Abstract
Objective
Levofloxacin has been proposed to replace clarithromycin for Helicobacter pylori treatment. Seven- and 10-day fluoroquinolone triple therapies have generally failed to achieve cure rates of ≥90%, whereas 14-day therapy has achieved 95% success. The aim was to assess the efficacy and effect of fluoroquinolone resistance on 14-day levofloxacin-containing triple therapy with or without the addition of bismuth.
Design
Helicobacter pylori-positive patients with functional dyspepsia or healed peptic ulcers were randomized to receive lansoprazole 30 mg b.i.d., amoxicillin 1000 mg b.i.d., and levofloxacin 500 mg daily with (B-LAL) or without (LAL) bismuth potassium citrate 220 mg b.i.d. for 14 days. Eradication was assessed by 13C-urea breath testing 4 weeks after completing treatment. Antimicrobial susceptibility was by the agar dilution method. Success was defined as PP success ≥90%.
Results
A total of 152 of 161 patients (81 LAL and 80 B-LAL) enrolled completed treatment. The PP rates were 94.6% (70/74; 95% CI, 86.9–97.9%) with B-LAL and 85.9% (95% CI, 76.5–91.9%) with LAL (p = .07); the ITT eradication rates were 87.5% (95% CI, 78.5–93.1%) with B-LAL and 82.7% (95% CI, 73–89.4%) with LAL (p = .39). Levofloxacin resistance was present in 30.3%. Treatment success was excellent with susceptible strains (97.5%) versus resistant strains (70.6%) for B-LAL and 97.3% versus 37.5% for LAL, respectively.
Conclusions
Fourteen-day fluoroquinolone therapy was highly effective when fluoroquinolone resistance rates are <12%. The addition of bismuth maintained effectiveness with fluoroquinolone resistance as high as 25%.
Keywords: Levofloxacin, eradication therapy, Helicobacter pylori, bismuth, amoxicillin
Many studies on the efficacy of clarithromycin-containing triple therapy have reported poor results in China [1–3]. Levofloxacin is a fluoroquinolone with a broad spectrum of activity both against Gram-positive and Gram-negative bacteria, and it has been proposed as a replacement for clarithromycin in H. pylori treatment [4–6]. However, prevalence of fluoroquinolone H. pylori resistance has increased rapidly in recent years. For example, in Shanghai, levofloxacin resistance increased from 10.3% in 2000 to 32.5% in 2009 [7], and studies from Beijing reported an increase from 27% in 2006–2007 to 63.5% in 2009 [8]. Worldwide [9,10] and in our area [1,11], 7-day fluoroquinolone-containing therapies have typically produced cure rates of less than 80%, and 10-day therapy has also typically failed to achieve 90% treatment success [12]. A recent study suggested that 14-day fluoroquinolone therapy provided excellent (i.e., 95%) results suggesting that higher cure rates could be obtained by prolonging the duration of therapy [13]. However, the effect of different levels of fluoroquinolone resistance on the outcome of 14-day therapy remains unknown, and such data are needed to judge the potential usefulness of a new regimen in any particular population where baseline prevalences of resistance are known [14]. This study was registered with ClinicalTrials.gov, number NCT0-1667718.
Materials and Methods
Patients and Study Design
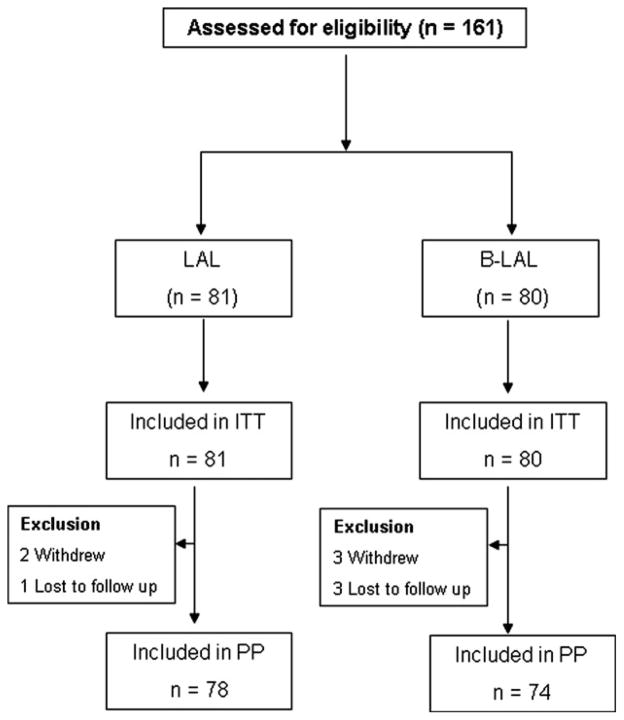
This was a prospective and open-label pilot study designed to attempt to confirm that: 1, prolonging fluoroquinolone triple therapy to 14 days reliably provided high cure rates; 2, to explore the effect of fluoroquinolone resistance on outcome of the 14-day therapy; and 3, to test whether the addition of bismuth would improve the efficacy of 14-day levofloxacin-containing triple therapy in the presence of fluoroquinolone resistance. To reduce selection bias of H. pylori-positive patients with functional dyspepsia or healed peptic ulcers, the selection of treatments was randomized. All subjects were recruited into the study in Renji Hospital, Shanghai, and underwent endoscopy with biopsy for rapid urease test, culture and histology prior to treatment (Fig. 1) in 2012. Helicobacter pylori infection was diagnosed by positive urease test and histology or by culture. Exclusion criteria included patients less than 18 years old, with a history of H. pylori infection treatment, with previous gastric surgery, pregnancy, lactation, major systemic diseases, administration of antibiotics, bismuth, antisecretory drugs in the preceding 8 weeks, or allergy to any one of the given medication in the regimens. The study was approved by the Ethics Committee of Shanghai Renji Hospital, and all enrolled patients gave written informed consent.
Figure 1.
Flow diagram of the progress of patients through the phases.
Randomization was made by reference to a computer-generated randomization list. Actual randomization was by sealed envelopes. The two different schemes were given for 2 weeks and consisted of lansoprazole (Takeda Pharmaceutical Company, Osaka, Japan) 30 mg b.i.d., amoxicillin (Zhuhai United Laboratories Co., Zhuhai, China) 1000 mg b.i.d. and levofloxacin (Daiichi Sankyo Pharmaceutical Beijing Co., LTD) 500 mg once daily (LAL group) or lansoprazole 30 mg b.i.d., bismuth potassium citrate (Livzon Pharmaceutical Group Inc., Zhuhai, China) 220 mg b.i.d., amoxicillin 1000 mg b.i.d., and levofloxacin 500 mg once daily (B-LAL group).
Six weeks after completion of therapy, H. pylori eradication was assessed by 13C-urea breath test. Eradication was defined as negative result from urea breath test (<4‰) (4‰ as the cutoff values). Side effects were scored as mild, moderate, or severe according to their influence on daily activities. Good compliance was defined as taking at least 90% of study drug by pill counting.
Helicobacter pylori Culture
Gastric mucosal biopsy specimens were cultured and maintained on brain heart infusion agar medium (OXOID, Basingstoke, UK) containing 5% defibrinated sheep blood under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37°C. We stored all isolates in brain heart infusion broth (Difco Laboratory, Detroit, MI, USA) supplemented with 30% glycerol at −80°C. Clinical isolates were identified as H. pylori using positive tests for urease, oxidase, catalase, and Gram staining.
Agar Dilution and Minimal Inhibitory Concentrations
Minimal inhibitory concentrations (MIC) of clarithromycin (Cla), amoxicillin (Amo), and levofloxacin (Lev) were determined by the twofold agar dilution method. H. pylori was suspended in saline and measured using spectrophotometer. The bacterial suspensions (108 colony-forming units per milliliter) were then plated with an inoculator (Sakuma Seisaku, Tokyo, Japan) onto agar plates containing various concentrations of above antibiotics. After 3 days of microaerophilic incubation, MIC was defined as the lowest drug concentration that prevented visible growth of bacteria. ATCC43504 were used as the quality control. Amo >8 μg/mL, Cla >2 μg/ mL, and Lev >2 μg/mL were defined as resistance breakpoints.
Statistical Analysis
As the actual rate of fluoroquinolone resistance in the population to be tested was unknown and the effects of extending the duration of the triple therapy were both unknown, the sample size was chosen based on a worst-case scenario with the understanding that the data from the trial would provide actual data regarding effectiveness in China and in the presence of fluoroquinolone resistance, which in subsequent studies could be used to reliably calculate sample sizes. One goal was to enter sufficient subjects to provide reliable 95% CI of the outcomes. In the worst-case scenario, we hypothesized that the outcome with 14-day triple therapy would not be worse than with 7- or 10-day therapy (e.g., ~75%) or 90% with the addition of bismuth. As calculated by the statistical program, the sample size was chosen to detect a difference of 15% in the eradication rate between the triple (assumed to have an eradication rate of 75%) and the quadruple (estimated to have an eradication rate of 90%) regimen, with a power of 0.8 and a significance level of 0.05. As noted above, the expected enrollment of at least 80 subjects in each arm would provide sufficient subjects to yield relatively tight 95% confidence intervals. The additional advantage was that if our assumptions were incorrect and the worst-case scenario was in fact realized, the number of individual receiving a regimen with an unacceptably low cure rate would be minimized.
The primary analysis for the exploratory pilot study was per protocol (PP). Intention-to-treat (ITT) analyses were also calculated to assess the eradication rates of H. pylori. Treatment success was calculated separately for those with fluoroquinolone-susceptible and fluoroquinolone-resistant strains. The 95% confidence intervals (95% CI) were also calculated. The eradication rates and frequencies of adverse effects were compared using the chi-squared test. The significance level was set at p < .05.
Results
A total of 161 subjects fulfilling the inclusion criteria were enrolled. Relevant demographic and endoscopic data at entrance to the study are given in Table 1. At entry, the two groups did not differ in terms of age, sex, smoking, or drinking habits. Nine subjects (three subjects in LAL group, and six subjects in B-LAL group) were lost to follow-up. Two subjects in LAL group and three subjects in B-LAL group discontinued treatment due to side effects.
Table 1.
Demographic and clinical data of subjects
| LAL group | B-LAL group | |
|---|---|---|
| Number of subjects | 81 | 80 |
| Gender (male/female) | 46/35 | 43/37 |
| Age, years (range) | 48.9 (23–75) | 46.7 (23–78) |
| Diagnosis | ||
| Functional dyspepsia | 49 | 50 |
| Peptic ulcer | 32 | 30 |
| Eradication rate | ||
| ITT analysis | 82.7% (67/81) | 87.5% (70/80) |
| 95% CI | 73.0–89.4% | 78.5–93.1% |
| PP analysis | 85.9% (67/78) | 94.6% (70/74) |
| 95% CI | 76.5–91.9% | 86.9–97.9% |
| Lost to follow-up | 3 | 6 |
| Stopped therapy due to adverse events | 2 | 3 |
| Adverse events (%) (n) | 7.4% (n = 6) | 5.1% (n = 4) |
Susceptibility Testing
One hundred and twelve strains from 161 subjects were successfully cultured and recovered. All clinical isolates were susceptible to amoxicillin. The resistance rate of H. pylori to levofloxacin was 30.3% (34/112), while the resistance rate of H. pylori to clarithromycin was 18.7% (21/112).
Helicobacter pylori Eradication Rates Overall
As shown in Table 1, treatment success by intentionto-treat (ITT) analysis was 82.7% (67/81; 95% CI, 73.0–89.4%) with LAL and 87.5% (70/80; 95% CI, 78.5–93.1%) with B-LAL. Per-protocol (PP) eradication results were 85.9% (67/78; 95% CI, 76.5–91.9%) with LAL and 94.6% (70/74; 95% CI, 86.9–97.9%) with B-LAL. Although the cure rate was higher in B-LAL group than those in LAL group, the difference between the two groups was not statistically significant (p = .39 for ITT comparison, and p = .07 for PP comparison).
Effect of Helicobacter pylori Resistance
Treatment success was markedly influenced by fluoroquinolone susceptibility (Table 2). In the susceptible group, success was 97.3% (95% CI, 86.5–99.5%) (37/ 38) with LAL triple therapy and 97.5% (95% CI, 87.1–99.6%) (39/40) with B-LAL quadruple therapy. With B-LAL quadruple therapy, the treatment success in resistant strains was 70.6% (95% CI, 46.9–86.7%) (12/ 17), which was significantly greater than 14-day LAL triple therapy in the presence of fluoroquinolone resistance (37.5%, 95% CI, 18.5–61.4%; 6/16) (p = .047) (Fig. 2).
Table 2.
Eradication rates of different groups
| LAL group | B-LAL group | |
|---|---|---|
| Number of subjects | 81 | 80 |
| Total eradication rate | ||
| ITT analysis | 82.7%(67/81) | 87.5% (70/80) |
| 95% CI | 73.0–89.4% | 78.5–93.1% |
| PP analysis | 85.9% (67/78) | 94.6% (70/74) |
| 95% CI | 76.5–91.9% | 86.9–97.9% |
| Susceptible strains | 38 | 40 |
| Eradication rate | 97.3% (37/38) | 97.5% (39/40) |
| 95% CI | 86.5–99.5% | 87.1–99.6% |
| Resistant strains | 17 | 16 |
| Eradication rate | 37.5% (12/17) | 70.6% (6/16) |
| 95% CI | 18.5–61.4% | 46.9–86.7% |
Figure 2.
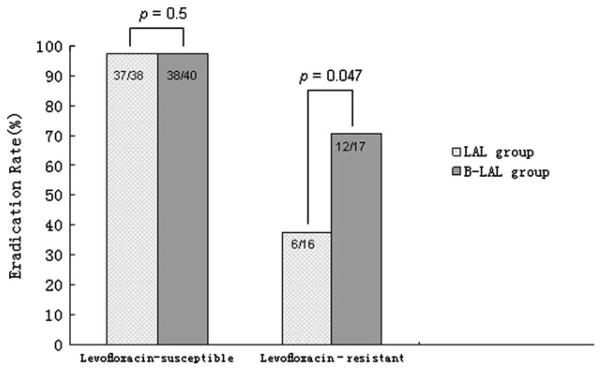
Effect of levofloxacin resistance on H. pylori eradication.
Side Effects
Side effects occurred in 7.4% (LAL group) and 5.1% (B-LAL group) including fatigue, stomachache, diarrhea, drowsiness, headache/dizziness, and skin rash and disappeared after cessation of medications. Five subjects (two in LAL group and three in B-LAL group) were withdrawn from the treatment because of nausea, drowsiness, insomnia, and skin allergy. There was no significant difference in the incidence of side effects between the two groups (Table 1).
Discussion
It has been suggested that clarithromycin-containing anti-H. pylori regimens should be abandoned as an empiric therapy in high clarithromycin resistance area [14]. Fluoroquinolones have been suggested as an alternative but generally the results of 7- or 10-day fluoroquinolone triple therapy have been less than the desired 90% (e.g., several meta-analyses have shown that 7-day fluoroquinolone triple therapy provides cure rates of typically <80% and that extending the duration to 10 days improves outcome but the treatment success has remained typically below 90%) [9,15]. In the Miehlke et. al study [10], 14-day fluoroquinolone therapy provided treatment success of 95%. Our results confirmed their results when treating fluoroquinolone-susceptible stains. We used 500 mg of levofloxacin daily. The optimal dose of fluoroquinolone is unknown; however, once-a-day therapy appears to be adequate [16], and comparative studies of 500 mg, 750 mg, and 1000 mg of levofloxacin for 7 days or 10 days confirmed that duration was more important than dosage [17].
In our study, the eradication rate was 82.7% for 2-week LAL triple therapy as an empiric therapy with the relatively low treatment success being largely due to the presence of fluoroquinolone resistance. Because fluoroquinolone resistance cannot be overcome by increasing the duration of therapy or dose and worldwide fluoroquinolone resistance is increasing [18–20], it seems unlikely that fluoroquinolone triple therapy will find success as an empiric regimen. However, treatment success was slightly greater with the addition of bismuth despite the local high levels of fluoroquinolone resistance. The most likely reason is triple therapy was less effective is because resistant strains effectively received only a PPI and amoxicillin dual regimen, whereas those receiving the bismuth-containing regimen received a PPI, amoxicillin, and bismuth triple therapy. If one assumes that the results of our study reflect the true eradication rates for each subgroup (i.e., approximately 97% success with fluoroquinolone-susceptible strains, 35% success with the triple therapy, and 70% with the quadruple bismuth-containing therapy with fluoroquinolone-resistant strains), one can calculate the expected results with different levels of fluoroquinolone resistance using the concepts proposed by Graham and Shiotani [15] and identify the cutoff level of resistance at which treatment success per protocol would fall below 90% (Fig. 3). Using that approach, treatment success would fall below 90% with 14-day fluoroquinolone triple therapy when fluoroquinolone resistance rates exceed approximately 12%, whereas 14-day bismuth-containing fluoroquinolone quadruple therapy could be used in areas with a fluoroquinolone resistance of up to approximately 26%. The high prevalence of fluoroquinolone resistance in China suggests that neither 14-day triple nor bismuth-containing quadruple fluoroquinolone therapy are generally not good choices as empiric therapies. We suggest that fluoroquinolone-containing therapy should be restricted to tailored therapy in patients with known fluoroquinolone-susceptible strains and to areas where fluoroquinolone resistance is still below the levels where treatment results fall below 90%. These results suggest that 14-day fluoroquinolone plus bismuth quadruple therapy should be especially useful in many regions where fluoroquinolone resistance is increasing but is still relatively low.
Figure 3.

Calculation of success rate.
Acknowledgments
This study was supported by the grant from Key Laboratory of Gastroenterology & Hepatology, Ministry of Health (Renji Hospital, Shanghai Jiao Tong University School of Medicine) and National Natural Science Foundation of China (81170355, 81200287). Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
Competing interests: Among the authors, only Dr. Graham declares conflicts of interest. Dr. Graham is a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and for Otsuka Pharmaceuticals regarding diagnostic testing.
Disclosures
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
References
- 1.Cheng H, Hu FL, Zhang GX, Shi RH, Du YQ, Li ZS, Han W, Li YQ, Wu QD, Qian KD. Levofloxacin-based triple therapy for first-line Helicobacter pylori eradication treatment: a multi-central, randomized, controlled clinical study] Zhonghua Yi Xue Za Zhi. 2010;90:79–82. [PubMed] [Google Scholar]
- 2.Yan X, Zhou L, Song Z, et al. Sequential therapy for Helicobacter pylori eradication in adults compared with triple therapy in China: a multi-center, prospective, randomized, control trial. Helicobacter. 2011;16(Suppl 1):87. [Google Scholar]
- 3.Zheng Q, Dai J, Li X, Lu H, Xiao S. Comparison of the efficacy of pantoprazole-based triple therapy versus quadruple therapy in the treatment of Helicobacter pylori infection; a single-center, randomized, open and parallel-controlled study. Chin J Gastroenterol. 2009;14:8–11. [Google Scholar]
- 4.Di Caro S, Assunta Zocco M, Cremonini F, Candelli M, Nista EC, Bartolozzi F, Armuzzi A, Cammarota G, Santarelli L, Gasbarrini A. Levofloxacin based regimens for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 2002;14:1309–12. doi: 10.1097/00042737-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe Y, Aoyama N, Shirasaka D, et al. Levofloxacin based triple therapy as a second-line treatment after failure of Helicobacter pylori eradication with standard triple therapy. Dig Liver Dis. 2003;35:711–5. doi: 10.1016/s1590-8658(03)00432-8. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O’Morain CA European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 7.Sun QJ, Liang X, Zheng Q, Gu WQ, Liu WZ, Xiao SD, Lu H. Resistance of Helicobacter pylori to antibiotics from 2000 to 2009 in Shanghai. World J Gastroenterol. 2010;16:5118–21. doi: 10.3748/wjg.v16.i40.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460–6. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 9.Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 10.Miehlke S, Krasz S, Schneider-Brachert W, et al. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori infection. Helicobacter. 2011;16:420–6. doi: 10.1111/j.1523-5378.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, Li Y, Qiu Y, Tang Q, Qian B, Yao L, Shi R, Zhang G. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32:2003–11. doi: 10.1016/j.clinthera.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488–96. doi: 10.1111/j.1572-0241.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 13.Chuah SK, Tai WC, Hsu PI, et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment–a pilot study. Helicobacter. 2012;17:374–81. doi: 10.1111/j.1523-5378.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY, Shiotani A. Which therapy for Helicobacter pylori infection? Gastroenterology. 2012;143:10–2. doi: 10.1053/j.gastro.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465–80. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Di Caro S, Franceschi F, Mariani A, Thompson F, Raimondo D, Masci E, Testoni A, La Rocca E, Gasbarrini A. Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig Liver Dis. 2009;41:480–5. doi: 10.1016/j.dld.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Hung KH, Sheu BS, Chang WL, Wu HM, Liu CC, Wu JJ. Prevalence of primary fluoroquinolone resistance among clinical isolates of Helicobacter pylori at a University Hospital in Southern Taiwan. Helicobacter. 2009;14:61–5. doi: 10.1111/j.1523-5378.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- 19.Glocker E, Stueger HP, Kist M. Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrob Agents Chemother. 2007;51:346–9. doi: 10.1128/AAC.00614-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaerts P, Berhin C, Nizet H, Glupczynski Y. Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter. 2006;11:441–5. doi: 10.1111/j.1523-5378.2006.00436.x. [DOI] [PubMed] [Google Scholar]