Abstract
Molecular mechanisms that distinguish self and non-self are fundamental in innate immunity to prevent infections in plants and animals. Recognition of the conserved microbial components triggers immune responses against a broad spectrum of potential pathogens. In Arabidopsis, bacterial flagellin was perceived by a leucine-rich repeat-receptor-like kinase (LRR-RLK) FLS2. Upon flagellin perception, FLS2 forms a complex with another LRR-RLK BAK1. The intracellular signaling events downstream of FLS2/BAK1 receptor complex are still poorly understood. We recently identified a receptor-like cytoplasmic kinase (RLCK) BIK1 that associates with flagellin receptor complex to initiate plant innate immunity. BIK1 is rapidly phosphorylated upon flagellin perception in an FLS2- and BAK1-dependent manner. BAK1 directly phosphorylates BIK1 with an in vitro kinase assay. Plants have evolved a large number of RLCK genes involved in a wide range of biological processes. We provided evidence here that additional RLCKs could also be phosphorylated by flagellin and may play redundant role with BIK1 in plant innate immunity.
Keywords: plant innate immunity, pattern recognition receptor, microbe-associated molecular pattern, BAK1, FLS2
Plants and animals are exposed to a diverse array of microorganisms in their living environment. To survive, both plants and animals have evolved sophisticated strategies to recognize microbial agents and defend themselves from pathogen damage. Lacking the specified immune cells and being sessile, plants largely rely on innate immunity to fend off pathogen attack.1-3 In general, the first line of innate immune response is triggered upon recognition of pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) such as bacterial flagellin and elongation factor EF-Tu through cell-surface pattern recognition receptors (PRRs).1,4 PAMP-triggered immunity (PTI) is also recognized as plant basal defense. The second branch of plant innate immune response is effector-triggered immunity (ETI) or gene-for-gene resistance, which is mediated by intracellular disease resistance (R) proteins directly or indirectly recognizing pathogen virulence effectors.5 Plant ETI is usually cultivar-specific and associated with hypersensitive response (HR). However, the boundary between PTI and ETI is becoming less distinct with the discovery that the genetically defined R gene can be PRR recognizing conserved microbial components6 and certain MAMP can be race-specific in triggering defense responses.7
The best-characterized plant MAMP receptor is Arabidopsis leucine-rich repeat receptor-like kinase (LRR-RLK) FLS2 that recognizes and directly binds to a conserved 22-amino acid peptide (flg22) from bacterial flagellin.8 Upon flagellin perception, FLS2 rapidly dimerizes with BAK1, another LRR-RLK, thereby initiating a complex defense responses, including changes in cytoplasmic Ca2+ levels, activation of MAP kinase (MAPK) cascades, induction of defense-related genes, and production of reactive oxygen species (ROS) and nitric oxide (NO).9,10 How these intracellular defense responses are activated by flagellin receptor complex is not clear.
BIK1 Relays MAMP Signaling from Receptor Kinase Complexes
We have recently identified a receptor-like cytoplasmic kinase (RLCK) BIK1 transducing MAMP signaling from receptor complexes to downstream intracellular events.11 In plants, RLCKs, lacking apparent transmembrane domain, have a common monophyletic origin with RLKs.12 Genetic and biochemical analyses have suggested the roles of some RLCKs in plant defense and development. For example, PBS1 is involved in plant race specific resistance to Pseudomonas syringae expressing avrPphB.13 BIK1 is an important component in Arabidopsis resistance to necrotrophic fungi Botrytis cinerea and Alternaria brassicicola.14 OX1 is involved in oxidative burst-mediated signaling in Arabidopsis.15 BSKs mediate Arabidopsis brassinosteroid (BR) signaling by interacting with BR receptor BRI1.16 However, the mode of action of RLCKs in plant innate immunity has not been established.
By using a combination of cellular, biochemical and genetic approaches, we provided the evidence that BIK1 is an essential component in MAMP signal transduction linking MAMP receptor complex to downstream intracellular signaling11 (Fig. 1). Our discovery was originated from the observation that BIK1 transcripts were induced by multiple MAMPs, including flagellin and EF-Tu. Significantly, BIK1 is rapidly phosphorylated upon flg22 perception in an FLS2- and BAK1-dependent manner. The phosphorylation of BIK1 could be easily detected by western blot as a mobility shift. BIK1 is a plasma membrane localized protein. In agreement with this, BIK1 associates with membrane-localized FLS2 and BAK1 in vivo and in vitro. The association of BIK1 with FLS2 or BAK1 is reduced after flagellin perception, suggesting that BIK1 might be released from receptor complex to propagate MAMP signaling upon phosphorylation (Fig. 1). Since both FLS2 and BAK1 are serine/threonine protein kinases, BIK1 is likely a substrate of FLS2 and/or BAK1. Indeed, BAK1 could directly phosphorylate BIK1 with an in vitro kinase assay. Surprisingly, BIK1 also phosphorylates BAK1 and FLS2, which likely enhances the kinase activity of FLS2 and BAK1. Extensive mutagenesis analysis identified T237 as an in vivo phosphorylation site of BIK1 by flg22. T237 is also required for BIK1 autophosphorylation and phosphorylation on BAK1 and FLS2 in vitro. Compared with wild-type plants, bik1 mutants display reduced flg22 responses as assayed by flg22-mediated inhibition of seedling growth and disease resistance to virulent and nonpathogenic bacterial infection. Moreover, BIK1 is also phosphorylated by the treatment of EF-Tu in addition to flg22, indicating that it may transduce the convergent signaling initiated from multiple MAMP receptor complexes, which is consistent with the involvement of BAK1 in different MAMP-triggered signaling.
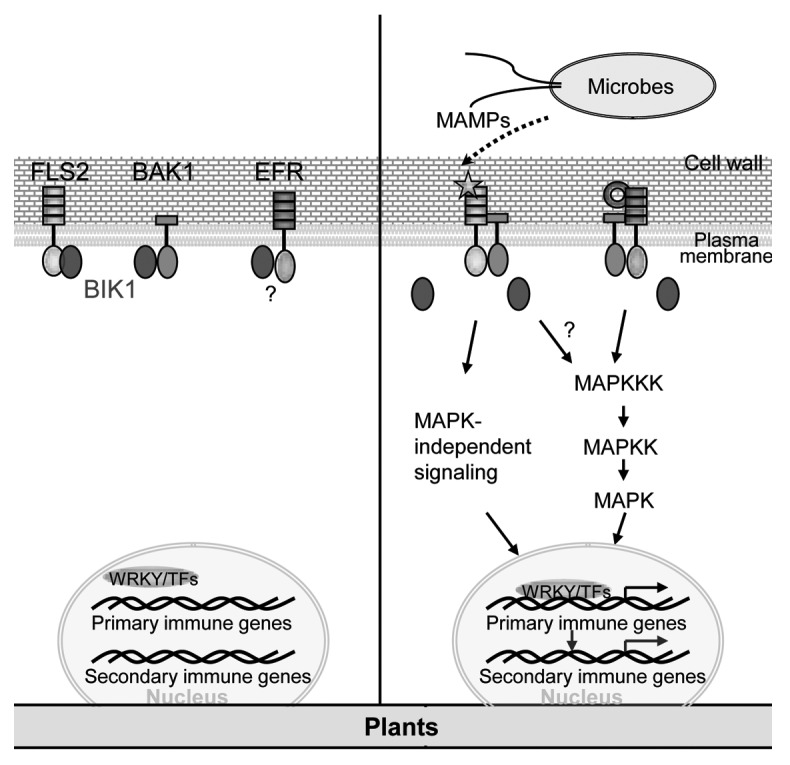
Figure 1. Phosphorylation of BIK1 by microbe-associated molecular pattern (MAMP) initiates plant innate immunity. In the absence of microbe signals, BIK1 associates with MAMP receptors and the signaling partner BAK (left). FLS2 is the receptor of flagellin, and EFR is the bacterial EF-Tu receptor. The association of BIK1 and EFR has not been determined experimentally. Upon MAMP perception, MAMP receptor associates with BAK1 and activates receptor complex probably through transphosphorylation (right). The phosphorylated BAK1 phosphorylates BIK1, which results in BIK1 disassociation from plasma membrane. BIK1 further transduces MAMP signaling through either MAP kinase-dependent or -independent pathways, thereby activating transcription factors (TF) and immune response genes. BIK1 could also transphosphorylate BAK1 and FLS2 to potentiate their kinase activity.
Our discovery not only advances the understanding of signaling events in plant innate immunity, but also suggests the striking similarity and obvious difference of innate immunity between plants and animals. In mammals, MAMPs are perceived by cell surface Toll-like receptors (TLRs).17 Signaling via all TLRs (except TLR3) leads to the recruitment of adaptor protein myeloid differentiation factor 88 (MyD88), and the Ser/Thr-specific protein kinases IL-1 receptor-associated protein kinase 1 (IRAK1) and IRAK 4. Once IRAK1 associates with MyD88, it is phosphorylated by the activated IRAK4, and subsequently released from MyD88 to propagate the signal by association with an E3 ubiquitin ligase.17,18 BIK1 appears to be analogous of IRAK1, in that it associates with BAK1 and it is released from MAMP receptor complex to transduce signal. The kinase domain of BAK1 or/and FLS2 may be similar to IRAK4,9 in that it phosphorylates BIK1. However, BIK1 differs from IRAK1 in that BIK1 also associates with MAMP receptor FLS2, and it transphosphorylates FLS2 and BAK1.
Redundant Function of RLCKs in Plant Innate Immunity
Although BIK1 was found to be an essential component in flg22-mediated signaling, notably, bik1 mutants still retain substantial sensitivity to flagellin.11 This might be the result of possible redundancy among related RLCKs that can work in concert with BIK1 in MAMP-mediated signaling. There are about 150 RLCKs in Arabidopsis genome.19 We further examined the possible involvement of other RLCKs in plant innate immunity. The BIK1 closest homolog BIK1-like kinase (At3g55450), BLK, which shows 77% identity with BIK1, was also phosphorylated by flg22 as detected by western blot (Fig. 2A). PBS1, which was originally identified as a component in gene-for-gene resistance, could also be phosphorylated upon flagellin treatment as indicated by the CIP sensitive mobility shift (Fig. 2B). Flg22-induced PBS1 phosphorylation was not dependent on BIK1, suggesting that PBS1 functions in parallel with BIK1 in flg22 signaling (Fig. 2C). It was reported that PBS1 is involved in P. syringae effector AvrPphB-triggered immunity mediated by plant R protein RPS5.20 PBS1 is directly targeted and cleaved by bacterial virulence effector AvrPphB, suggesting its role in plant PTI.13 PBS1 falls into the same RLCK subfamily RLCK-VII as BIK1 and they share 51% identity in the kinase domain.12,14 There are 46 members of RLCK VII subfamily in Arabidopsis genome with largely unknown functions.12,14 Further systemic characterization of these subfamily members may reveal additional components in plant PTI.
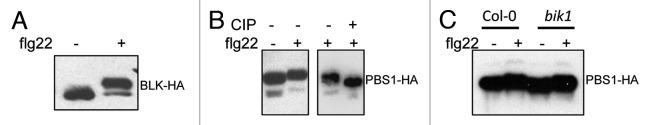
Figure 2. flg22 induces RLCKs phosphorylation. (A) flg22 induces BLK phosphorylation. (B) flg22 induces PBS1 phosphorylation. (C) PBS1 phosphorylation is independent of BIK1. Protoplasts were isolated from Col-0 or bik1 mutants, and transfected with HA epitope-tagged BLK or PBS1 for 6 h and treated with 1 μM flg22 for 10 min. The CIP treatment was performed at 37 °C for 1 h. The protein was analyzed by western blot with an anti-HA antibody.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11500
References
- 1.Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–4. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–14. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 4.He P, Shan L, Sheen J. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol. 2007;9:1385–96. doi: 10.1111/j.1462-5822.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 5.DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol. 2006;7:1243–9. doi: 10.1038/ni1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science. 2009;326:850–3. doi: 10.1126/science.1173438. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF. Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell. 2006;18:764–79. doi: 10.1105/tpc.105.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–11. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 9.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 10.Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A. 2007;104:12217–22. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci U S A. 2001;98:10763–8. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science. 2003;301:1230–3. doi: 10.1126/science.1085671. [DOI] [PubMed] [Google Scholar]
- 14.Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell. 2006;18:257–73. doi: 10.1105/tpc.105.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–61. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- 16.Tang W, Kim TW, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, Wang R, Burlingame AL, Wang ZY. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–60. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Smith H, Peggie M, Campbell DG, Vandermoere F, Carrick E, Cohen P. Identification of the phosphorylation sites on the E3 ubiquitin ligase Pellino that are critical for activation by IRAK1 and IRAK4. Proc Natl Acad Sci U S A. 2009;106:4584–90. doi: 10.1073/pnas.0900774106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiu SH, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–43. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren RF, Merritt PM, Holub E, Innes RW. Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics. 1999;152:401–12. doi: 10.1093/genetics/152.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]


