Abstract
Rationale: Delirium is common in intensive care unit (ICU) patients and is a predictor of worse outcomes and neuroinflammation is a possible mechanism. The antiinflammatory actions of statins may reduce delirium.
Objectives: To determine whether critically ill patients receiving statin therapy had a reduced risk of delirium than those not on statins.
Methods: A prospective cohort analysis of data from consecutive ICU patients admitted to a UK mixed medical and surgical critical care unit between August 2011 and February 2012; the Confusion Assessment Method for ICU was used to determine the days each patient was assessed as being free of delirium during ICU admission.
Measurements and Main Results: Delirium-free days, daily administration of statins, and serum C-reactive protein (CRP) were recorded. Four hundred and seventy consecutive critical care patients were followed, of whom 151 patients received statins. Using random-effects multivariable logistic regression, statin administration the previous evening was associated with the patient being assessed as free of delirium (odds ratio, 2.28; confidence interval, 1.01–5.13; P < 0.05) and with lower CRP (β = −0.52; P < 0.01) the following day. When the association between statin and being assessed as free of delirium was controlled for CRP, the effect size became nonsignificant (odds ratio, 1.56; confidence interval, 0.64–3.79; P = 0.32).
Conclusions: Ongoing statin therapy is associated with a lower daily risk of delirium in critically ill patients. An ongoing clinical trial, informed by this study, is investigating if statins are a potential therapy for delirium in the critically ill.
Keywords: delirium, statin, inflammation, C-reactive protein, critical care
At a Glance Commentary
Scientific Knowledge on the Subject
Neuroinflammation is believed to be a significant factor in delirium pathophysiology. Statins have a number of antiinflammatory properties and have been investigated as a potential therapy for conditions thought to be related to systemic inflammation.
What This Study Adds to the Field
This observational study found a link between ongoing administration of statins and a reduction in risk of delirium, which could be mediated through a reduction in systemic inflammation.
Delirium is a form of acute brain dysfunction, with a prevalence of up to 65% in critically ill patients requiring mechanical ventilation in the United Kingdom (1). It is associated with significantly worse clinical outcomes. Delirium is independently associated with a threefold increased risk of mortality at 6 months, and for survivors a 10-fold increased risk of cognitive impairment at 12 months (2, 3). Long-term cognitive impairment after critical illness reduces quality of life, increases healthcare costs, and leads to institutionalization (4, 5).
Although the pathogenesis of delirium remains poorly understood, there is evidence for ongoing neuroinflammation driving oxidative damage and apoptosis, which is hypothesized to drive the development of cognitive impairment (6). Irrespective of whether or not patients have sepsis, during critical illness, higher levels of procalcitonin is associated with patients being free of delirium, whereas higher C-reactive protein (CRP) levels showed trends toward fewer delirium-free days (7). This implicates systemic inflammation in the pathophysiology of delirium in the intensive care unit (ICU).
Statins exert pleiotropic effects independent of inhibiting cholesterol synthesis, and a significant proportion of these effects are antiinflammatory and may be evident within 24 hours (8, 9). Simvastatin is known to decrease systemic inflammation as measured by serum CRP in healthy volunteers and in critically ill patients with acute lung injury (10, 11). In adult mice, surgical stress causes inflammation-mediated, hippocampal-dependent, cognitive dysfunction. Postoperative elevation of serum inflammatory cytokines was associated with memory impairment, reactive microgliosis, and increased interleukin-1b expression in the hippocampus (12). For these reasons, it has been suggested that statins might have a clinical effect on reducing delirium (13). Although statins have been investigated in clinical trials to modify organ dysfunction in critically ill patients, none have assessed delirium as an outcome (11, 14).
The aim of this study was to determine if statin use in critically ill patients was associated with delirium, as assessed using the Confusion Assessment Method-ICU (CAM-ICU) (15). The hypothesis to be tested was that statin usage would be associated with less delirium, and would be associated with a reduction in systemic inflammation.
Methods
This was a prospective cohort study. The cohort comprised all consecutive patients admitted to a 19-bed mixed medical and surgical adult critical care unit in Watford General Hospital, a UK district general hospital. The study started on August 1, 2012 and included all patients admitted from that date on until February 29, 2012. Data were from ICU admission collected up until discharge from ICU. There were no exclusions, and therefore the study population included a variety of admissions including elective emergency, medical, and surgical admissions. All patients were allocated a study number and data collected were anonymized. The ICU clinical staff was not informed regarding the study or hypothesis, although they were aware data were being collected.
To derive propensity score additional information was collected: age, sex, primary hypercholesterolemia, ischemic heart disease, diabetes, peripheral and cerebrovascular disease, and admission after aortic aneurysm surgery. In addition, the admitting diagnosis, data required to calculate daily severity of illness scores, presence of sepsis, number of ventilator days, emergency or elective admission, and preadmission statin use were also documented. Additional detail regarding the collection of the data is provided in the online supplement.
Outcome Measures
For ICU patients the number of days without delirium or not in coma (any cause including sedation) is an indicator of normal cognitive status (i.e., awake and no delirium). The presence of delirium was assessed using the CAM-ICU, a delirium screening tool developed for use by the bedside nurse as part of routine patient assessment. It has been validated in intubated critically ill patients against the reference Diagnostic and Statistical Manual of Mental Disorders-IV criteria (16).
Patients were allocated to the “never statin” group if they did not receive statins throughout ICU admission regardless of whether they had been on statins before admission. For daily comparison the patients in the “statin” group were then allocated to the “no statin received” group on an individual day if they did not receive their statin medication for whatever reason.
Clinical Assessments
Patients were routinely assessed by nurses first for level of sedation using the Richmond Agitation Sedation Scale and then for delirium using the CAM-ICU (17).
All daily nursing charts of study patients were reviewed to determine the number of days when an individual patient was assessed throughout a 24-hour period (8:00 a.m. to 8:00 a.m.) as CAM-ICU negative (i.e., free of delirium). Patients are assessed using the CAM-ICU on an average of two times per 12-hour shift; the number of daily assessments of individual patients was not collected. If any CAM-ICU assessment on a given day recorded as positive or the patient was unable to be assessed because of lack of response, that day was counted as not delirium- or coma-free. Routine delirium screening was introduced in 2007 and there is ongoing nurse-led training.
Statins were given according to our standard practice, where in patients previously taking statins as soon as the attending physician made the decision that enteral therapy could be started the statin was administered if there were no contraindications (e.g., elevated liver enzymes or ongoing macrolide therapy). It was not necessary for enteral feeding to be fully established. In keeping with evidence that simvastatin is more effective in cholesterol reduction if given in the evening, all statins were administered at approximately 22:00 (18). Blood for serum CRP measurement was drawn at 06:00. A member of the research team collected the data regarding statin administration (X.B.Z.). Additional detail regarding the assessments and measurement of CRP is provided in the online supplement.
Statistical Analysis
All analyses were conducted using STATA 12.1 (Stata Corp, College Station, TX). Distributions of each variable were first examined in relation to statin use. The distribution of CRP was positively skewed, necessitating log-transformation for analyses. Crude differences in continuous variables were assessed with t test and Mann-Whitney U test; differences in categorical variables were assessed using chi-square and Fisher exact test. Associations were assessed with 95% confidence intervals (CI) and considered significant at P less than 0.05.
Demonstrating mediation requires four steps: (1) the effect of the independent variable (statin) on the dependent variable (delirium-free) must be significant, (2) the path from the independent variable (statin) to the mediator (CRP) must be significant, (3) the path from the mediator (CRP) to the dependent variable (delirium-free) must be significant, and (4) the independent variable (statin) has a reduced or no effect on the dependent variable after adjustment for the mediator (CRP) (19). Accordingly, the analysis sought to address the following questions:
-
1.
Is statin use associated with being delirium-free (when not controlling for CRP)?
-
2.
Is statin use associated with serum CRP levels?
-
3.
Is CRP associated with being delirium-free?
-
4.
Does the association between statin use and being delirium-free change when simultaneously adjusting for CRP so as to suggest CRP mediates this relationship?
Three regression models were used to test each of the hypotheses, with numbers 3 and 4 tested simultaneously (20). Random-effects accounted for the clustered nature of the data. The association between being free of delirium and CRP the following day was assessed using a linear mixed-effect model. Three mixed-effects logistic regression models tested (1) the lagged association between statin use and being free of delirium the following day, (2) the association between CRP and being free of delirium the same day, and (3) the lagged association between statin use and being free of delirium the following day controlling for CRP. The term “lagged” refers to the temporal structure of the data (i.e., that statins were routinely given the previous evening). Thus, the statin (exposure) was used to estimate the odds of being delirium-free the next day (outcome). Without this lag, exposure and outcome would have been assigned to the same day and because statins were administered after delirium assessment would not have been a causally appropriate model for the purposes of this study.
To reduce confounding introduced by nonrandom patient-associated factors for statin therapy, a propensity score analysis was included. Variables for the propensity score were selected from among demographic and clinical variables associated with statin prescription: age, sex, primary hypercholesterolemia, ischemic heart disease, diabetes, peripheral and cerebrovascular disease, and admission after aortic aneurysm surgery. In addition, all models were adjusted by age, sex, daily modified daily Sequential Organ Failure Assessment (SOFA) score (excluding the neurologic component, Glasgow Coma Score [GCS]), sepsis on admission, propensity score, need for ventilator support (known risk factor for delirium), emergency admission, and preadmission statin use. Any day of observation on which a participant died was excluded from the analysis. Data were assumed to be missing at random, because any predictors of missingness were included as covariates in the random-effects models to account for this.
Results
In total, 470 consecutive critical care patients with 2,927 person-days follow-up were included in the analysis (median, 5 d). There were no exclusions. Clinical characteristics of the 151 (32.1%) who received statins during their stay, compared with the 319 (67.9%) who did not, are given in Table 1. There were no patients started on statins as a new therapy; statins were only prescribed for patients who had been on statins before admission. The group receiving statins was more likely to be older and have lower median CRP levels (70 vs. 88 mg/L). Patients who were not on statins before admission were more likely to require ventilatory support. For reasons for admission see Table E1 in the online supplement. In total 167 patients were assessed as having delirium at least once of which 44 had delirium throughout the admission. The median duration of delirium was 2 days (interquartile range, 1–5). Thirty-one patients were also recruited to an ongoing phase 2 randomized controlled trial to determine if early intravenous haloperidol, as compared with placebo, modified delirium in critically ill patients. The study, Hope-ICU, showed that there was no difference in the number of days spent in delirium or sedation-induced coma between patients in the two groups (21).
Table 1:
Clinical Characteristics of Study Population, Stratified by Statin Use
| Statin Use |
|||
|---|---|---|---|
| No (n = 319) | Yes (n = 151) | P Value | |
| Age, yr (SD) | 63 (19) | 77 (11) | <0.01 |
| Sex, male (%) | 165 (52) | 86 (57) | 0.51 |
| Days delirium-free (IQR) | 3 (1–5) | 2 (1–5) | 0.81 |
| Delirium Y/N*, n (%) | 125/188 (40%/60%) | 50/100 (33%/67%) | 0.17 |
| CRP, median, mg/L (IQR)† | 88 (47–207) | 70 (33–193) | <0.01 |
| ICU length of stay, d (IQR) | 5 (3–8) | 4 (2–7) | 0.07 |
| APACHE II score (SD) | 17 (7) | 18 (7) | 0.32 |
| ICU mortality, % | 63 (20) | 27 (18) | 0.71 |
| Ventilated, n (%) | 148 (46) | 49 (32) | <0.01 |
| Ventilated, d (IQR) | 2 (0–9) | 1 (0–13) | 0.75 |
| Indication for statin (%)‡ | <0.01 | ||
| IHD or CVD | 62 (18) | 67 (56) | |
| Peripheral vascular disease | 9 (3) | 4 (3) | |
| Abdominal aortic aneurysm | 10 (3) | 6 (5) | |
| Diabetes mellitus | 21 (6) | 15 (13) | |
| Hypercholesterolemia | 4 (1) | 20 (19) | |
| None | 202 (57) | 0 (0) | |
| Missing | 44 (13) | 7 (6) | |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation score; CRP = C-reactive protein; CVD = cerebrovascular disease; ICU = intensive care unit; IHD = ischemic heart disease; IQR = interquartile range.
Y, assessed with delirium on at least one occasion; N, unable to be assessed (coma) or assessed free of delirium throughout admission.
CRP on admission (not adjusted).
Percentages are given for the columns, by statin use.
Table 2 shows each pair of associations between statins, CRP, and being free of delirium on any given day, using a random-effects logistic regression model to adjust for the covariates age, sex, daily severity of illness, sepsis, requiring ventilator support, emergency admission, and the propensity score. The difference between the number of subjects in Table 2 used to calculate the pair-wise associations results from missing data (see Tables E2 and E3). The decision was made to use random-effects model as one technique that estimates robust standard errors where covariates are predictors of data missing (i.e., missing at random). As expected patients were more likely to develop delirium if they were admitted as an emergency and if more seriously ill and required ventilatory support. CRP levels were higher in patients with sepsis.
Table 2:
Random-Effects Logistic Regression Model Showing Pairwise Associations between Statin, CRP, and Being Free of Delirium
| Statin: Free of Delirium | Statin: CRP | |||||
|---|---|---|---|---|---|---|
| (n = 228; Person-Days = 1,246) |
(n = 226; Person-Days = 1,123) |
|||||
| OR | 95% CI | P Value | β | 95% CI | P Value | |
| Statin | 2.28 | 1.01 to 5.13 | <0.05 | −0.52 | −0.70 to −0.33 | <0.01 |
| Age, per yr | 0.99 | 0.96 to 1.03 | 0.71 | −0.00 | −0.01 to 0.00 | 0.35 |
| Sex, women vs. men | 0.48 | 0.20 to 1.19 | 0.11 | 0.03 | −0.19 to 0.24 | 0.81 |
| mSOFA, per point | 0.59 | 0.47 to 0.73 | <0.01 | 0.13 | 0.09 to 0.18 | <0.01 |
| Sepsis, yes vs. no | 1.53 | 0.62 to 3.76 | 0.36 | 0.29 | 0.07 to 0.51 | 0.01 |
| Propensity score* | 2.90 | 0.35 to 23.83 | 0.32 | 0.02 | −0.49 to 0.53 | 0.94 |
| Ventilated, yes vs. no | 0.78 | 0.72 to 0.84 | <0.01 | −0.01 | −0.03 to 0.00 | 0.14 |
| Emergency vs. elective | 12.81 | 3.05 to 53.82 | <0.01 | 0.36 | 0.05 to 0.66 | 0.02 |
Definition of abbreviations: CI = confidence interval; CRP = C-reactive protein; mSOFA = modified Sequential Organ Failure Assessment (minus Glasgow Coma Score); OR = odds ratio.
β is the slope from linear regression.
CRP quantities log(e) transformed to obtain normal distribution. When used as a dependent variable, OR is per increase in standard deviation of log(e) CRP.
All models allow for random-effects for each individual.
Propensity score according to age, sex, primary hypercholesterolemia, ischemic heart disease, diabetes, peripheral and cerebrovascular disease, and admission for aortic aneurysm surgery.
There was variability in statin administration such that statins were omitted 46% of total admission days for patients who had been receiving statins preadmission. This was for a variety of reasons including unable to receive or absorb medication, liver enzyme rise, or concurrent macrolide prescription.
Association between Statin Use and Being Free of Delirium
For ongoing statin therapy, the independent variable was statin (yes or no) administered the previous evening with being free of delirium the following day as the dependent variable. When accounting for this dosing schedule, an association between statin and being free of delirium was observed (odds ratio [OR], 2.28; 95% CI, 1.01–5.13; P < 0.05) (Table 2).
Association between Statin Use and CRP
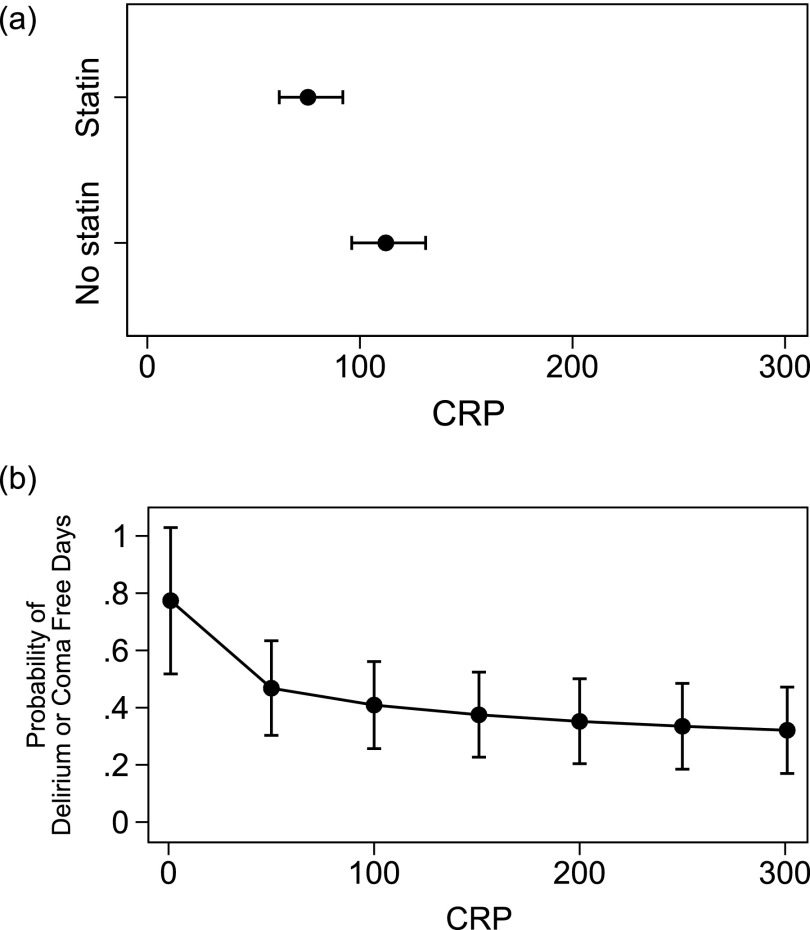
The independent variable was statin use the previous evening (yes or no) with log CRP as the dependent variable. Statin use was associated with lower CRP levels (Figure 1a). Linear regression demonstrated a significant association with statin administration (β = −0.52; 95% CI, −0.7 to −0.33; P < 0.01) (Table 2). This reduction in log CRP when statins were given the previous evening is the equivalent to CRP 100 mg/L lowering to 59 mg/L or CRP 250 mg/L lowering to 148 mg/L on the natural scale.
Figure 1.
(a) The relationship between levels of C-reactive protein (CRP) according to statin use the previous evening. Each point shows the average CRP after adjustments for covariates in each group along with 95% confidence intervals (whiskers above and below the point estimate). There is little overlap in the width of the confidence intervals in each group, suggesting that statin use significantly influences CRP (P < 0.01). (b) The relationship between adjusted CRP and probability of being delirium-free, adjusting by statin use. Each point shows the probability of being free of delirium along with 95% confidence intervals (whiskers above and below point estimate). The higher the CRP, the lower the probability of being free of delirium. For example, if CRP is 20 mg/L the probability of being delirium-free on a given day is 65%; if CRP is 100 mg/L, the probability is 42%. Together, these figures show that statin use is associated with lower CRP, and this in turn is associated with a higher probability of being free of delirium.
Association between Statin Use and Being Free of Delirium after Adjustment for CRP
The independent variables were statin use the previous evening (yes or no) and log CRP with being free of delirium as the dependent variable. The observed effect size of statin administration on the probability of being free of delirium was reduced (OR, 1.56; 95% CI, 0.64–3.79) and became nonsignificant (P = 0.32) when accounting for CRP (Table 3). A significant relationship between CRP and being free of delirium remained (OR, 0.68; 95% CI, 0.51–0.90) (Table 3). The probability of being free of delirium had a strongest association with CRP when CRP is less than 100 mg/L; there is an attenuated decrease in the probability of being delirium-free at the highest levels of CRP.
Table 3:
Random-Effects Logistic Regression Model Showing the Relationship between Statin and Free of Delirium, Adjusted by CRP
| Statin + CRP: Free of Delirium |
|||
|---|---|---|---|
| (n = 225; Person-Days = 1,117) |
|||
| OR | 95% CI | P Value | |
| Statin | 1.56 | 0.64–3.79 | 0.32 |
| CRP | 0.68 | 0.51–0.90 | 0.01 |
| Age, per year | 0.99 | 0.96–1.02 | 0.46 |
| Sex, women vs. men | 0.62 | 0.25–1.53 | 0.30 |
| mSOFA, per point | 0.60 | 0.48–0.76 | <0.01 |
| Sepsis, yes vs. no | 2.46 | 0.95–6.36 | 0.06 |
| Propensity score* | 3.63 | 0.41–31.8 | 0.24 |
| Ventilated, yes vs. no | 0.74 | 0.68–0.81 | <0.01 |
| Elective vs. emergency | 17.6 | 3.96–78.1 | <0.01 |
Definition of abbreviations: CI = confidence intervals; CRP = C-reactive protein; mSOFA = Sequential Organ Failure Assessment (minus Glasgow Coma Score); OR = odds ratio.
CRP quantities log(e) transformed to obtain normal distribution. When used as a dependent variable, OR is per increase in standard deviation of log(e) CRP.
All models allow for random-effects for each individual.
Propensity score according to age, sex, primary hypercholesterolemia, ischemic heart disease, diabetes, peripheral and cerebrovascular disease, and admission for aortic aneurysm surgery.
For every day a statin user continued to receive a statin the odds of being delirium-free and coma-free increased by 39% (OR, 1.39; 95% CI, 1.18–1.63; P < 0.001) (Table 4).
Table 4:
Relationship between Statin Continuation and the Odds of Being Delirium-Free Coma-Free in Persons Prescribed Statins before Admission
| Statin Continuation |
|||
|---|---|---|---|
| (n = 89; Person-Days = 405) |
|||
| OR | 95% CI | P Value | |
| Days on statin, per day | 1.39 | 1.18–1.63 | <0.01 |
| Age, per year | 0.97 | 0.91–1.02 | 0.22 |
| Sex, women vs. men | 0.64 | 0.19–2.10 | 0.460 |
| mSOFA, per point | 0.79 | 0.56–1.11 | 0.17 |
| Sepsis, yes vs. no | 3.23 | 1.01–10.47 | 0.05 |
| Propensity score* | 2.03 | 0.07–62.99 | 0.69 |
| Ventilated, yes vs. no | 0.71 | 0.62–0.80 | <0.01 |
| Emergency vs. elective | 28.2 | 5.20–153 | <0.01 |
Definition of abbreviations: CI = confidence intervals; mSOFA = modified Sequential Organ Failure Assessment (excluding Glasgow Coma Scale); OR = odds ratio.
Propensity score accounting for age, sex, primary hypercholesterolemia, ischemic heart disease, diabetes, peripheral and cerebrovascular disease, and admission for aortic aneurysm surgery.
Discussion
In this population of patients admitted to critical care, after adjusting for age, sex, and daily illness severity, ongoing statin therapy was associated with a lower daily risk of delirium and a concomitant reduction in serum CRP. These findings are the first to suggest that ongoing statin use reduces brain dysfunction as assessed using the CAM-ICU in consecutive critical care admissions. These data raise the suggestion that an antiinflammatory action may form part of the basis of the statin-delirium relationship and are consistent with the neuroinflammatory hypothesis of delirium. However, additional research is required to confirm this suggestion.
Statins are known to have several antiinflammatory properties in addition to their lipid-lowering actions (8, 9, 22). In a rat model, it has been demonstrated that surgery under anesthesia but not anesthesia alone causes inflammation-mediated, hippocampal-dependent, cognitive dysfunction (23). Moreover, in statin-treated animals there is preservation of memory retrieval after head injury, whereas in a separate study after unilateral nephrectomy there was a decrease in functional neurologic deficits in animals who received statins (24, 25). Published studies on the use of statins and delirium in ICU patients have conflicting results (26–28). They are limited to preoperative use of statins in patients after cardiac surgery. Importantly in all these studies, it is not specified whether the statins were withdrawn on ICU admission.
Two small trials support a plausible antiinflammatory effect of statins used at clinically relevant doses over the short-term. One study in 42 patients comparing one dose of 80 mg simvastatin with placebo demonstrated significant reductions in serum median and mean CRP concentrations measured 48 hours later (29). Another trial compared 17 patients who received standard therapy for unstable angina or non–Q wave myocardial infarction with 13 patients also given one dose of cerivastatin (30). At 24 hours cerivastatin-treated patients had significantly lowered CRP levels.
There is increasing evidence neuroinflammation has a major part in the development and maintenance of delirium (31–34). Preadmission statins did not affect the risk of delirium, but this study did show a risk reduction in developing delirium on a day-by-day basis with the administration of statins, suggesting that statins actively protect against delirium rather than indicating this was a statin withdrawal syndrome. The observed effect after statin administration suggests a biologically plausible causal pathway whereby a reduction in systemic inflammation mediates the statin-delirium relationship. In the analyses the criteria for mediation were largely met: statin use (independent variable) was associated with being free of delirium (dependent variable), statin use was associated with CRP (mediating variable), and adjusting the association between statin use and being free of delirium by CRP showed a reduction in effect size. Previous studies assessing markers of inflammation and delirium during critical illness have had conflicting results; a study of 138 critically ill patients measuring CRP on enrollment and Day 5 did not demonstrate an association with delirium (35).
The main strength of this study is that a large number of consecutive patients with data on daily mental status assessment, CRP measurements, and administration of statin therapy in a general ICU were included. In addition, data regarding severity of illness over time were collected using a modified SOFA score. The SOFA is a well-established measure of severity of illness in the critically ill; however, we did not include the neurologic component (GCS). This is consistent with other analyses (36). It is important to recognize that the inclusion of a neurologic measure of arousal (GCS), which is a cardinal component of delirium, as a covariate for illness severity would lead to overadjustment. In other words, it would not be appropriate to have GCS represented as both a covariate and outcome measure.
The models were also adjusted for daily severity of illness using a modified SOFA score to address the concern that the attending physician’s decision to commence with administration of enteral therapy medication with or without nutrition may have coincided with clinical improvement and consequently a lower delirium risk. By estimating multiple separate models, one per individual day of admission for each patient, it could be shown that the reduction in delirium occurred on the days in which a statin had been given the previous evening. This takes into account the patients on statins before admission who did not receive them during ICU admission because those days were analyzed as no statin given.
The patient group in this study is broadly representative of the case mix of patients admitted to a mixed critical care unit in contrast to other studies of delirium in the critical care environment where the population recruited has largely been postoperative cardiac patients. Previous studies of statin and CRP have measured plasma CRP levels at specified time points during patients admission, the most frequent being a recent study in patients with severe sepsis in which CRP was measured every other day for the first week and only twice in the second (37). Our study has a robust data set based on longitudinal observations providing a depth that studies with less frequent observations have lacked.
Several limitations should be acknowledged. First, this is a single-site study, and as with any observational study, despite the multiple adjustments, residual confounding remains a possibility. The possibility that the administration of statins is more likely as a patient’s clinical condition improves is a potential confounding factor. However, given the relation between statin usage and delirium persisted when adjusted for the daily severity of illness means this is unlikely. Data collected regarding patients receiving statins were limited to the daily administration of statins while on ICU, not whether the patients had been taking statins before admission.
Confounding by indication is another potential limitation; patients prescribed statins before admission may have differing risks for delirium, compared with those not on statins. In our analysis we have used a propensity score based on several variables relating to the likelihood of statin prescription. It may be that, despite including the obvious variables, such as diabetes and ischemic heart disease, we have not captured other factors, for instance one that would make it less likely a patient would seek medical attention (38).
There were three statins prescribed (simvastatin, atorvastatin, and pravastatin), although most (134 of 151) of the patients were given simvastatin. It is not clear if a specific statin may be superior particularly given statins are known to have varying degrees of brain penetration (39). In addition, information regarding the dose of individual statins was lacking such that dose–response thresholds could not be examined. With regard to effective doses, clinical trials have found that high-dose, compared with conventional-dose, statin therapy reduces the risk of cardiovascular events in patients with stable coronary heart disease and acute coronary syndromes (40). There were no data collected regarding other known confounding factors, particularly the sedative agents used or doses administered. The standard sedation protocol for ventilated patients at our hospital uses fentanyl and propofol infusions with daily sedation interruption as clinically appropriate. Midazolam is used occasionally and antipsychotics are reserved for patients who have hyperactive delirium. Although it is likely the sedation exposure was similar in all patients, this cannot be assumed.
We did not investigate trajectories of CRP change to determine a rate or limit of reduction. Instead we estimated a separate model for each day with statin, SOFA, CRP, and delirium information linked together using random-effects for each individual to demonstrate the association between statin and delirium.
We used delirium-free days as the outcome (i.e., when patients could be assessed as not in delirium using the CAM-ICU) rather than the incidence of delirium in patients. The limitations of cognitive assessments in critically ill patients are recognized. Although the CAM-ICU has been shown to lack sensitivity it is one of the only two tools validated and recommended for use by the recent Pain, Analgesia and Delirium Clinical Practice Guidelines from the American College of Critical Care Medicine (41). It indicates normal brain function in critically ill patients, rather than a patient being in coma (whether sedative induced or caused by a medical cause), or having delirium. For the purposes of this observational trial, being assessed as delirium-free is clinically desirable and therefore relevant for critically ill patients.
In conclusion, this is the first report to indicate a beneficial effect of ongoing statin therapy on delirium in a UK critically ill population. These results suggest that in patients receiving statins before ICU admission, statin therapy should be continued to prevent delirium, albeit with appropriate safety monitoring. To test the hypothesis generated by this study that daily statin therapy reduces delirium in the critically ill, a phase 2 randomized placebo-controlled trial in critically ill ventilated patients is ongoing (ISRCTN89079989). It is underpinned by investigations to determine the mechanisms by which statins may be effective.
Acknowledgments
Acknowledgment
The authors thank Hannah Payne, R.N. (Watford General Hospital) for her assistance in compiling the database, and Alessandro Morandi, M.D., M.P.H. (Department of Rehabilitation and Aged Care Unit, Ancelle della Carità Hospital, Cremona, Italy; Geriatric Research Group, Brescia, Italy; Center for Quality of Aging, Vanderbilt) and Tim Girard, M.D., M.S.C.I. (Vanderbilt University Medical Center, Nashville; Center for Quality of Aging, Vanderbilt) for their advice and support on the design and analysis of this project.
Footnotes
Supported by grant funding from the Wellcome Trust (090661/Z/09/Z to D.D.); the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (G0700704/84698 to D.D); the BBSRC, EPSRC, ESRC, and MRC; and in part by NIHR CLAHRC for Cambridgeshire and Peterborough (S.N.). Support of the UK Intensive Care Foundation is gratefully acknowledged.
Author Contributions: V.J.P. contributed to the study conception, study design, acquisition of data, and drafting of article. D.D., S.N., and D.F.M. contributed to analysis and interpretation of data and drafting of article. X.B.Z., A.C., and T.B. contributed to acquisition of the data. E.W.E., D.D., and D.F.M. contributed to study conception and study design. S.N., D.D., X.B.Z., A.C., T.B., E.W.E., and D.F.M. critically revised the article and all authors approved the final version to be published.
Originally Published in Press as DOI: 10.1164/rccm.201306-1150OC on January 13, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Page VJ, Navarange S, Gama S, McAuley DF. Routine delirium monitoring in a UK critical care unit. Crit Care. 2009;13:R16. doi: 10.1186/cc7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eeles EM, Hubbard RE, White SV, O’Mahony MS, Savva GM, Bayer AJ. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing. 2010;39:470–475. doi: 10.1093/ageing/afq052. [DOI] [PubMed] [Google Scholar]
- 5.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 7.McGrane S, Girard TD, Thompson JL, Shintani AK, Woodworth A, Ely EW, Pandharipande PP. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:R78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22:165–170. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- 9.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 10.Shyamsundar M, McKeown ST, O’Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 12.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP. Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest. 2011;140:580–585. doi: 10.1378/chest.10-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, Amichay D, Almog Y. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4th edition, text revision. Washington: American Psychiatric Association; 2000 [Google Scholar]
- 17.Sessler C, Gosnell M, Grap M, Brophy G, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale (RASS) Am J Respir Crit Care Med. 2002;166:1339–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 18.Lund TM, Torsvik H, Falch D, Christophersen B, Skårdal R, Gullestad L. Effect of morning versus evening intake of simvastatin on the serum cholesterol level in patients with coronary artery disease. Am J Cardiol. 2002;90:784–786. doi: 10.1016/s0002-9149(02)02614-0. [DOI] [PubMed] [Google Scholar]
- 19.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 20.MacKinnon DP.Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008
- 21.Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206:59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Penn JW, Vizcaychipi MP, Zhuang L, Pac-Soo C, Ma D. Atorvastatin attenuates cognitive impairment following nephrectomy in mice. Intensive Care Med. 2010;36:S383. [Google Scholar]
- 26.Katznelson R, Djaiani GN, Borger MA, Friedman Z, Abbey SE, Fedorko L, Karski J, Mitsakakis N, Carroll J, Beattie WS. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110:67–73. doi: 10.1097/ALN.0b013e318190b4d9. [DOI] [PubMed] [Google Scholar]
- 27.Mariscalco G, Cottini M, Zanobini M, Salis S, Dominici C, Banach M, Onorati F, Piffaretti G, Covaia G, Realini M, et al. Preoperative statin therapy is not associated with a decrease in the incidence of delirium after cardiac operations. Ann Thorac Surg. 2012;93:1439–1447. doi: 10.1016/j.athoracsur.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Mathew JP, Grocott HP, McCurdy JR, Ti LK, Davis RD, Laskowitz DT, Podgoreanu MV, Swaminathan M, Lynch J, Stafford-Smith M, et al. Preoperative statin therapy does not reduce cognitive dysfunction after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2005;19:294–299. doi: 10.1053/j.jvca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Li JJ, Wang Y, Nie SP, Zhang CY, Li YS, Hui RT, Zhen X. Reduction of C-reactive protein by a single 80 mg of simvastatin in patients with unstable angina. Clin Chim Acta. 2007;376:163–167. doi: 10.1016/j.cca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Ostadal P, Alan D, Hajek P, Horak D, Vejvoda J, Trefanec J, Mates M, Vojacek J. The effect of early treatment by cerivastatin on the serum level of C-reactive protein, interleukin-6, and interleukin-8 in the patients with unstable angina and non-Q-wave myocardial infarction. Mol Cell Biochem. 2003;246:45–50. [PubMed] [Google Scholar]
- 31.Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 34.van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 35.Girard TD, Ware LB, Bernard GR, Pandharipande PP, Thompson JL, Shintani AK, Jackson JC, Dittus RS, Ely EW. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. 2012;38:1965–1973. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zygun DA, Doig CJ, Gupta AK, Whiting G, Nicholas C, Shepherd E, Conway-Smith C, Menon DK. Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003;18:238–244. doi: 10.1016/j.jcrc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B, Jones D, Joyce C, Kostner K, et al. for the ANZ-STATInS Investigators-ANZICS Clinical Trials Group. A multicenter randomized trial of atorvstatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 38.Koh GC, Vlaar AP, Hofstra JJ, de Jong HK, van Nierop S, Peacock SJ, Wiersinga WJ, Schultz MJ, Juffermans NP. In the critically ill patient, diabetes predicts mortality independent of statin therapy but is not associated with acute lung injury: a cohort study. Crit Care Med. 2012;40:1835–1843. doi: 10.1097/CCM.0b013e31824e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierra S, Ramos MC, Molina P, Esteo C, Vázquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23:307–318. doi: 10.3233/JAD-2010-101179. [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 41.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]