Abstract
A methyl-detected ‘out-and-back’ NMR experiment for obtaining simultaneous correlations of methyl resonances of Valine and Isoleucine/Leucine residues with backbone carbonyl chemical shifts, SIM-HMCM(CGCBCA)CO, is described. The developed pulse-scheme serves the purpose of convenience in recording a single data set for all ILV methyl positions instead of acquiring two separate spectra selective for Valine or Leucine/Isoleucine residues. The SIM-HMCM(CGCBCA)CO experiment can be used for ILV methyl assignments in moderately sized protein systems (up to ~100 kDa) where the backbone chemical shifts of 13Cα, 13Cβ and 13CO are known from prior NMR studies and where some losses in sensitivity can be tolerated for the sake of an overall reduction in NMR acquisition time.
Keywords: chemical shift assignments, methyl labeling, Enzyme I, methyl TROSY, isotope shifts
Selective 13CH3 labeling of Ileδ1, Leuδ and Valγ (ILV) methyl positions on a deuterated background (Goto et al. 1999; Ruschak and Kay 2010; Sheppard et al. 2010; Tugarinov et al. 2006; Tugarinov and Kay 2004) in combination with Methyl-TROSY (Amero et al. 2009; Ollerenshaw et al. 2003; Tugarinov et al. 2003) have had a profound impact on NMR studies of structure and dynamics in high-molecular-weight proteins (Gelis et al. 2007; Hamel and Dahlquist 2005; Religa et al. 2010; Rosenzweig et al. 2013; Ruschak et al. 2010; Sprangers and Kay 2007; Sprangers et al. 2007; Tugarinov et al. 2004; Velyvis et al. 2007). The success of NMR studies that make use of the ILV labeling methodology is predicated upon the availability of methyl resonance assignments. While a variety of approaches have been proposed for assignment of methyl resonances in very large (>> 100-kDa) proteins and protein complexes over the past decade (John et al. 2007; Sprangers and Kay 2007; Sprangers et al. 2007; Velyvis et al. 2009; Venditti et al. 2011; Xu et al. 2009), methyl-detected ‘out-and-back’ NMR experiments that correlate the (1H, 13C) chemical shifts of ILV methyl groups with the backbone and 13Cβ carbon positions (Sheppard et al. 2009; Sheppard et al. 2009; Tugarinov et al. 2004; Tugarinov and Kay 2003), provide the most sensitive and robust methodology for unambiguous methyl assignments in medium-sized (< ~100 kDa) protein systems without recourse to site-directed mutagenesis and/or analysis of NOE data. These experiments benefit from isotope labeling strategies that ‘linearize’ the 13C spin-systems of Leu and Val side-chains via the use of α-keto-acid (Tugarinov et al. 2006) or aceto-lactate (Gans et al. 2010) biosynthetic precursors that have only one of their methyl positions labeled with 13CH3, while the other remains at natural abundance and deuterated (12CD3). The ‘linearization’ of carbon spin-systems of (branched) LV side-chains has been shown to simplify NMR spectra and provide significant sensitivity gains in methyl resonance assignment experiments (Tugarinov and Kay 2003).
Commonly, the HMCM[CG]CBCA experiment that correlates methyl chemical shifts with the chemical shifts of 13Cα/13Cβ nuclei (Tugarinov and Kay 2003) is sufficient for reliable assignments of ILV methyls in proteins with a moderate content of ILV methyl groups. The degeneracy of 13Cα/13Cβ pairs of chemical shifts that inevitably occurs for some residues, even in medium-sized proteins, can be resolved by recording spectra that correlate ILV methyl resonances with carbonyl chemical shifts: one experiment selective for Ile and Leu, ILE/LEU-HMCM(CGCBCA)CO, and a separate data set for Val, VAL-HMCM(CBCA)CO (Tugarinov and Kay 2003). Here, we describe a three-dimensional methyl-detected ‘out-and-back’ NMR experiment developed for simultaneously correlating the methyl resonances of Val and Ile/Leu residues with the backbone carbonyl chemical shifts, SIM-HMCM(CGCBCA)CO. The pulse scheme provides the convenience of acquiring only a single data set for all ILV methyl positions instead of two separate spectra for Val and Ile/Leu residues. The SIM-HMCM(CGCBCA)CO experiment can be used for ILV methyl assignments in medium-sized proteins (up to ~100 kDa) where the backbone chemical shifts of 13Cα, 13Cβ and 13CO are known from prior NMR studies and where some losses in sensitivity can be tolerated for the sake of overall reduction in NMR acquisition time. We primarily address the problem of how carbon magnetization in homonuclear 13C spin-systems of different topologies (as in Val versus Ile/Leu) can be manipulated to obtain the desired correlations for all ILV positions with minimal losses in sensitivity. As a corollary result, we report the assignments of ILV methyl resonances for the 70-kDa homo-dimeric C-terminal domain of E. coli Enzyme I (EIC) (Venditti et al. 2012) obtained via combined analysis of methyl-detected ‘out-and-back’ HMCM[CG]CBCA and SIM-HMCM(CGCBCA)CO data sets.
Despite the fact that Val and Ile/Leu side-chains have different topologies, careful manipulation of magnetization via application of selective pulses allows one to ‘store’ the magnetization terms of (shorter) Val side-chains to ensure the subsequent ‘read-out’ of carbonyl frequencies of Val in synchrony with that of Ile/Leu residues. In the following, we briefly describe how this can be achieved in practice.
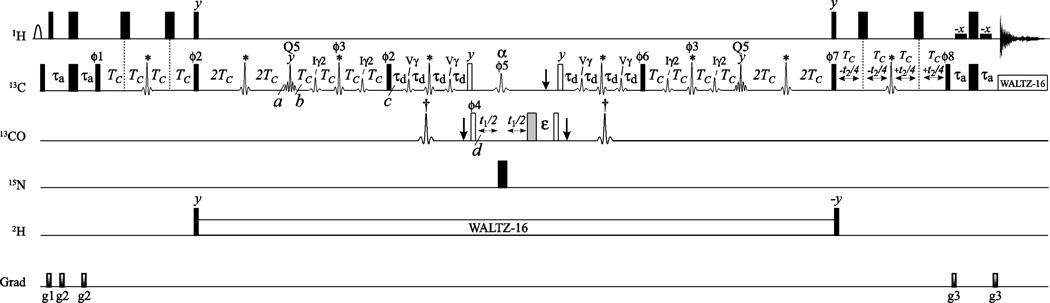
Figure 1 shows the SIM-HMCM(CGCBCA)CO pulse-scheme that allows methyl-carbonyl correlations in Val and Ile/Leu residues to be obtained simultaneously. The pulse-scheme in Figure 1 utilizes the Methyl-TROSY principle (Tugarinov et al. 2003) where possible by keeping the methyl magnetization in a 1H-13C multiple-quantum state as long as it resides on methyl groups (i.e. before the 13Cϕ2/Hy pair of pulses and after the 13Cϕ7/Hy pair of pulses). At time-point a of the scheme, the generated magnetization terms are of the form for Val and for Ile/Leu residues, where are product operators for carbon nucleus i and the three methyl proton nuclei, respectively, r = x, y, z, and the signs of the terms are arbitrary. Fortuitously, the region of Val 13Cα chemical shifts is well separated from the rest of the carbon chemical shifts in the terms above. Therefore, a selective 90° pulse can be chosen that excites all carbon nuclei in these terms except for 13Cα of Val. Application of a universal Gaussian Cascade (Q5) pulse (Emsley and Bodenhausen 1987) with phase ‘y’ centered at 33 ppm with an excitation bandwidth of ± 12 ppm (600 MHz) converts the terms above to for Val and for Ile/Leu (time point b; Figure 1). Thus, the magnetization of Val residues is effectively ‘stored’ in a triple-spin-order state that does not evolve due to 13C-13C couplings during the following 4TC period. Subsequently, the evolution of magnetization proceeds via the following pathways:
| (1) |
for Val and,
| (2) |
for Ile/Leu, where the letters in parentheses correspond to the time points in the pulse scheme (Figure 1) and the signs of the terms are neglected. The 13Cβ-13Cγ2 couplings in Ile side-chains are refocused by application of a pair of ‘Iγ2’ pulses selective for the Ile 13Cγ2 methyl region between time-points b and c, while evolution due to 13Cβ-13Cγ couplings in Val between time-points c and d is eliminated by application of a pair of ‘Vγ’ pulses selective for the Val 13C methyl region. The terms on the right-hand side of pathways (1) and (2) evolve during the t1 period, with the chemical shifts of 13Cα nuclei of Val refocused by the 13C 180° pulse ‘α’ applied in the middle of t1 and selective for the 13Cα chemical shifts of all ILV residues (Figure 1). The selectivity of this pulse also ensures that the 13Cα-13Cβ J couplings in Val are refocused during the t1 evolution period. After the terms generated at time point d of pathways (1) and (2) are labeled with 13CO chemical shifts, the magnetization is transferred back to methyl carbons that evolve during the time t2, and subsequently to methyl protons for detection by reversing the pathways.
Figure 1.
The SIM-HMCM(CGCBCA)CO pulse-scheme for simultaneous methyl-carbonyl correlations in Val and Ile/Leu residues. All narrow and wide rectangular pulses are applied with flip angles of 90° and 180°, respectively. All the pulses (including shaped pulses) are applied along the x-axis unless indicated otherwise. The 1H, 2H and 15N carrier positions were set to 4.7, 2.5 and 119 ppm, respectively. The 13C carrier is positioned at 18 ppm at the beginning of the scheme, switched to 33 ppm before the 13C pulse with phase ϕ1, and returned to 18 ppm after the 13C pulse with phase ϕ8. All the pulses shown with black rectangles are applied with the highest possible power except for the ~1.4-ms long water-selective rectangular pulses flanking the last 180 1H pulse and the water-selective ~7.0-ms 1H E-BURP1 pulse (Geen and Freeman 1991) shown with the open arc. 13C WALTZ-16 decoupling (Shaka et al. 1983) during acquisition is achieved using a 2.5 kHz field, while 2H WALTZ-16 decoupling uses a 0.8 kHz field. The 13C shaped pulses marked with asterisks are high-power 350-µs (600 MHz) RE-BURP pulses (Geen and Freeman 1991) applied on-resonance. The 13C shaped pulses marked with daggers are high-power 350-µs RE-BURP pulses applied at 176 ppm by phase modulation of the carrier (Boyd and Soffe 1989; Patt 1992). The 13C pulses marked with ‘Iγ2’ are 4.0 ms RE-BURP pulses selective for Ile 13Cγ2 positions and are applied at 16 ppm by phase-modulation of the carrier. The 13C pulses marked with ‘Vγ’ are 3.0 ms RE-BURP pulses selective for 13Cγ methyls of valine residues and are applied at 21 ppm by phase modulation of the carrier. The 13C pulse labeled ‘α’ is a 1.8 ms RE-BURP pulse (600 MHz) selective for 13Cγ positions of ILV residues and applied at 60 ppm by phase modulation of the carrier. The 13C pulses labeled ‘Q5’ are 1.4 ms Q5 Gaussian Cascade pulses (Emsley and Bodenhausen 1987) applied on-resonance (see text for details). 13C and 13CO 90° pulses shown with open rectangles are applied at 45 and 176 ppm, respectively, by phase modulation of the carrier with a field strength of Δ/√15 where Δ is the difference (in Hz) between 13Cα/β (45 ppm) and 13CO (176 ppm) chemical shifts (Kay et al. 1990). The 180° 13CO pulse following the t1 period shown with the shaded rectangle is applied at 176 ppm by phase modulation of the carrier with the same field strength as the 90° ‘open’ pulses. This ensures complete refocusing of ‘Bloch-Siegert’ shifts of both 13Cα and 13CO nuclei during t1. Vertical arrows at the start and end of the 4τd periods and after the t1 period indicate the position of Bloch-Siegert compensation pulses (Kay et al. 1990). Delays are: τa = 2.0 ms; TC = 3.5 ms; τd = 2.6 ms; the delay ε is adjusted to accommodate the shaped Bloch-Siegert compensation pulse ‘α’. The phase-cycle is: ϕ1 = x,-x; 2 = 2(y),2(−y); ϕ3 = x; ϕ4 = 2(x),2(−x); ϕ5 = 4(x),4(−x); ϕ6 = 4(y),4(−y); ϕ7 = 8(y),8(−y); ϕ8 = x; receiver = x,-x,-x,x. Note that if the phase of one of the 13Cϕ3 pulses is shifted by 90° (±y instead of x), the signs of Ile/Leu correlations will be inverted while retaining the signs of Val correlations. Quadrature detection in F1 snd F2 is achieved by States-TPPI (Marion et al. 1989) of ϕ4 and ϕ8, respectively. Durations and strengths of the pulsed-field gradients in units of (ms; G/cm) are: g1 = (1.0; 15), g2 = (0.3; 8), g3 = (0.45; 10).
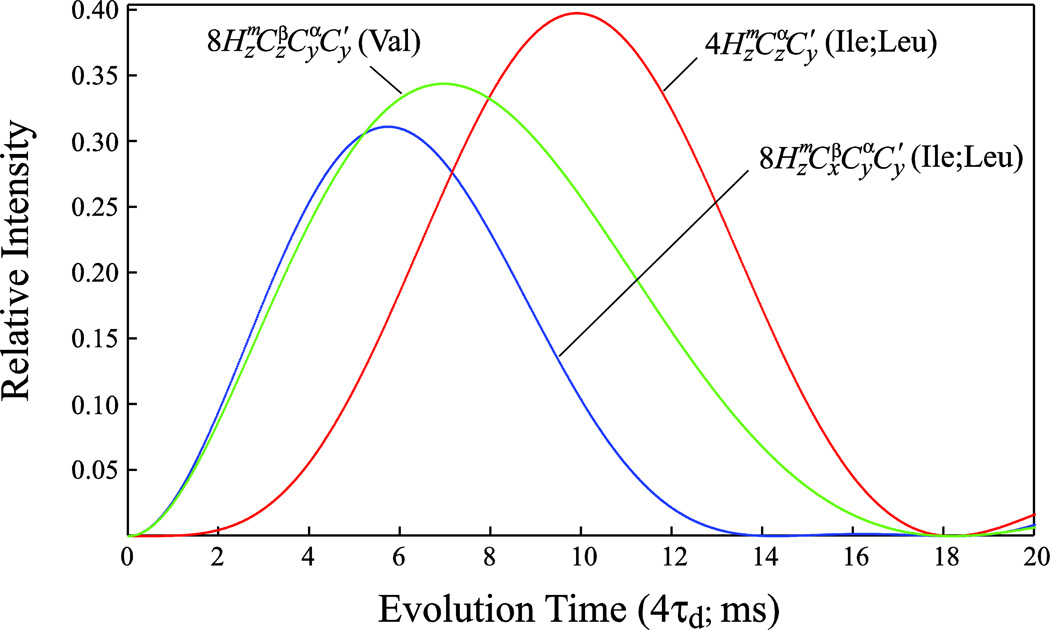
Figure 2 shows plots of the magnetization transfer function for Val methyl (green curve) and Ile/Leu methyl (red curve) (1H,13C)-13CO correlations as a function of the delay 4τd in the SIM-HMCM( CGCBCA)CO pulse scheme. These plots take into account the fact that Val signals relax faster during the 4τd period than Ile/Leu signals as Val magnetization is present in a 13C-13C multiple-quantum state, with both 13Cα and 13Cβ nuclei relaxing in the transverse plane. This is also true for Val residues with respect to the t1 evolution period where the multiple-quantum 13Cα-13CO state relaxes. The duration of the τd delays should be chosen to ensure the desired compromise between the intensities of Val and Ile/Leu correlations (τd = 2.6 ms in this work; Figure 2), and the time t1,max should be adjusted with faster relaxation of Val correlations in mind (t1,max ≈ 15 ms at 600 MHz allows the observation of all Val correlations for EIC). The terms in Ile/Leu residues are omitted from the end of pathway (2) above but are not filtered out before the t1 evolution period. These terms (blue curve in Figure 2) are, however, much lower in intensity for the duration of τd chosen here, providing correlations are observed for only a few very intense peaks in the 3D spectra of EIC at the frequencies ΩCO ± ΩCβ, where Ωi is the offset of nucleus i from the carbon carrier. We note that the same transfer function as shown for the terms of Ile/Leu in Figure 2 also applies for the correlations of Val residues in the ILE/LEU-HMCM( CGCBCA)CO data sets optimized for observation of Ile, Leu methyl-(1H,13C)-13CO correlations. That is why the correlations of Val residues are either not observed or observed with low intensities in the ILE/LEU-HMCM(CGCBCA)CO data sets.
Figure 2.
Plots comparing the magnetization transfer functions for Valine methyl (1H,13C)-13CO correlations (green curve; [sin(4πτd1JCα-CO)exp(−4RCατd)exp(−4RCβτd)]2, where 1JCα-CO is the one bond 13Cα-13CO J coupling (55 Hz), and RCα and RCβ are the relaxation rates of 13Cα and 13Cβ nuclei, respectively, both assumed to be equal to 30 s−1 for deuterated EIC), and Ile/Leu methyl (1H,13C)-13CO correlations (red and blue curves corresponding to [sin(4πτd1JC1-CO)sin(4πτd1JCα-Cβ)exp(™4RCατd)]2 and [sin(4πτd1JCα-CO)cos(4πτd1JCα-Cβ)exp(−4RCατd)]2, respectively, where 1JCα-Cβ is the one bond 13Cα-13Cβ J coupling (35 Hz) and the rest of the notation defined above) as a 18 function of the delay 4τd (ms) in the SIM-HMCM(CGCBCA)CO experiment of Figure 1. Methyl (1H,13C)-13CO correlations arise from the magnetization terms indicated on the plot created at time point d and evolving during the t1 evolution period of the scheme in Figure 1.
Analysis of magnetization flow for the pulse-scheme in Figure 1 shows that shifting the phase of one of the 13C 180° pulses with phase ϕ3 (13Cϕ3; in the time interval between points b and c in Figure 1) by 90° inverts the sign of the correlations belonging to Ile/Leu residues while preserving the sign of Val correlations. Although this may seem as an especially attractive feature of the SIM-HMCM( CGCBCA)CO experiment, in practice, Val residues are easily distinguished from Ile/Leu by the relative signs of the peaks obtained in the 3D HMCM[CG]CBCA data sets (Tugarinov and Kay 2003). Nevertheless, in the regions of the 3D HMCM[CG]CBCA spectra where Val correlations may overlap with Leu peaks (an unlikely event due to significantly different 13Cα and 13Cβ chemical shifts for Val and Leu), it may prove useful to be able to differentiate between the two types of residues from the SIM-HMCM(CGCBCA)CO data.
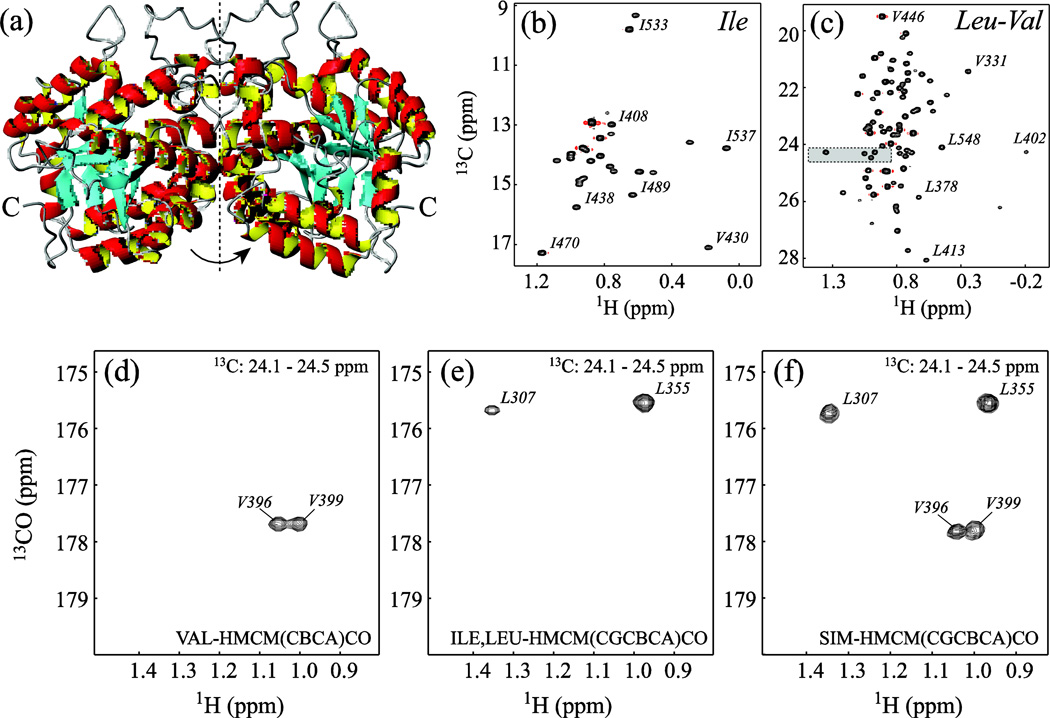
The SIM-HMCM(CGCBCA)CO experiment was tested on the C-terminal domain of Enzyme I (EIC) from E. coli, a symmetric 70-kDa homodimer. Enzyme I (PDB access code 2hwg (Teplyakov et al. 2006)) is the first enzyme in the phosphotransferase system (PTS), a signal transduction pathway that couples phosphoryl transfer to active sugar transport across the cell membrane (Meadow et al. 1990). Auto-phosphorylation of Enzyme I by phosphoenolpyruvate (PEP) in a Mg2+-dependent manner is followed by the transfer of the phosphoryl group to the histidine phosphocarrier protein HPr (Weigel et al. 1982; Weigel et al. 1982). Recently, robust protocols for expression and purification of EIC, as well as the backbone assignments and conformational transitions occurring in EIC upon PEP binding have been reported by our laboratory (Venditti and Clore 2012; Venditti et al. 2012). NMR analysis and earlier crystallographic data show that the isolated C-terminal domain of enzyme I adopts a stable tertiary fold and exists in a monomer/dimer equilibrium that is modulated by ligand binding (Chauvin et al. 1996; Chauvin et al. 1994; Patel et al. 2006; Seok et al. 1996; Seok et al. 1998; Venditti and Clore 2012)). The symmetric homo-dimer of EIC is shown in Figure 3a. Under the sample conditions used in this study (0.5 mM protein and 4 mM Mg2+ concentration), ~95% of the protein is present in solution in the dimeric state (Venditti and Clore 2012). The Ile and Leu/Val regions of the high-resolution 1H-13C methyl constant time (CT)-HMQC spectrum (Bax et al. 1983; Mueller 1979; Tugarinov et al. 2003) of [U-2H,15N,13C; Ileτ1-{13CH3}; Leu,Val-{13CH3/12CD3}]-labeled EIC are shown in Figures 3b–c. Among 27 Ile τ1, 56 Leu τ and 36 Val γ methyl groups present in the EIC monomer, all Ile, 52 Leu and 26 Val methyls have been unambiguously assigned via combined analysis of 3D HMCM[CG]CBCA and 3D SIM-HMCM(CGCBCA)CO data sets. The backbone chemical shifts of the remaining 5 Val methyls (Val278, Val281, Val428, Val430 and Val446) were not assigned in the previous NMR study of EIC (Venditti and Clore 2012). A pair of resonances with outstanding 13C and/or 1H chemical shifts from this subset of methyls (Val430 and Val446) could be assigned tentatively based on the magnitude of the ring-current effects (Haigh and Mallion 1979; Koradi et al. 1996; Kuszewski et al. 1995) calculated from the x-ray coordinates (Teplyakov et al. 2006).
Figure 3.
(a) Ribbon diagram representation of the structure of the biological assembly of the C-terminal domain of Enzyme I (EIC dimer; pdb code 2hwg (Teplyakov et al. 2006)). The molecular dyad axis of the homodimer is shown with a dashed vertical line. (b–c) Regions of the 1H-13C methyl CT-HMQC correlation map of [U-2H,15N,13C; Ileδ1-{13CH3}; Leu,Val-{13CH3/12CD3}]-labeled EIC (90% H2O/ 10% D2O; 600 MHz; 37 °C) featuring the correlations of (b) Ileδ1 and (c) Valγ/Leuδ methyl groups. The high-resolution CT-HMQC correlation spectrum was acquired with the constant time period adjusted to 2/1JCC = 56 ms. Selected residues are labeled with methyl peak residue types and numbers. The methyl-containing residues are numbered in the context of full-length EI. The assignments of Val430 and Val446 are tentative. (d–f) Superposition of a selected region of the 2D 1H-13CO planes plotted from the 1H-13C chemical shift area highlighted in the Leu-Val region of the methyl 1H-13C correlation map in panel (c) from (d) 3D VAL-HMCM( CBCA)CO, (e) 3D ILE/LEU-HMCM(CGCBCA)CO and (f) 3D SIM-HMCM( CGCBCA)CO data sets. The sample comprised 0.5 mM EIC dissolved in 20 mM Tris buffer, pH = 7.4, 100 mM NaCl, 2 mM DTT, 4 mM MgCl2, and 1 mM EDTA (Venditti and Clore 2012). All experiments were performed on a Bruker Avance III 600 MHz spectrometer equipped with a cryogenic probe operating at 37 °C. Typically, the 3D data sets were recorded with 18*, 40* and 512* complex points in the 13CO, 13C and 1H dimensions, respectively, corresponding to respective acquisition times of 15, 14, and 64 ms. The recovery delay of 1.4 s and 32 scans per fid led to total acquisition times of ~40 hrs per 3D experiment. NMR spectra were processed with NMRPipe/NMRDraw software (Delaglio et al. 1995) and analyzed with NMRView (Johnson and Blevins 1994) using a tcl/tk interface written in-house.
Figures 3d–f show the superposition of a selected region of the 2D 1H-13CO planes from the 3D VAL-HMCM(CBCA)CO (Figure 3d), ILE/LEU-HMCM(CGCBCA)CO (Figure 3e) and SIM-HMCM(CGCBCA)CO (Figure 3f) spectra corresponding to the area of 1H-13C chemical shifts highlighted in the Leu-Val region of the methyl 1H-13C correlation map in Figure 3c. Clearly, the cross-peaks in the Val-selective VAL-HMCM(CBCA)CO (Figure 3d) and Ile/Leu-selective ILE/LEU-HMCM(CGCBCA)CO (Figure 3e) experiments represent only subsets of the correlations obtained in the SIM-HMCM(CGCBCA)CO data set. The convenience of recording a single data set comes at a relatively low price: we estimate that the sensitivity of the SIM-HMCM( CGCBCA)CO data set is 25% lower on average for Ile/Leu correlations and 50% lower for Val correlations compared to the separate (Ile/Leu- and Val-selective) experiments recorded for the same measurement time. These losses in signal-to-noise per unit time in the simultaneous data set are associated with (i) faster relaxation of the multiple-quantum magnetization terms present during the t1 evolution period for Val residues, (ii) the non-idealities as well as relaxation and phase evolution during the long Q5 Gaussian Cascade pulses (Emsley and Bodenhausen 1987) for all residues, and (iii) the need to adjust the length of the delay 4τd to achieve the desired compromise in sensitivity of Val versus Ile/Leu correlations. We note that even the loss of about half of the signal for Val residues in the SIM-HMCM(CGCBCA)CO data set is tolerable as the Val-selective VAL-HMCM(CBCA)CO spectra are usually much more sensitive than their Ile,Leu-selective counterparts (4.6-fold more sensitive in the 82-kDa Malate Synthase G at the same temperature (Tugarinov and Kay 2003)) and can therefore be recorded with shorter total acquisition times.
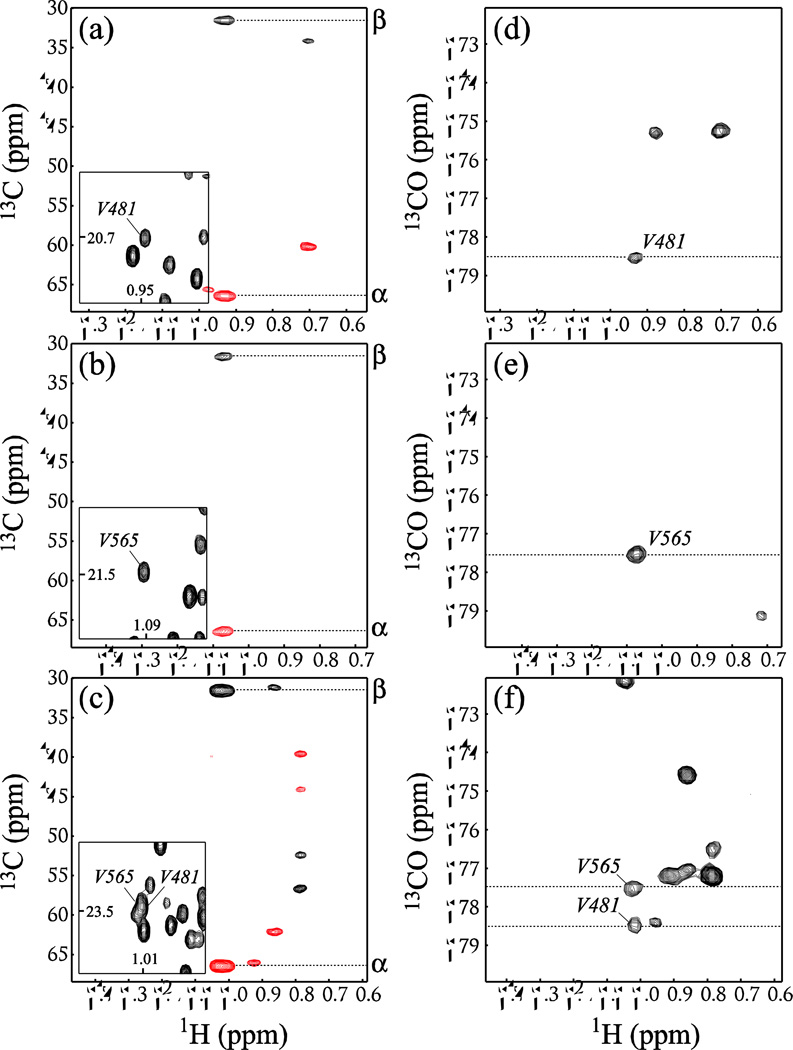
Although a single HMCM[CG]CBCA dataset is usually sufficient for assignments of the vast majority of methyl sites, and correlations of methyl resonances with backbone carbonyls are obtained primarily for verification purposes, even in such medium-sized systems as the EIC dimer, some cases can be identified when the correlations with carbonyl chemical shifts are absolutely indispensable for unambiguous assignments. For example, Val481 and Val565 of the EIC dimer have practically identical 13Cα and 13Cβ chemical shifts (31.2 and 66.4 ppm, respectively). Figures 4a–c show selected regions of the 2D 1H-13C planes plotted from the 3D HMCM[CG]CBCA spectrum of the [U-2H,15N,13C; Ileδ1-{13CH3}; Leu,Val-{13CH3/12CD3}]-EIC at the methyl 13C chemical shifts of these two residues. The peaks of one of the two methyl sites of these two valines overlap in the 1H-13C CT-HMQC map (Figure 4c). The correlations provided by the SIM-HMCM(CGCBCA)CO data set is the sole means of distinguishing between the two methyl positions, as the chemical shifts of backbone 13CO nuclei of this pair of valines happen to be different by 1 ppm, as can be seen in the selected region of the 2D 1H-13CO planes from the SIM-HMCM(CGCBCA)CO data set plotted at the methyl 13C chemical shifts of Val481 and Val565 shown in Figures 4d–f.
Figure 4.
(a–c) Selected regions of the 2D 1H-13C planes drawn from the 3D HMCM(CG)CBCA spectrum of [U-2H,15N,13C; Ileδ1-{13CH3}; Leu,Val-{13CH3/12CD3}]-EIC (90% H2O/ 10% D2O; 600 MHz; 37 °C) at the methyl 13C chemical shifts of (a) one of the two Val481 methyls of EIC, (b) one of the two Val565 methyls of EIC, and (c) the position where the methyl peaks of Val481 and Val565 overlap. The insets in (a–c) show the regions of the 2D 1H-13C CT-HMQC correlation map of EIC centered at the methyl positions in question. Negative contours are shown in red. 13C chemical shift positions of 1H-13Cα and 1H-13Cβ correlations are indicated on the right side of each plot. (d–f) Selected regions of the 2D 1H-13CO planes from the SIM-HMCM(CGCBCA)CO data set plotted at the methyl 13C chemical shifts of (d) one of Val481 methyls, (e) one of Val565 methyls, and (f) the region where the methyl peak of Val481 overlaps with that of Val565.
Methyl assignments based on HMCM[CG]CBCA data sets are complicated by the fact that 13Cδ (and to a smaller extent 13Cα) chemical shifts of ILV residues in 13CH3-labeled protein samples are different from those of fully deuterated proteins by three times the value of the multiple-bond deuterium isotope shifts (Gardner and Kay 1998; Rosen et al. 1996; Venters et al. 1996). Usually, however, 13Cβ and 13Cα chemical shifts are available from the assignment experiments acquired on fully deuterated protein samples as was the case in the previous study of EIC (Venditti and Clore 2012). For example, deuterium isotope shifts of 13Cβ nuclei arising from the substitution of three methyl deuterons for protons, nΔCβ (Dm) where n is the number of bonds between the carbon β and the site of the substitution (n = 2 for Val and n = 3 for Ile/Leu), are measured to be 250 ± 50 ppb for Val and 80 ± 30 ppb for Ile/Leu residues in EIC from a comparison of shifts in the 3D TROSY-HNCACB spectra acquired on the 13CH3-labeled and fully deuterated protein samples. The variability of these isotope effects may compromise the exact ‘matching’ of 13Cβ and 13Cα frequencies in the HMCM[CG]CBCA and HNCACB data sets if recorded on different samples. Moreover, these deuterium isotope effects on 13Cβ nuclei may be somewhat offset by three-bond isotope shifts resulting from backbone amide 1HN-to-DN substitutions, 3ΔCβ (ND) (~37 ppb on average ranging from 2 to 65 ppb in model proteins (Zhang and Tugarinov 2013)), when the HMCM[CG]CBCA spectra are recorded in D2O. The assignments based on correlations with carbonyl shifts, however, do not suffer from these isotope effects as (i) carbonyl carbons are removed by 4 (in Val) and 5 (in Ile/Leu) bonds from the sites of the methyl 1H-to-D replacement, and (ii) generally, deuterium isotope effects of carbonyl nuclei tend to be much smaller (Garrett et al. 1997; Sun and Tugarinov 2012; Zhang and Tugarinov 2013). In this context, we note that 13CO chemical shifts in the SIM-HMCM(CGCBCA)CO experiments recorded in H2O and D2O solvents are different by the sum of two- and three-bond deuterium isotope shifts arising from the 1H-to-D substitutions at backbone amide positions (2ΔCO(Ni+1D) + 3ΔCO(NiD); equal on average to 102 ± 25 ppb in EIC in good agreement with the measurements reported earlier in model protein systems (Feeney et al. 1974; Kainosho et al. 1987; LiWang and Bax 1996; Tuchsen and Hansen 1991; Zhang and Tugarinov 2013)).
In summary, we describe a methyl-detected ‘out-and-back’ NMR experiment for obtaining simultaneous correlations of methyl resonances of Val and Ile/Leu residues with backbone carbonyl chemical shifts. The developed pulse-scheme serves the purpose of convenience in recording a single data set for all ILV methyl positions instead of acquiring two separate spectra for Val and Ile/Leu correlations. The SIM-HMCM(CGCBCA)CO experiment can be used for ILV methyl assignments in moderately sized protein systems (up to ~100 kDa) where the backbone chemical shifts of 13Cα, 13Cβ and 13CO are known from prior NMR studies and where some losses in sensitivity can be tolerated for the sake of reduction in net NMR acquisition time. We note that though Val and Leu side-chains have been non-stereospecifically labeled in EIC via the use of [13CH3/12CD3]-labeled α-keto-isovalerate (Tugarinov et al. 2006; Tugarinov and Kay 2004), the developed methodology is equally applicable to stereospecifically [13CH3/12CD3]-labeled Val and Leu isopropyl moieties, i.e. with 13CH3 labels restricted to either pro-R or pro-S methyl positions as obtained via the use of appropriately labeled aceto-lactate precursors (Gans et al. 2010).
Supplementary Material
Acknowledgments
This work was supported by funds from the Intramural Program of the NIH, NIDDK, and the Intramural AIDS Targeted Antiviral Program of the Office of the Director of the NIH (to G.M.C.).
References
- Amero C, Schanda P, Durá MA, Ayala I, Marion D, Franzetti B, Brutscher B, Boisbouvier J. Fast two-dimensional NMR spectroscopy of high molecular weight protein assemblies. J Am Chem Soc. 2009;131:3448–3449. doi: 10.1021/ja809880p. [DOI] [PubMed] [Google Scholar]
- Bax A, Griffey RH, Hawkings BL. Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J Magn Reson. 1983;55:301–315. [Google Scholar]
- Boyd J, Soffe N. Selective excitation by pulse shaping combined with phase modulation. J Magn Reson. 1989;85:406–413. [Google Scholar]
- Chauvin F, Brand L, Roseman S. Enzyme I: the first protein and potential regulator of the bacterial phosphoenolpyruvate: glycose phosphotransferase system. Res Microbiol. 1996;147:471–479. doi: 10.1016/0923-2508(96)84001-0. [DOI] [PubMed] [Google Scholar]
- Chauvin F, Brand L, Roseman S. Sugar transport by the bacterial phosphotransferase system. Characterization of the Escherichia coli enzyme I monomer/dimer transition kinetics by fluorescence anisotropy. J Biol Chem. 1994;269:20270–20274. [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Emsley L, Bodenhausen G. Gaussian pulse cascades: new analytical functions for rectangular selective inversion and in-phase excitation in NMR. Chem Phys Lett. 1987;165:469–476. [Google Scholar]
- Feeney J, Partington P, Roberts GCK. The assignment of carbon-13 resonances from carbonyl groups in peptides. J Magn Reson. 1974;13:268–274. [Google Scholar]
- Gans P, Hamelin O, Sounier R, Ayala I, Durá MA, Amero CD, Noirclerc-Savoye M, Franzetti B, Plevin MJ, Boisbouvier J. Stereospecific isotopic labeling of methyl groups for NMR spectroscopic studies of high-molecular-weight proteins. Angew Chem Int Ed Engl. 2010;49:1958–1962. doi: 10.1002/anie.200905660. [DOI] [PubMed] [Google Scholar]
- Gardner KH, Kay LE. The use of 2H, 13C, 15N multidimensional NMR to study the structure and dynamics of proteins. Annu Rev Biophys Biomol Struct. 1998;27:357–406. doi: 10.1146/annurev.biophys.27.1.357. [DOI] [PubMed] [Google Scholar]
- Garrett DS, Seok YJ, Liao DI, Peterkofsky A, Gronenborn AM, Clore GM. Solution structure of the 30 kDa N-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system by multidimensional NMR. Biochemistry. 1997;36:2517–2530. doi: 10.1021/bi962924y. [DOI] [PubMed] [Google Scholar]
- Geen H, Freeman R. Band-selective radiofrequency pulses. J Magn Reson. 1991;93:93–141. [Google Scholar]
- Gelis I, Bonvin AM, Keramisanou D, Koukaki M, Gouridis G, Karamanou S, Economou A, Kalodimos CG. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell. 2007;131:756–769. doi: 10.1016/j.cell.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-,13C-,2H-labeled proteins. J Biomol NMR. 1999;13:369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- Haigh CW, Mallion RB. Ring current theories in nuclear magnetic resonance. Prog Nucl Magn Reson Spectrosc. 1979;13:303–344. [Google Scholar]
- Hamel DJ, Dahlquist FW. The contact interface of a 120 kD CheA-CheW complex by methyl TROSY interaction spectroscopy. J Am Chem Soc. 2005;127:9676–9677. doi: 10.1021/ja052517m. [DOI] [PubMed] [Google Scholar]
- John M, Schmitz C, Park AY, Dixon NE, Huber T, Otting G. Sequence-specific and stereospecific assignment of methyl groups using paramagnetic lanthanides. J Am Chem Soc. 2007;129:13749–13757. doi: 10.1021/ja0744753. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Kainosho M, Nagao H, Tsuji T. Local structural features around the C-terminal segment of Streptomyces subtilisin inhibitor studied by carbonyl carbon nuclear magnetic resonances of three phenylalanyl residues. Biochemistry. 1987;26:1068–1075. doi: 10.1021/bi00378a013. [DOI] [PubMed] [Google Scholar]
- Kay LE, Ikura M, Tschudin R, Bax A. Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson. 1990;89:496–514. doi: 10.1016/j.jmr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Koradi R, Billeter M, Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Kuszewski J, Gronenborn AM, Clore GM. The impact of direct refinement against proton chemical shifts in protein structure determination by NMR. J Magn Reson Series B. 1995;107:293–297. doi: 10.1006/jmrb.1995.1093. [DOI] [PubMed] [Google Scholar]
- LiWang AC, Bax A. Equilibrium protium/deuterium fractionation of backbone amides in U-13C/15N labeled human ubiquitin by triple resonance NMR. J Am Chem Soc. 1996;118:12864–12865. [Google Scholar]
- Marion D, Ikura M, Tschudin R, Bax A. Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange. J Magn Reson. 1989;85:393–399. [Google Scholar]
- Meadow ND, Fox DK, Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Mueller L. Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc. 1979;101:4481–4484. [Google Scholar]
- Ollerenshaw JE, Tugarinov V, Kay LE. Methyl TROSY: explanation and experimental verification. Magn Reson Chem. 2003;41:843–852. [Google Scholar]
- Patel HV, Vyas KA, Savtchenko R, Roseman S. The monomer/dimer transition of enzyme I of the Escherichia coli phosphotransferase system. J Biol Chem. 2006;281:17570–17578. doi: 10.1074/jbc.M508965200. [DOI] [PubMed] [Google Scholar]
- Patt SL. Single- and multiple-frequency-shifted laminar pulses. J Magn Reson. 1992;96:94–102. [Google Scholar]
- Religa TL, Sprangers R, Kay LE. Dynamic regulation of archaeal proteasome gate opening as studied by TROSY NMR. Science. 2010;328:98–102. doi: 10.1126/science.1184991. [DOI] [PubMed] [Google Scholar]
- Rosen MK, Gardner KH, Willis RC, Parris WE, Pawson T, Kay LE. Selective methyl group protonation of perdeuterated proteins. J Mol Biol. 1996;263:627–636. doi: 10.1006/jmbi.1996.0603. [DOI] [PubMed] [Google Scholar]
- Rosenzweig R, Moradi S, Zarrine-Afsar A, Glover JR, Kay LE. Unraveling the mechanism of protein disaggregation through a ClpB-DnaK interaction. Science. 2013;339:1080–1083. doi: 10.1126/science.1233066. [DOI] [PubMed] [Google Scholar]
- Ruschak AM, Kay LE. Methyl groups as probes of supra-molecular structure, dynamics and function. J Biomol NMR. 2010;46:75–87. doi: 10.1007/s10858-009-9376-1. [DOI] [PubMed] [Google Scholar]
- Ruschak AM, Religa TL, Breuer S, Witt S, Kay LE. The proteasome antechamber maintains substrates in an unfolded state. Nature. 2010;467:868–871. doi: 10.1038/nature09444. [DOI] [PubMed] [Google Scholar]
- Seok YJ, Lee BR, Zhu PP, Peterkofsky A. Importance of the carboxyl-terminal domain of enzyme I of the Escherichia coli phosphoenolpyruvate: sugar phosphotransferase system for phosphoryl donor specificity. Proc Natl Acad Sci USA. 1996;93:347–351. doi: 10.1073/pnas.93.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok YJ, Zhu PP, Koo BM, Peterkofsky A. Autophosphorylation of enzyme I of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system requires dimerization. Biochem Biophys Res Commun. 1998;250:381–384. doi: 10.1006/bbrc.1998.9323. [DOI] [PubMed] [Google Scholar]
- Shaka AJ, Keeler J, Frenkiel T, Freeman R. An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson. 1983;52:335–338. [Google Scholar]
- Sheppard D, Guo C, Tugarinov V. 4D 1H-13C NMR spectroscopy for assignments of alanine methyls in large and complex protein structures. J Am Chem Soc. 2009;131:1364–1365. doi: 10.1021/ja808202q. [DOI] [PubMed] [Google Scholar]
- Sheppard D, Guo C, Tugarinov V. Methyl-detected 'out-and-back' NMR experiments for simultaneous assignments of Alaβ and Ileδ2 methyl groups in large proteins. J Biomol NMR. 2009;43:229–238. doi: 10.1007/s10858-009-9305-3. [DOI] [PubMed] [Google Scholar]
- Sheppard D, Sprangers R, Tugarinov V. Experimental approaches for NMR studies of sidechain dynamics in high-molecular-weight proteins. Prog Nucl Magn Reson Spectrosc. 2010;56:1–45. doi: 10.1016/j.pnmrs.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Sprangers R, Kay LE. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature. 2007;445:618–622. doi: 10.1038/nature05512. [DOI] [PubMed] [Google Scholar]
- Sprangers R, Velyvis A, Kay LE. Solution NMR of supramolecular complexes: providing new insights into function. Nat Methods. 2007;4:697–703. doi: 10.1038/nmeth1080. [DOI] [PubMed] [Google Scholar]
- Sun H, Tugarinov V. Precision measurements of deuterium isotope effects on the chemical shifts of backbone nuclei in proteins: correlations with secondary structure. J Phys Chem B. 2012;116:7436–7448. doi: 10.1021/jp304300n. [DOI] [PubMed] [Google Scholar]
- Teplyakov A, Lim K, Zhu PP, Kapadia G, Chen CC, Schwartz J, Howard A, Reddy PT, Peterkofsky A, Herzberg O. Structure of phosphorylated enzyme I, the phosphoenolpyruvate:sugar phosphotransferase system sugar translocation signal protein. Proc Natl Acad Sci USA. 2006;103:16218–16223. doi: 10.1073/pnas.0607587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüchsen E, Hansen PE. Hydrogen bonding monitored by deuterium isotope effects on carbonyl 13C chemical shift in BPTI: intra-residue hydrogen bonds in antiparallel β-sheet. Int J Biol Macromol. 1991;13:2–8. doi: 10.1016/0141-8130(91)90002-c. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Hwang PM, Kay LE. Nuclear magnetic resonance spectroscopy of high-molecular-weight proteins. Annu Rev Biochem. 2004;73:107–146. doi: 10.1146/annurev.biochem.73.011303.074004. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kanelis V, Kay LE. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc. 2006;1:749–754. doi: 10.1038/nprot.2006.101. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kay LE. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc. 2003;125:13868–13878. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kay LE. An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR. 2004;28:165–172. doi: 10.1023/B:JNMR.0000013824.93994.1f. [DOI] [PubMed] [Google Scholar]
- Velyvis A, Schachman HK, Kay LE. Assignment of Ile, Leu, and Val methyl correlations in supra-molecular systems: an application to aspartate transcarbamoylase. J Am Chem Soc. 2009;131:16534–16543. doi: 10.1021/ja906978r. [DOI] [PubMed] [Google Scholar]
- Velyvis A, Yang YR, Schachman HK, Kay LE. A solution NMR study showing that active site ligands and nucleotides directly perturb the allosteric equilibrium in aspartate transcarbamoylase. Proc Natl Acad Sci USA. 2007;104:8815–8820. doi: 10.1073/pnas.0703347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti V, Clore GM. Conformational selection and substrate binding regulate the monomer/dimer equilibrium of the C-terminal domain of Escherichia coli enzyme I. J Biol Chem. 2012;287:26989–26998. doi: 10.1074/jbc.M112.382291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti V, Fawzi NL, Clore GM. Automated sequence- and stereo-specific assignment of methyl-labeled proteins by paramagnetic relaxation and methyl-methyl nuclear Overhauser enhancement spectroscopy. J Biomol NMR. 2011;51:319–328. doi: 10.1007/s10858-011-9559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti V, Fawzi NL, Clore GM. An efficient protocol for incorporation of an unnatural amino acid in perdeuterated recombinant proteins using glucose-based media. J Biomol NMR. 2012;52:191–195. doi: 10.1007/s10858-012-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters RA, Farmer BT, Fierke CA, Spicer LD. Characterizing the use of perdeuteration in NMR studies of large proteins: 13C, 15N and 1H assignments of human carbonic anhydrase II. J Mol Biol. 1996;264:1101–1116. doi: 10.1006/jmbi.1996.0699. [DOI] [PubMed] [Google Scholar]
- Weigel N, Kukuruzinska MA, Nakazawa A, Waygood EB, Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium . J Biol Chem. 1982;257:14477–14491. [PubMed] [Google Scholar]
- Weigel N, Waygood EB, Kukuruzinska MA, Nakazawa A, Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of enzyme I from Salmonella typhimurium . J Biol Chem. 1982;257:14461–14469. [PubMed] [Google Scholar]
- Xu Y, Liu M, Simpson PJ, Isaacson R, Cota E, Marchant J, Yang D, Zhang X, Freemont P, Matthews S. Automated assignment in selectively methyl-labeled proteins. J Am Chem Soc. 2009;131:9480–9481. doi: 10.1021/ja9020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tugarinov V. Accurate measurements of the effects of deuteration at backbone amide positions on the chemical shifts of 15N, 13Cα, 13Cβ, 13CO and 1Hα nuclei in proteins. J Biomol NMR. 2013;56:169–182. doi: 10.1007/s10858-013-9733-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.