis during pregnancy (11, 12).
We recently reported a novel murine model of L. monocytogenes infection during pregnancy that uses bioluminescence technology. The latter allowed us to perform real-time in vivo imaging and semi-quantify the outcome of intragastric infection, which resulted in maternal sepsis, fetal infection, and abortion (12). Bioluminescence technology has also been used with intravenous infection of the gerbil to study fetal L. monocytogenes infection (13). In this study, we use bioluminescence technology to visualize potential sources for transmission of L. monocytogenes to infants during parturition and lactation.
Breast milk feeding is the standard of care for infants because of the short and long term medical and neurodevelopmental advantages for the infant (14). Because of the distinct health benefits, breastfeeding is now considered a public health issue, not solely a lifestyle choice, by the American Association of Pediatrics (14). Historically, breast milk is not considered a significant risk factor for transmission of L. monocytogenes, or other bacterial pathogens, to nursing infants. This is surprising considering the high awareness of human breast milk transmission of viral pathogens such as hepatitis B, cytomegalovirus, herpes simplex, Epstein Barr virus, and human immunodeficiency virus (HIV) (15–18). There are rare reports that Listeria and Salmonella contamination of human breast milk resulted in neonatal illness (19, 20). Furthermore, transmission of bacterial pathogens via milk is widely recognized as causing infection of offspring in domesticated mammalian species. For example, L. monocytogenes contaminated milk and colostrum can cause neonatal sepsis in foals, calves, and crias (21, 22). It is also widely known that sheep and cattle intermittently shed L. monocytogenes in their milk, which is a major concern for zoonotic transmission to people via consumption of unpasteurized dairy products (23, 24).
Listeria monocytogenes is well adapted to survive in and on human breast milk collection and storage equipment, as the organism tolerates high and low temperatures, as well as high salt and low pH concentrations (25). It is common for nursing mothers to collect and store milk in suboptimal storage conditions (e.g. small coolers) when away from home where thorough cleaning with sanitizers is not available. Considering that exposure and transient colonization of the gastrointestinal tract is relatively common (44% of healthy pregnant women shed L. monocytogenes in their feces when repeatedly cultured, 12% culture positive when a single sample is taken), contamination of breast milk with the pathogen is a distinct possibility (26).
The study presented here uses bioluminescence to test the hypothesis that intragastric L. monocytogenes infection of peri-parturient mice results in shedding of bacteria in vaginal and mammary secretions.
Methods
Strains of L. monocytogenes
Bioluminescent L. monocytogenes strain 10403SLUX, serotype 1/2a, was originally obtained from Dr. Christopher Contag (Stanford, CA). This construct was made bioluminescent by transformation with the plasmid pAUL-A Tn4001 luxABCDE Kmr, optimized for expression in Gram-positive bacteria (27). The addition of the lux-kan transposon cassette decreased virulence, evidenced by increased LD50 compared to the parent strain. This is theorized to be due to ectopic expression of novel proteins, to which L. monocytogenes is known to be sensitive (28).
Preparation of L. monocytogenes
L. monocytogenes cells were stored at −20°C on Cryobank™ Cryobeads (Copan Diagnostics, Inc., Corana, CA). For each experiment, a bead was placed into 5 ml of brain heart infusion (BHI) broth (BD Biosciences, Franklin Lakes, NJ) and incubated overnight with shaking at 37°C. Bacterial cells were harvested by centrifugation (3,500 × g for 5 minutes), washed three times in phosphate buffered saline and kept on ice prior to inoculating mice. The bacterial suspensions were diluted to the desired concentration, and numbers of viable L. monocytogenes confirmed by plating serial dilutions onto tryptic soy agar with 5% sheep blood (BD® Biosciences).
Infection of mice
Female inbred A/J mice were obtained from the Jackson Laboratories (Bar Harbor, ME) at six weeks of age and housed under microisolator caps at the UW-Madison Microbial Sciences animal care facility. The inbred A/J mouse strain was used because it is a prototypical susceptible strain of mouse regulated, in part, by the Hc locus on chromosome two (29). Mice were acclimated for one week in this facility prior to being paired with a breeding male. Female mice were infected at various times during pregnancy and after parturition. Pre-partum infections were done at 10–14 days gestation (start of 3rd trimester) or 18–20 days gestation (just prior to whelping). For post-parturition infection experiments, lactating mice were infected three days post-whelping. Mice received food and water ad libitum until five hours prior to an intragastric infection experiment, at which time food was removed from the cage. This was done to minimize the risk of delivering the bacterial inoculum into stomachs engorged with mouse chow, which could lead to aspiration of the inoculum into the lungs. Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). When the mice were sedated, the listerial inoculum (106 colony forming units (CFU)) was introduced (in a total volume of 0.1 ml 0.9% NaCl) via a 1.5 in.-long, 24 gauge, stainless steel oral esophageal tube attached to a 1-ml syringe.
Bioluminescence imaging of mammary glands and vaginal secretions
Bioluminescence imaging was performed using an IVIS® 200 Imaging System (Caliper Life Sciences, Hopkinton, MA) as instructed by the manufacturer. Two hours prior to imaging, dams were removed from the pups to allow mammary engorgement. Five minutes prior to imaging, the mice were injected with 10 USP units of oxytocin (Bimeda, Dublin, Ireland) to stimulate milk let down to the gland cistern. Imaging the vaginal secretions of mice experiencing abortion after infection were imaged without prior preparation. All imaged mice were anesthetized with isoflurane and bioluminescence was recorded for one to three minutes at a pixel binning of eight. Blood agar plates were imaged for ten seconds at a pixel binning of eight. Bioluminescence was measured as total photon flux (photons/s/cm2) by the Living Image® software package (Caliper Life Sciences, Hopkinton, MA).
Recovery of L. monocytogenes from luminescent mammary glands
Milk was collected from luminescent mammary glands immediately after imaging while mice remained unconscious from isoflurane anesthesia. The gland was expressed by hand onto a sterile, 5 mm diameter piece of filter paper. The filter paper was immediately placed into 5 ml of BHI broth and incubated overnight with shaking at 37°C. Following 18 hours of incubation, the culture was streaked (100 μl) onto tryptic soy agar with 5% sheep blood (BD® Biosciences). Plates were imaged for luminescent signal 24 and 48 hours later using the IVIS 200 system with Living Image Software.
Results
Pre-partum infection with luminescent L. monocytogenes results in luminescent vaginal secretions and mammary glands
Bioluminescence imaging revealed infection of maternal and fetal tissues in pregnant mice inoculated intragastrically with 106 CFU of L. monocytogenes at 10–14 days of gestation. Repeated imaging for five days after intragastric infection revealed luminescent vaginal discharge consistent with actively multiplying L. monocytogenes cells (Fig. 1A). No live pups were born in mice infected at 10–14 days of gestation. It is unknown if pups were born alive and died, were stillborn, or if they were liquefied by necrosis and discharged. Serosanguinous discharge was noted in the cage but we could not rule out the dam eating sick or dead pups at birth (whelping tends to occur at night). When mice were infected later in gestation (18–20 days) and imaged post-parturition every 24 h for five days, luminescent vaginal discharge was evident, as were luminescent foci in what was presumed to be the axillary mammary glands (Fig. 1B). Live pups were whelped in these experiments, but no pup lived for more than five days. We do know whether pups died from sepsis, or because the dams were systemically ill and did not nurse pups effectively. Bacterial culture of dead pups was not performed because they were autolyzed or destroyed by the dam. Clinically, all pregnant mice infected during pregnancy showed signs of systemic illness including decreased grooming, feeding, and hunched posture. Any mice that showed signs consistent with severe sepsis were humanely euthanized as per our approved animal use protocol.
Figure 1. Pre-partum L. monocytogenes infection causes fetal death and contaminated vaginal secretions.
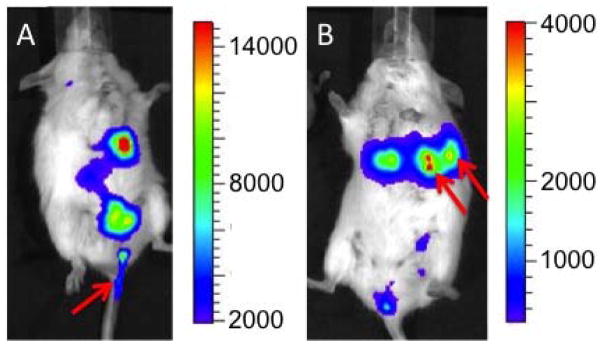
Mice were inoculated intragastrically with 106 CFU of LM10403S. (A) Mouse was infected at 10–14 days of gestation and imaged five days later. Bioluminescence illustrates discharge of contaminated vaginal discharge (arrow). No live pups were born to mice infected at 10–14 days gestation. (B) Mouse was infected approximately 48 h before its calculated whelping date and imaged five days later (three days after delivering live pups – all of which died within five days of parturition). Bioluminescence illustrates contaminated vaginal secretions and infected axillary mammary glands (arrows). Images are representative from those obtained in four separate experiments with four mice per experiment.
Post-partum infection causes luminescent mammary glands and shedding of L. monocytogenes in breast milk
The preceding experiments suggested that L. monocytogenes disseminated to the mammary glands in pregnant mice. We next asked whether this occurred in mice infected after parturition. Bioluminescent imaging showed active L. monocytogenes infection in mammary tissue of mice infected ig with 106 CFU L. monocytogenes at three days after parturition. This time point was chosen to allow mammary development and increased milk production. Seventy-two and ninety-six hours after intragastric infection, mice were imaged and milk samples taken from luminescent glands (Fig. 2A, C). After enrichment, the plated samples exhibited luminescent L. monocytogenes colonies intermixed with non-luminescent colonies (presumably normal skin microflora) (Fig. 2B, D). Unlike mice infected during pregnancy, mice infected post-partum showed few, if any, clinical signs associated with systemic disease such as decreased grooming, hunched posture, lethargy, and lack of feed intake. Nor did any of the dams exhibit clinical signs that would have required euthanasia during any experiment. Histopathology was performed but despite using several staining methods (Gram stain and immunohistochemistry), L. monocytogenes cells were not visualized (data not shown). This was presumably due to the low sensitivity of histopathology to find bacterial organisms in tissues, which requires approximately 105 CFU/g of tissue to visualize L. monocytogenes.
Figure 2. Post-partum L. monocytogenes infection translocates to mammary glands and breast milk.
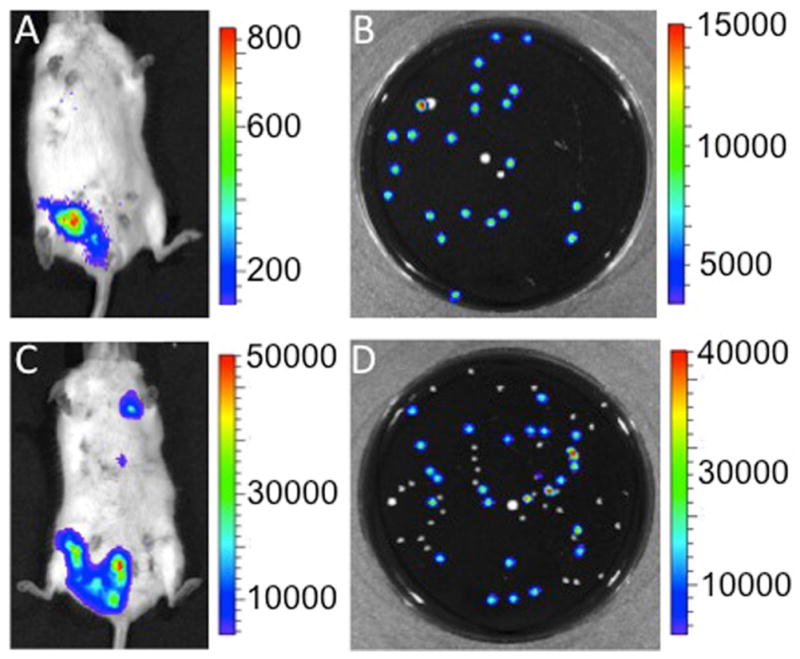
Mice were inoculated intragastrically with 106 CFU of LM10403S 3 days after parturition. The dams were removed from their pups for two hours to allow for mammary engorgement and injected intraperitoneally with 10 units of oxytocin to stimulate milk letdown to the gland cistern at (A) 72 h and (C) 96 h after infection with L. monocytogenes. Milk was expressed from luminescent glands onto sterile filter paper and placed in BHI media for a 24 h enrichment culture prior to serial dilution in phosphate buffered saline and plating on blood agar. Plates incubated at 37°C for 24 h and were imaged 24 h later. Panel B illustrates post enrichment 10−8 dilution of milk expressed 72 h after infection and Panel D a post enrichment 10−7 dilution of milk expressed 96 h after inoculation. Plates exhibit luminescent L. monocytogenes colonies amongst non-luminescent colonies of bacteria. Pup mortality was not observed in dams infected 72 h post-partum. Results are representative of five experiments with two mice per experiment.
Discussion
We have previously reported the use of bioluminescent bacteria to study hematogenous spread of L. monocytogenes to the uterus and fetoplacental units in pregnant mice (12). Here, we use in vivo imaging to demonstrate that intragastric L. monocytogenes infection results in infection of the birth canal visualized by contaminated vaginal discharge, as well as infection of mammary tissue and milk secretions. Presence of L. monocytogenes in vaginal discharge should raise awareness of risk for transmission to the neonate or other high risk family members by inadequate sanitation in the maternity ward or at home. Shedding of infective bacteria also represents a risk for nosocomial infection of other neonates in shared rooms or group wards, especially considering the ability of L. monocytogenes to persist in the environment. Post-parturient mice are susceptible to infection and can shed L. monocytogenes in breast milk for at least 96 hours after infection without clinical signs of systemic illness.
A weakness of our study is that, because of the small volume of milk that we were able to collect, we cannot directly quantitate the bacterial load in murine breast milk. We directly inoculated tryptic soy agar plates with milk secretions but were not able to isolate L. monocytogenes, presumably due to competitive inhibition from normal milk and skin flora. Instead we enriched for L. monocytogenes in milk secretions by incubation overnight in BHI broth. This is a standard method used with other mammalian species, including ruminants. Recovery of L. monocytogenes from biological fluids such as cerebrospinal fluid or milk is uncommon without the use of enrichment or molecular biology techniques (30). This is not always the case with human L. monocytogenes infection, in which the organism can be isolated from cerebrospinal fluid, blood, and placenta without the need for enrichment or selective media (31).
We cannot rule out contamination of the teat by skin, fecal, or vaginal secretions because sterile preparation of the teats is not possible. Collection of human breast milk for culture is commonly contaminated with skin flora due to anatomy and culture method. This has been reported with methicillin-resistant Staphylococcus aureus (MRSA) and Group B streptococci, which are both known skin flora and shed in human breast milk (32, 33). We also do not show luminescence of individual organs, which would require euthanasia and dissection and our goal was to show luminescence of bodily secretions over time. Here we show luminescent L. monocytogenes from fluids secreted by the vagina and mammary gland over multiple days, which is highly suggestive that the birth canal and mammary tissue was or had recently been infected with L. monocytogenes.
We make the interesting observation that mice can become infected with a single dose of bacteria after parturition, and shed L. monocytogenes in breast milk. Typically, non-pregnant humans and animals are considered relatively low risk for infection with L. monocytogenes. The typical at risk human populations have some degree of immune suppression and are exposed to a single infective dose such as contaminated fresh cheese made from raw milk at a family gathering. These include adults treated with immuno-suppressive drugs, neonates with a developing immune system, and the fetus during pregnancy. Reasons for fetal susceptibility are not completely understood. Our results show that post-parturient mice are susceptible to infection and shed listerial cells in their milk in the absence of overt signs of septic illness. However they will be exposing their nursing pups to L. monocytogenes cells. These findings raise the question of whether some cases of late onset neonatal listeriosis are transmitted via contaminated breast milk from infected mothers. Further study would be required to more completely assess the clinical status of infected compared to uninfected lactating mice including food and water intake, body weight, pup growth, and pup survival rates. Future studies should also attempt transmission from post-partum infected lactating mice to pups. However, this will be difficult considering we do not have evidence that mice shed large numbers of L. monocytogenes in breast milk.
Transmission of bacterial pathogens via contaminated breast milk is commonly encountered in veterinary cases of neonatal listeriosis, but currently is not considered a risk for human neonatal listeriosis. This is not the case for viruses, such as HIV, for which breastfeeding is a known risk factor for neonatal transmission (16). Transmission of bacterial pathogens via breast milk has been reported in human neonatal cases of L. monocytogenes and Salmonella species, including S. typhimurium DT104 (19, 20, 34). If lactating humans shed L. monocytogenes in breast milk, it could be a factor in the pathogenesis of late onset neonatal listeriosis (several days to weeks after birth), which is typically associated with uncomplicated pregnancies, normal maternal history, and healthy babies at birth (2).
Efforts to define the risk of L. monocytogenes contamination of breast milk of nursing mothers with listeriosis, or mothers with infants suffering from neonatal listeriosis are needed. The standard of care is to feed neonates fresh or fresh-frozen breast milk without testing or processing. Furthermore, maternal shedding of L. monocytogenes in the hospital environment could increase nosocomial infections (35). This is particularly important with this bacterium because it is able to survive readily in the environment. In extreme examples, L. monocytogenes can persist in a food processing plant for years despite vigorous sanitation and disinfection efforts to eliminate it (36–38).
Bacteriological analysis of breast milk as a potential source of L. monocytogenes in infants diagnosed with listeriosis is relatively simple. It only requires a small amount of milk, which is easily collected and stored for qualitative analysis via culture or PCR. Clinical intervention for positive samples would then decrease exposure of the nursing infant and the potential for environmental contamination.
Acknowledgments
Statement of Financial Support:
USDA Special Cooperative Agreement 58-1935-1-128
Comparative Biomedical Sciences Training Grant, T32 RR023916
Walter and Martha Renk Endowed Laboratory of Food Safety Research
Michael and Winona Foster Wisconsin Distinguished Fellowship Award
The authors would like to thank Dr. Christopher Contag (Stanford, CA) for generously providing L. monocytogenes strain LM10403S with bacterial luciferase. We are particularly grateful to our colleague Dr. Laura Knoll (Madison, WI) for providing access to the IVIS 200 Imaging System.
Footnotes
Disclosure Statements
Financial Disclosure Statement: None of the authors have any potential or actual interests relevant to the topics discussed in this manuscript.
Human Research Statement: No human subjects were used in generation of data for this manuscript.
Animal Research Statement: All animal experiments were done under an approved protocol from the Research Animal Care Committee at the University of Wisconsin-Madison.
References
- 1.Gellin BG, Broome CV. Listeriosis. JAMA. 1989;261(9):1313–20. [PubMed] [Google Scholar]
- 2.Posfay-Barbe KM, Wald ER. Listeriosis. Semin Fetal Neonatal Med. 2009;14(4):228–33. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Filice GA, Cantrell HF, Smith AB, Hayes PS, Feeley JC, Fraser DW. Listeria monocytogenes infection in neonates: Investigation of an epidemic. J Infect Dis. 1978;138(1):17–23. doi: 10.1093/infdis/138.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Posfay-Barbe KM, Wald ER. Listeriosis. Pediatr Rev. 2004;25(5):151–9. doi: 10.1542/pir.25-5-151. [DOI] [PubMed] [Google Scholar]
- 5.Facinelli B, Varaldo PE, Casolari C, Fabio U. Cross-infection with Listeria monocytogenes confirmed by DNA fingerprinting. Lancet. 1988;2(8622):1247–8. doi: 10.1016/s0140-6736(88)90834-3. [DOI] [PubMed] [Google Scholar]
- 6.Albritton WL, Wiggins GL, Feeley JC. Neonatal listeriosis: distribution of serotypes in relation to age at onset of disease. J Pediatr. 1976;88(3):481–3. doi: 10.1016/s0022-3476(76)80273-9. [DOI] [PubMed] [Google Scholar]
- 7.Lamont RF, Sobel J, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Kim SK, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med. 39(3):227–36. doi: 10.1515/JPM.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecuit M. Human listeriosis and animal models. Microbes Infect. 2007;9(10):1216–25. doi: 10.1016/j.micinf.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Zachar Z, Savage DC. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun. 1979;23(1):168–74. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecuit M, Cossart P. Genetically-modified-animal models for human infections: the Listeria paradigm. Trends Mol Med. 2002;8(11):537–42. doi: 10.1016/s1471-4914(02)02413-9. [DOI] [PubMed] [Google Scholar]
- 11.Barbour AH, Rampling A, Hormaeche CE. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect Immun. 2001;69(7):4657–60. doi: 10.1128/IAI.69.7.4657-4660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulsen KP, Faith NG, Steinberg H, Czuprynski CJ. Pregnancy reduces the genetic resistance of C57BL/6 mice to Listeria monocytogenes infection by intragastric inoculation. Microb Pathog. 50(6):360–6. doi: 10.1016/j.micpath.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disson O, Nikitas G, Grayo S, Dussurget O, Cossart P, Lecuit M. Modeling human listeriosis in natural and genetically engineered animals. Nature protocols. 2009;4(6):799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- 14.Breastfeeding and the Use of Human Milk. Pediatrics. 2012;129:827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 15.Hayes K, Danks DM, Gibas H, Jack I. Cytomegalovirus in human milk. N Engl J Med. 1972;287(4):177–8. doi: 10.1056/NEJM197207272870407. [DOI] [PubMed] [Google Scholar]
- 16.Thiry L, Sprecher-Goldberger S, Jonckheer T, Levy J, Van de Perre P, Henrivaux P, et al. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet. 1985;2(8460):891–2. doi: 10.1016/s0140-6736(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 17.Dunkle LM, Schmidt RR, O’Connor DM. Neonatal herpes simplex infection possibly acquired via maternal breast milk. Pediatrics. 1979;63(2):250–1. [PubMed] [Google Scholar]
- 18.Junker AK, Thomas EE, Radcliffe A, Forsyth RB, Davidson AG, Rymo L. Epstein-Barr virus shedding in breast milk. Am J Med Sci. 1991;302(4):220–3. doi: 10.1097/00000441-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Drhova A, Dobiasova V, Stefkovicova M. Mother’s milk--unusual factor of infection transmission in a salmonellosis epidemic on a newborn ward. J Hyg Epidemiol Microbiol Immunol. 1990;34(4):353–5. [PubMed] [Google Scholar]
- 20.Svabic-Vlahovic M, Pantic D, Pavicic M, Bryner JH. Transmission of Listeria monocytogenes from mother’s milk to her baby and to puppies. Lancet. 1988;2(8621):1201. doi: 10.1016/s0140-6736(88)90276-0. [DOI] [PubMed] [Google Scholar]
- 21.Dolente BA, Lindborg S, Palmer JE, Wilkins PA. Culture-positive sepsis in neonatal camelids: 21 cases. J Vet Intern Med. 2007;21(3):519–25. doi: 10.1892/0891-6640(2007)21[519:csincc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Vaala W, House JK, Lester GD. Neonatal Infection. In: BPS, editor. Large Animal Internal Medicine. 4. St. Louis: Mosby Elsevier; 2009. p. 282. [Google Scholar]
- 23.George L. Listeriosis. In: Smith B, editor. Large Animal Internal Medicine. 4. St. Louis: Mosby Elsevier; 2009. p. 1045. [Google Scholar]
- 24.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9(10):1236–43. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Southwick FS, Purich DL. Intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334(12):770–6. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- 26.Kampelmacher EH, van Noorle Jansen LM. Isolation of Listeria monocytogenes from faeces of clinically healthy humans and animals. Zentralbl Bakteriol Orig. 1969;211(3):353–9. [PubMed] [Google Scholar]
- 27.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68(6):3594–600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy J, Francis KP, DeBoer M, Chu P, Gibbs K, Contag CH. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science. 2004;303(5659):851–3. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 29.Czuprynski CJ, Canono BP, Henson PM, Campbell PA. Genetically determined resistance to listeriosis is associated with increased accumulation of inflammatory neutrophils and macrophages which have enhanced listericidal activity. Immunology. 1985;55(3):511–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Czuprynski CJKS, Poulsen KP. Listeria. In: Gyles CLPJ, Songer JG, Thoen CO, editors. Pathogenesis of Bacterial Infections in Animals. 4. Ames, IA: Blackwell; 2010. pp. 167–87. [Google Scholar]
- 31.Driscoll SG, Gorbach A, Feldman D. Congenital listeriosis: diagnosis from placental studies. Oncologia. 1962;20:216–20. [PubMed] [Google Scholar]
- 32.Christiansen H, Leth H. Breast milk as a cause of group B streptococcal sepsis. Ugeskr Laeger. 173(2):129–30. [PubMed] [Google Scholar]
- 33.Novak FR, Da Silva AV, Hagler AN, Figueiredo AM. Contamination of expressed human breast milk with an epidemic multiresistant Staphylococcus aureus clone. J Med Microbiol. 2000;49(12):1109–17. doi: 10.1099/0022-1317-49-12-1109. [DOI] [PubMed] [Google Scholar]
- 34.Qutaishat SS, Stemper ME, Spencer SK, Borchardt MA, Opitz JC, Monson TA, et al. Transmission of Salmonella enterica serotype typhimurium DT104 to infants through mother’s breast milk. Pediatrics. 2003;111(6 Pt 1):1442–6. doi: 10.1542/peds.111.6.1442. [DOI] [PubMed] [Google Scholar]
- 35.Jean D, Croize J, Hirtz P, Legeais C, Pelloux I, Favier M, et al. Listeria monocytogenes nosocomial infection in the maternity ward. Arch Fr Pediatr. 1991;48(6):419–22. [PubMed] [Google Scholar]
- 36.Chasseignaux E, Toquin MT, Ragimbeau C, Salvat G, Colin P, Ermel G. Molecular epidemiology of Listeria monocytogenes isolates collected from the environment, raw meat and raw products in two poultry- and pork-processing plants. J Appl Microbiol. 2001;91(5):888–99. doi: 10.1046/j.1365-2672.2001.01445.x. [DOI] [PubMed] [Google Scholar]
- 37.Latorre AA, Van Kessel JA, Karns JS, Zurakowski MJ, Pradhan AK, Zadoks RN, et al. Molecular ecology of Listeria monocytogenes: evidence for a reservoir in milking equipment on a dairy farm. Appl Environ Microbiol. 2009;75(5):1315–23. doi: 10.1128/AEM.01826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunden JM, Autio TJ, Sjoberg AM, Korkeala HJ. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J Food Prot. 2003;66(11):2062–9. doi: 10.4315/0362-028x-66.11.2062. [DOI] [PubMed] [Google Scholar]


