Abstract
Disorganized speech, or thought disorder, in schizophrenia may reflect abnormal processing of meaningful concepts. To examine whether schizophrenia involves abnormalities in how a meaningful context influences processing of concepts strongly, weakly, or not related to it, we used the N400, an event-related brain potential (ERP) index of semantic relatedness. ERPs were recorded from schizophrenia patients (n=18) and normal controls (n=18) while they viewed category definitions (e.g., a type of fruit), each followed by a target word that was either a high-typicality category exemplar (apple), low-typicality exemplar (cherry), or non-exemplar (clamp). Participants' task was to indicate via button-press whether or not the target belonged to the category. In both patients and controls, N400 amplitude was largest (most negative) for non-exemplars, intermediate for low-typicality exemplars, and smallest (least negative) for high-typicality exemplars. Compared to controls, patients showed a trend toward reduced N400 amplitude differences between non-exemplars and low-typicality exemplars. Most importantly, within patients, reduced N400 amplitude differences between high- and low-typicality exemplars were correlated with psychotic symptoms. In schizophrenia patients, an N400 index of semantic processing was associated with psychotic symptoms. Psychosis may be associated with greater similarity in how concepts strongly and weakly meaningfully related to their context are processed.
Keywords: Schizophrenia, Semantic priming, Thought disorder, Psychosis, Event-related potentials, N400
1. Introduction
“Disturbance of associations” was identified as a core feature of schizophrenia by Bleuler (1911/1950), who observed that in schizophrenic language “associations lose their continuity” and “appear odd, bizarre, distorted” or “senseless.” He inferred from this that:
thinking operates with ideas and concepts which have no, or a completely insufficient, connection with the main idea…The result is that thinking becomes confused, bizarre, incorrect, abrupt.
Disorganized speech in schizophrenia has thus been thought to objectively reflect underlying conceptual disorganization, or “thought disorder.”
Bleuler's (1911/1950) notion of abnormalities in “pathways of association and inhibition” presaged more recent theories proposing that schizophrenic disorganization results from abnormalities in how concepts activate one another in semantic memory (McCarley et al., 1999; Nestor et al., 1998; Spitzer, 1997). These theories assume a model of semantic long-term memory in which concepts are nodes in a neural network, and meaningful associations among concepts reflect connectivity among these nodes (Anderson and Pirolli, 1984; Collins and Loftus, 1975; Neely, 1977). When a concept node is activated, as by its corresponding word stimulus, this activation spreads through the network to associated nodes. The degree to which the stimulus concept activates another concept and facilitates its processing is presumably related to the strength of the links between them.
One hypothesis for how abnormal activation in semantic memory may lead to disorganized speech postulates a broader spread of activation to weakly or remotely related items. For instance, Spitzer (1997) proposed that indirect associates - those related through at least one other concept, like CAT and CHEESE (mediated by MOUSE) - activate one another more strongly in schizophrenia, thereby leading to speech containing sequences of apparently unrelated or weakly related concepts.
Some semantic priming data support this hypothesis. Semantic priming refers to the facilitation of response to a target item - e.g., faster reaction time (RT) in a lexical-decision task - when it is preceded by a meaningfully related prime stimulus rather than an unrelated one. Greater priming is thought to reflect greater activation of the target by the prime. Consistent with increased spread of activation to weak associates, an abnormally large priming effect for indirectly related words has been found in thought-disordered schizophrenia patients, although only when the prime-target interval (or stimulus-onset asynchrony (SOA)) is relatively short (≤300 ms) (Moritz et al., 2001; Moritz et al., 2003; Spitzer et al., 1993).
A different hypothesis attributes disorganized speech in schizophrenia to impaired ability to use context to activate related items, or to inhibit unrelated items (Cohen and Servan-Schreiber, 1992; McCarley et al., 1999). Importantly, this abnormality is not necessarily mutually exclusive with increased spreading activation, as they could occur in sequence. In fact, whereas RT priming evidence for increased spreading activation has come from word-pair studies employing relatively short SOAs (≤300 ms), most RT priming evidence for impaired context use has come either from word-pair studies employing longer SOAs, or from studies using sentence contexts, which also build up over a longer period. For instance, schizophrenia patients displayed less priming than normal controls for closely related words at a long SOA of 950 ms, but not at shorter (200–700 ms) SOAs (Barch et al., 1996). In a sentence-context study (Kuperberg et al., 1998), both schizophrenia patients and controls were slower to recognize a sentence-final word when it was semantically incongruent with the context than when it was congruent, but patients with high thought-disorder ratings were delayed less than were either controls, or patients with low thought-disorder ratings – consistent with impaired use of context.
Semantic priming effects also have been investigated using the N400 component of scalp-recorded event-related brain potentials (ERPs). The N400 is a negativity occurring from approximately 200 to 500 ms, and peaking at approximately 400 ms, after presentation of any potentially meaningful stimulus such as a word or picture. N400 amplitude is reduced by factors facilitating an item's processing, such as linguistic word frequency or stimulus repetition (reviewed in Kutas and Federmeier (2000)). Of relevance here is that N400 amplitude elicited by a target stimulus is reduced (i.e., made less negative) by increasing semantic relatedness between the target and a preceding prime stimulus (Holcomb and Neville, 1990; Holcomb and Neville, 1991; Kutas, 1985; Kutas and Hillyard, 1980; Stelmack and Miles, 1990). In other words, N400 amplitude to a target is smaller (less negative) when it is more related to the prime. N400 amplitude has thus been used to measure the degree to which concepts activate one another in semantic memory, with reduced (less negative) amplitude corresponding to greater activation.
Results of N400 studies in schizophrenia have been mixed. Prime-target word pairs with SOA=450 (Kostova et al., 2005; Kostova et al., 2003) and 600 ms (Strandburg et al., 1997)) were associated with larger N400 amplitudes to related targets in schizophrenia patients than in normal controls, while amplitudes to unrelated targets did not differ between patients and controls. These results suggest decreased activation of related targets in schizophrenia, consistent with the impaired context use hypothesis. In another word-pair experiment, Condray et al. (2003) reported a reduced N400 priming effect (i.e., reduced difference in N400 amplitude between related and unrelated targets) in patients versus controls, at SOAs of both 350 and 950 ms, although N400 amplitudes did not significantly differ between patients and controls for either related or unrelated targets. In contrast, Spitzer et al. (1997) reported that N400 amplitude for indirectly related words (SOA=200 ms) was smaller in patients compared to controls, consistent with relatively greater activation of weak associates. In a picture-word matching task (SOA=250 ms) (Mathalon et al., 2002), target words referring to an item from the same category as the picture elicited a smaller N400 in patients than in controls, consistent with increased activation of related items. Studies with sentence-final word paradigms (Kostova et al., 2003; Ohta et al., 1999) showed larger N400 amplitudes to congruent words in patients than controls, consistent with impairment in using context to activate semantically congruent items.
Overall, then, the results of these N400 studies, like those of RT priming studies, suggest that schizophrenia patients generally show abnormal semantic priming, although hypothetically for different reasons at different prime-target intervals. Specifically, at relatively short SOAs (approximately ≤ 300 ms), there may be increased or more broadly-spreading activation of related concepts, as reflected in smaller N400 amplitudes for related targets. However, at longer SOAs, including within a sentence context, schizophrenia patients may be impaired in using context to activate related or expected items, as reflected in larger N400s to these items.
One relationship in the semantic network that has been extensively studied in normal participants is the relation between categories and their exemplars. Behavioral norming studies have documented the typicality of different exemplars for a wide range of categories (Battig and Montague, 1969; Hunt and Hodge, 1971; McEvoy and Nelson, 1982; Shapiro and Palermo, 1970). For example, apple is a high-typicality exemplar of the category fruit, whereas cherry is a low-typicality exemplar, meaning that individuals rate apple as being a more typical fruit than cherry, and, when asked to name fruits, are more likely to say apple than cherry. Higher typicality is thought to reflect greater semantic relatedness between category and exemplar (Hampton, 1979; McCloskey and Glucksberg, 1978). After a category name, exemplars elicit smaller N400 amplitudes than do non-exemplars (Federmeier and Kutas; Heinze et al., 1998; Iragui et al., 1996); in addition, high-typicality exemplars elicit smaller N400 amplitudes than do low-typicality exemplars (Federmeier and Kutas; Heinze et al., 1998; Stuss et al., 1988).
In the present study, we used the N400 to examine whether categories activate their exemplars in the semantic network abnormally in schizophrenia, and whether any such abnormalities are reliably associated with particular symptoms. To that end, we presented schizophrenia patients and normal control participants (NCPs) with category definition phrases followed by target nouns, which were either high-typicality exemplars, low-typicality exemplars, or non-exemplars. Participants were asked to indicate whether or not the target belonged to the category. Thus, where previous N400 studies in schizophrenia used only two types of targets differing in their degree of relatedness to the prime, we aimed to more finely characterize how schizophrenia and its symptoms modulate the effect of relatedness on semantic priming, by distinguishing between targets strongly related (high-typicality exemplars), weakly related (low-typicality exemplars), and unrelated (non-exemplars) to the prime.
We hypothesized that in controls, the N400 would be largest to non-exemplars, intermediate to low-typicality exemplars, and smallest to high-typicality exemplars, consistent with previous findings, and that this general pattern would also hold for patients. In addition, we hypothesized that if schizophrenia is generally accompanied by a broader spread of activation to weaker associates, then categories might activate their less typical exemplars relatively more strongly, as reflected in smaller N400s to low-typicality exemplars in patients versus controls. Consequently, in patients compared to controls, the difference in N400 amplitude between low- and high-typicality exemplars (i.e., the N400 typicality effect) would be reduced, and the N400 effect between unrelated non-exemplars and low-typicality exemplars (the N400 low-typicality category effect) would be larger. On this account, the difference between unrelated non-exemplars and high-typicality exemplars (the N400 high-typicality category effect) would not change.
We also hypothesized that if schizophrenia patients generally make less efficient use of context, then both high- and low-typicality exemplars, as related words, would be less activated, i.e. elicit larger N400s, in patients than in controls. Additionally, non-exemplars might also be relatively less inhibited, or more activated, eliciting smaller (less negative) N400s in patients than in controls. Overall, these abnormalities would lead to smaller high- and low-typicality N400 category effects in the patients. In addition, in patients compared to controls, the N400 typicality effect would either remain unchanged (if activations of both high- and low-typicality exemplars are reduced by a similar magnitude) or decrease (if the activation decrease is larger in magnitude for high-typicality exemplars compared to low-typicality exemplars, in proportion to the difference in their absolute activation levels).
2. Methods and Materials
2.1. Participants
Participants included 18 schizophrenia patients and 18 NCPs. Patients were all outpatients, recruited through community residential facilities and physician referral. NCPs were recruited through newspaper advertisements, and flyers posted at the University of California, San Diego (UCSD) Medical Center. All participants were assessed on their capacity to provide informed consent, and gave written informed consent via the UCSD Institutional Review Board approved form (#030510). Participants were compensated in cash.
Patients were assessed with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995), and were screened to rule out any other Axis I diagnosis including substance abuse. NCPs were assessed using the SCID (non-patient version) to rule out any past or present Axis I or II diagnoses including substance abuse, and were also excluded if they were taking any psychotropic medications. Other exclusion criteria for all participants included: exposure to a language other than English at home as a child; and current or past neurological disorder. Handedness was assessed by the Edinburgh Inventory (Oldfield, 1971), and parental socioeconomic status (SES) was computed (Hauser and Warren, 1996). Participants completed the Peabody Picture Vocabulary Test (PPVT) (Dunn and Dunn, 1997) as a measure of receptive vocabulary. Demographic characteristics of the study sample are shown in Table 1.
Table 1. Demographic characteristics of the study sample (means ± SD given where applicable).
| Schizophrenia Patients | Healthy Controls | |
|---|---|---|
| Age, years | 46.3±10.5 | 43.2±8.4 |
| Sex | 13 male, 5 female | 12 male, 6 female |
| Handedness | 17 right, 1 left | 16 right, 2 left |
| Parental SES | 42.9±19.3 | 43.3±17.3 |
| Years of Educationa | 12.6±2.2 | 16.1±2.4 |
| PPVTb | 173.1±20.9 | 187.9±8.2 |
Patients differed significantly from controls, p<.0001
Patients differed significantly from controls, p=.008
Twelve patients were prescribed second-generation antipsychotic medications (as defined by Lohr and Braff (2003)), 2 were prescribed first-generation antipsychotics, and 2 were prescribed a combination of first- and second-generation antipsychotics. Two patients reported not taking antipsychotics for at least one month before testing.
2.2. Assessments
Clinical symptoms in patients were assessed with the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984) and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984). Based on these ratings, we calculated scores for the Psychotic symptom factor (Hallucinations + Delusions), Negative factor (Affective Flattening + Avolition/Apathy + Anhedonia/Asociality) and Disorganized factor (Positive Formal Thought Disorder + Bizarre Behavior) (Miller et al., 1993). Patients' clinical characteristics are shown in Table 2.
Table 2. Clinical characteristics of schizophrenia patients (means ± SD given where applicable).
| Age of onset, years | 23.7±7.3 |
| Duration of illness, years | 22.1±10.9 |
| Number of previous hospitalizations | 5.9±5.4 |
| SANS Total | 8.9±4.2 |
| SAPS Total | 3.5±3.4 |
| Negative Factor | 8.3±3.7 |
| Psychotic Factor | 2.2±2.7 |
| Disorganized Factor | 1.7±2.5 |
2.3. Stimuli
Stimuli were based on existing category production norms (Battig and Montague, 1969; Hunt and Hodge, 1971; McEvoy and Nelson, 1982; Shapiro and Palermo, 1970). For each of 120 category phrases, the associated target words included: a) a high-typicality exemplar, b) a low-typicality exemplar and c) an unrelated non-exemplar. Targets were matched overall for length and word frequency (Francis and Kucera, 1982) across these conditions. The high-typicality exemplar chosen was the exemplar most frequently produced by individuals in the category norms, except where its very high frequency would prevent the conditions from being matched for word frequency. In such cases an exemplar of as high a rank as possible was used. Low-typicality exemplars were chosen from among those ranked 9th or lower by frequency of generation. Examples are shown in Table 3.
Table 3. Sample categories and corresponding target stimuli.
| Category | High-Typicality Exemplars | Low-Typicality Exemplars | Non-Exemplars |
|---|---|---|---|
| A type of fruit | apple | cherry | clamp |
| A weapon | gun | revolver | musician |
| A string instrument | guitar | banjo | platform |
| A farm animal | cow | goat | antenna |
| A disease | cancer | alcoholism | pottery |
| Something worn on the feet | shoes | slippers | freight |
Using these stimuli, 3 different stimulus lists were generated. In each list, each of the 120 categories appeared once, and 40 categories were paired with each of the 3 target types. Each of the possible target types for each category occurred in one of the 3 lists. Within each list, length and frequency were matched across the 3 target types. Each list also included 40 additional filler categories followed by an unrelated target. Filler stimuli were not analyzed but were included so that each list included an equal number of exemplar and non-exemplar targets (80 each).
2.4. Task
Participants were tested in a single session. They were seated comfortably in a chair, 100 cm in front of a video monitor on which stimuli were visually presented, with each letter in a word subtending on average approximately 0.36° of visual angle horizontally, and up to 0.55° vertically. Words were displayed in yellow letters on a black background.
Each participant was presented with one of the 3 lists, with the categories presented in a fixed randomized order. Each of the 3 lists was seen by 6 patients and 6 controls. Each list was divided into four blocks of 40 trials each, separated by breaks during which participants were permitted to rest until ready to continue. Each trial consisted of the following sequence: a) category (e.g. a type of fruit) for 2150 ms, b) blank screen for an interval varied pseudorandomly between 250 and 650 ms (to avoid the superimposition of anticipatory ERP effects which occur when the timing of onset of the target is invariant), c) target (e.g. cherry) for 1000 ms, d) blank screen for 2000 ms, e) the prompt Yes or No? until participants responded with a button-press (see below), f) blank screen for 3000 ms until onset of the next trial. All stimuli were presented centered on the screen horizontally and vertically. A central fixation point remained visible throughout, positioned 0.5° below the bottom-most edge of where the words were presented.
Upon presentation of the prompt, participants were required to press one of two buttons on a keypad, with their right and left thumbs, respectively. One button (labeled “Yes”) signaled that the word was a true exemplar of the preceding category, while the other button (labeled “No”) signaled that it was a non-exemplar. The assignment of buttons was reversed for half the participants in each group.
2.5. Electrophysiological data collection and analysis
The electroencephalogram was recorded from 34 sintered Ag/AgCl electrodes in an electrode cap (EasyCap, Herrsching-Breitbrunn, Germany). Electrode sites corresponded to the International 10/20 system (Figure 1). Electrodes placed at the tip of the nose and at Fpz served as reference and ground, respectively. Blinks and eye movements were monitored via electrodes placed on the supraorbital ridge and infraorbital ridge of the left eye, and on the outer canthi of both eyes. Electrode impedances were below 5 kΩ. The EEG was processed through a Neuroscan NuAmps amplifier (Compumedics, El Paso, TX) set at a bandpass of 0.5–100 Hz, continuously digitized at 1 kHz, and stored on hard disk for later analysis.
Figure 1.
Schematic diagram of the electrode array.
The EEG was re-referenced off-line to the algebraic mean of the left and right mastoids (TP9/TP10). Continuous data were algorithmically corrected for eyeblink artifact (Semlitsch et al., 1986). ERPs were computed for epochs extending from 100 ms pre-stimulus to 924 ms post-stimulus. Individual trials containing artifacts due to eye movement, excessive muscle activity or amplifier blocking were rejected off-line by visual inspection before time-domain averaging; the mean percentage of trials lost to such artifacts was 17% for patients and 6% for controls.
For each trial, N400 latency was defined as the interval between stimulus onset and the largest negative peak between 250 and 650 ms post-stimulus. N400 amplitude was measured as the mean voltage from 350-550 ms post-stimulus (this window chosen because it was centered approximately around the grand mean N400 peak latency). For each participant, N400 effects were derived from the difference waves formed by subtracting the average ERPs between each pair of target types: non-exemplar minus high-typicality exemplar (high-typicality category effect), non-exemplar minus low-typicality exemplar (low-typicality category effect), and low-typicality exemplar minus high-typicality exemplar (typicality effect). Latency of N400 effects was defined as the interval between stimulus onset and the largest negative peak between 250 and 650 ms post-stimulus. Amplitudes of N400 effects were measured as the mean voltage of the appropriate difference wave from 300-500 ms post-stimulus (this window chosen because it was centered approximately around the grand mean N400 effect peak latency).
2.6. Statistical analysis
In general, all p-values in analyses of variance (ANOVA) with within-subject factors are reported after Greenhouse-Geisser Epsilon correction. Pairwise comparisons of factor-level means were made using the Tukey procedure for simultaneous pairwise comparisons, with a family confidence co-efficient of 0.95. Reported p-values for all statistical analyses are two-tailed.
Percentage of correct responses was analyzed in a repeated-measures ANOVA, with Group (schizophrenia vs. control) as a between-subject variable, and Target (high-typicality vs. low-typicality vs. non-exemplar) as a within-subject variable.
N400 latency and N400 amplitude were analyzed in repeated-measures ANOVAs with Group (schizophrenia vs. control) as between-subject variable, and Target (high-typicality vs. low-typicality vs. non-exemplar) and Electrode (34 levels, corresponding to all recording sites) as within-subject variables. To test for between-group differences in N400 effects, each of these effects (high-typicality category effect, low-typicality category effect and typicality effect) was analyzed in an ANOVA with Group (schizophrenia vs. control) as between-subject variable, and Electrode (15 levels, corresponding to a contiguous array of sites where differences in N400 effects were most prominent: Fz, F3, F4, FC1, FC2, FC5, FC6, Cz, C3, C4, T7, T8, CP1, CP2, Pz) as within-subject variable.
To examine the relationship between symptoms and N400 effects in patients, correlation co-efficients were calculated between the high-typicality category effect, low-typicality category effect, and typicality effect at Cz; and SANS and SAPS total and Psychotic, Negative, and Disorganized factor ratings. For correlations involving SAPS total and Psychotic and Disorganized factor ratings, we used Spearman's rank-order co-efficient ρ, since these scores were not normally distributed; otherwise, Pearson's co-efficient r was used.
3. Results
3.1. Behavioral data
Percentages of correct responses for schizophrenia patients and controls for the different conditions are shown in Table 4. Overall, the high rate of correct responses indicates that participants were attending to the stimuli. There was a significant Target effect (F2,68=32.73, p<0.0001), with correct response rate differing significantly between high- and low-typicality exemplars, and between low-typicality exemplars and non-exemplars, but not between high-typicality exemplars and non-exemplars. There was no Group effect (F1,34=1.47, p=0.23), or Group × Target interaction (F2,68=0.65, p=0.52), suggesting that patients and controls did not differ in their attention to the stimuli.
Table 4. Percentage of correct categorization responses, by participant group and target condition.
| Schizophrenia Patients (n=18) | Healthy Controls (n=18) | |||||
|---|---|---|---|---|---|---|
| Target | Mean | SD | Range | Mean | SD | Range |
| High-Typicality | 96.0 | 6.9 | 78-100 | 98.2 | 3.7 | 85-100 |
| Low-Typicality | 85.4 | 9.9 | 55-98 | 89.0 | 6.8 | 70-98 |
| Non-Exemplar | 94.3 | 6.7 | 78-100 | 95.0 | 6.2 | 80-100 |
3.2. Grand average ERPs
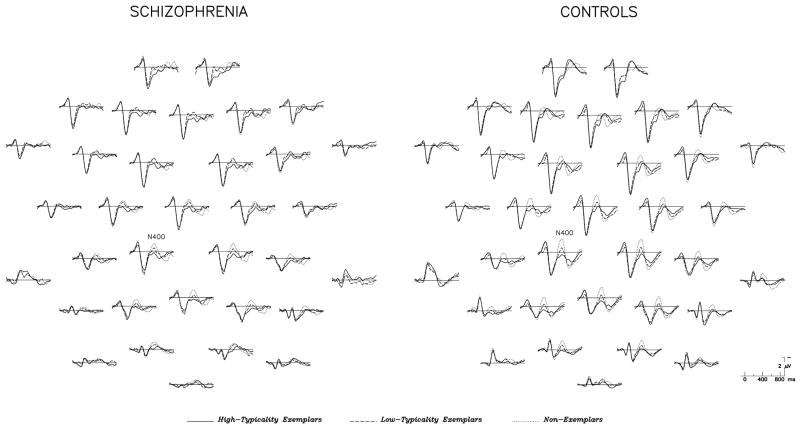
Grand average ERPs at all electrodes are shown separately for schizophrenia and control groups in Figure 2.
Figure 2.
Grand average ERPs for the three target types, at all electrode sites, for the schizophrenia and control groups (n=18 per group). As in Figure 1, the front of the head is oriented upward.
3.3. N400 latency
Mean N400 peak latency across the combined patient and control groups for all target types was 452 ms. Mean latency did not vary significantly by Group (F1,34=0.19, p=0.67) or Target (F2,68=2.01, p=0.22), and there was no Group × Target interaction (F2,68=2.01, p=0.14).
Mean peak latency for N400 effects across the combined patient and control groups for all target types was 411 ms. Mean latency was longer for the low-typicality category effect (419 ms) than for the high-typicality category effect (406 ms) or the typicality effect (408 ms) (F2,68=4.51, p=0.02). There was no significant Group effect (F1,34=0.43, p=0.52) or Group × Target interaction (F2,68=2.01, p=0.14).
3.4. N400 amplitude
Across patient and control groups, N400 amplitude was largest (most negative) for non-exemplars, intermediate for low-typicality exemplars, and smallest for high-typicality exemplars (main effect of Target: F2,68=12.22, p<0.0001) (see Figure 2). N400 effects were broadly distributed over the scalp although largest medially and centrally, consistent with the distribution seen in previous N400 studies of word reading (Federmeier and Kutas, 1999; Kutas and Van Petten, 1994) (Target × Electrode interaction: F50,1100=9.86, p<0.0001). There was no Group effect (F1,34=0.01, p=0.99) on N400 amplitude, nor any Group × Target interaction (F2,68=0.23, p=0.80), indicating that patients and controls did not differ significantly in N400 amplitude to the different target types.
3.5. N400 effects
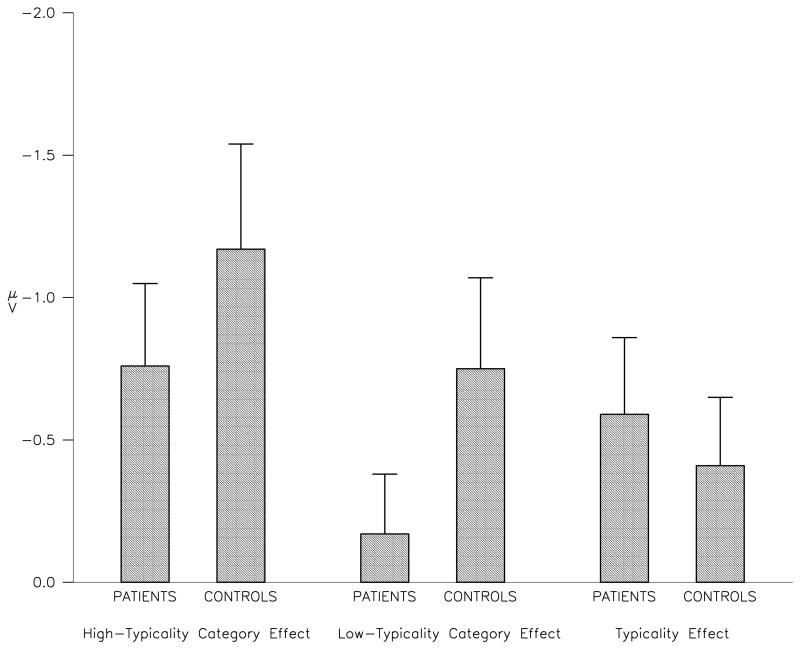
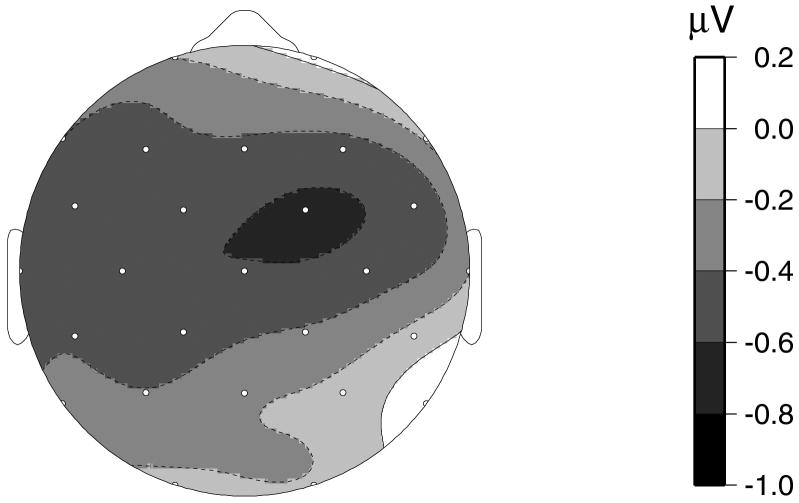
There was a trend for the N400 low-typicality category effect to be reduced in patients compared to controls (F1,34=2.79, p=0.10). This difference was decreased slightly when PPVT scores were included as a covariate (F1,33=2.32, p=0.14). Patients and controls did not differ significantly in the amplitude of the high-typicality category effect (F1,34=0.89, p=0.35) or the typicality effect (F1,34=0.24, p=0.63). Amplitudes of these N400 effects for patients and controls at a representative electrode, Cz, are shown in Figure 3. The scalp distribution of the difference in the low-typicality category effect between patients and controls is shown in Figure 4, using the spherical spline interpolation technique (Perrin et al., 1989).
Figure 3.
Amplitude of N400 effects at Cz. Bars represent mean and standard error.
Figure 4.
Spherical spline interpolation of the scalp distribution of the difference in the low-typicality category effect between the schizophrenia and control groups.
N400 effects were not significantly correlated with PPVT scores, either for patients (high-typicality category effect: ρ=-0.08, p=0.64; low-typicality category effect: ρ=-0.21, p=0.22; typicality effect: ρ=0.16, p=0.35) or for controls (high-typicality category effect: r=0.24, p=0.33; low-typicality category effect: r=0.16, p=0.53; typicality effect: r=0.16, p=0.53).
3.6. Relationship between N400 effects and patient characteristics and symptom ratings
Within patients, N400 effects were not significantly correlated with onset of illness (high-typicality category effect: r=-0.17, p=0.50; low-typicality category effect: r=-0.05, p=0.86; typicality effect: r=-0.13, p=0.62) or with its duration (high-typicality category effect: r=0.03, p=0.90; low-typicality category effect: r=0.05, p=0.85; typicality effect: r=-0.02, p=0.95).
Correlation co-efficients between N400 effects and symptom ratings for patients are shown in Table 5. Since the N400 has a negative amplitude, positive correlation co-efficients indicate that the N400 effect was smaller with higher symptom ratings. The Psychotic Factor was correlated with reduced N400 typicality effects. In addition, there was a trend for the Psychotic Factor to be correlated with reduced N400 high-typicality category effects, and for the Negative Factor to be correlated with reduced N400 high- and low-typicality category effects.
Table 5. Correlations of patient symptom ratings with N400 effects (difference wave mean amplitudes from 300-500 ms) at Cz (n=18).
| SANS Total | SAPS Total | SANS/SAPS Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Psychotic | Disorganized | ||||||||
| r | p | ρ | p | r | p | ρ | p | ρ | p | |
| High-typicality category effect | 0.43 | 0.08 | 0.38 | 0.12 | 0.45 | 0.06 | 0.42 | 0.08 | 0.05 | 0.85 |
| Low-typicality category effect | 0.39 | 0.11 | 0.19 | 0.46 | 0.42 | 0.08 | -0.26 | 0.29 | -0.10 | 0.70 |
| Typicality effect | 0.14 | 0.59 | 0.50 | 0.04 | 0.14 | 0.59 | 0.64 | 0.005 | 0.05 | 0.86 |
4. Discussion
This study used the N400 component of the ERP as a direct, brain-based method of assessing the functional organization of semantic memory in schizophrenia. Schizophrenia patients and NCPs matched for age, sex and parental SES were presented with prime phrases that were category definitions, each followed by a target noun that was either a high-typicality exemplar, a low-typicality exemplar or an unrelated non-exemplar of the category. As hypothesized, in both patients and controls, the N400 amplitude elicited by targets was largest (most negative) for non-exemplars, intermediate for low-typicality exemplars, and smallest (least negative) for high-typicality exemplars. Contrary to our hypotheses, patients and controls did not differ significantly in the N400 amplitudes elicited by each target type, or in amplitude differences between target types (N400 effects); however, there was a trend toward reduced N400 amplitude differences between non-exemplars and low-typicality exemplars (i.e. the N400 low-typicality category effect) for patients versus controls. Moreover, within the patient group, more severe psychotic symptoms were correlated with reduced N400 amplitude differences between high- and low-typicality exemplars (the N400 typicality effect).
The absence of significant between-group N400 effect differences in the present study contrasts with other studies where such differences were found to be associated with schizophrenia (Condray et al., 2003; Condray et al., 1999; Kostova et al., 2005; Kostova et al., 2003; Mathalon et al., 2002; Mitchell et al., 1991; Ohta et al., 1999; Strandburg et al., 1997), or with schizotypal personality (Kiang and Kutas, 2005; Kimble et al., 2000; Niznikiewicz et al., 2002), which is thought to share genetic and neurophysiological substrates with schizophrenia (Siever and Davis, 2004). Our results, however, are not an isolated instance, as there are other published reports in which no such differences were detected between schizophrenia patients and controls (Andrews et al., 1993; Koyama et al., 1994; Olichney et al., 1997). As with neurophysiological abnormalities in schizophrenia in general (Johannesen et al., 2005), there is considerable overlap in N400 effects between patients with the disorder and healthy individuals (Grillon et al., 1991), and variation in sample characteristics may thus have contributed to these inconsistent results across studies. For example, in our study, patients' average age was older than in any of the studies cited above except that of Olichney et al. (1997), which also did not find N400 effect differences between patients and controls. Since age is associated in general with smaller N400 effects (Kutas and Iragui, 1998), it might also attenuate differences in these effects between patients and controls. In addition, studies that found N400 effect differences between patients and controls may have examined more severely ill patients than studies that did not find such differences. In support of this hypothesis, some studies that found patient-control differences included a mixture of inpatients and outpatients (Kostova et al., 2005; Kostova et al., 2003; Mathalon et al., 2002; Ohta et al., 1999), whereas studies that found no such differences examined either outpatients only (our study and that of Olichney et al. (1997)), patients with “no acute illness” (Koyama et al., 1994), or patients of unspecified admission status (Andrews et al., 1993). Nevertheless, comparing illness severity among these studies directly is difficult, as they used a variety of symptom rating scales. Further research is necessary to clarify the effects of these and other patient characteristics on the extent of N400 effect abnormalities.
The trend we observed for patients to exhibit reduced low-typicality category effects compared to controls is consistent with the hypothesis that schizophrenia is associated with decreased use of context to activate items related to it, or to inhibit items unrelated to it. According to this hypothesis, either less than normal activation (i.e. larger N400s) for weakly related targets, or less than normal inhibition (i.e. smaller N400s) for unrelated targets, or both, would cause a reduction in the low-typicality category effect. Our results, however, do not fully support this hypothesis, which also predicts less than normal activation for strongly related targets, and hence significantly reduced high-typicality category effects in patients relative to controls.
The lower vocabulary of patients compared to controls raises the question of whether the patients' smaller low-typicality category effects could be explained by unfamiliarity with some of the low-typicality exemplars. Unfamiliar words might be processed as pseudowords, which tend to elicit larger N400 amplitudes than known words (Carreiras et al., 2005), thereby decreasing the low-typicality category effect. However, when vocabulary scores were included as a covariate, the difference in the low-typicality category effect between patients and controls only decreased slightly, suggesting that it is not caused primarily by vocabulary differences.
Our results also do not support the hypothesis of broader spread of activation to weakly related concepts in schizophrenia. Were this true, it would have led to smaller N400s to low-typicality exemplars, in turn causing smaller than normal typicality effects and larger than normal low-typicality category effects, which we did not observe. It is important to note, however, that we employed a relatively long SOA between prime and target (2400–2800 ms), giving participants sufficient time to read the longer category definitions. Thus, although we found no ERP evidence for more broadly spreading activation of related concepts in schizophrenia at long SOAs, our study design does not allow us to determine whether this might occur at short SOAs.
Within patients, the observed association between the SAPS-derived Psychotic factor and reduced N400 typicality effects might reflect an association of psychotic symptoms with either decreased context use or a broader spread of activation. If decreased context use leads to proportionally reduced activation for high-typicality exemplars compared to low-typicality exemplars, proportional to their different absolute activation levels (as predicted by Cohen and Servan-Schreiber's (1992) model of decreased gain in a context-representing neural network), this would decrease the activation difference between them, reducing the typicality effect. Broader spread of activation, by reducing the activation difference between high- and low-typicality exemplars, would likewise reduce the typicality effect. However, decreased context use, but not broader spread of activation, predicts a reduction in the high-typicality category effect as well. Therefore, the additional trend we observed toward a correlation of psychotic symptoms with reduced high-typicality category effects suggests that, in our experimental paradigm, decreased context use may be more likely than increased spread of activation to mediate the association between psychotic symptoms and abnormal N400 categorical priming effects.
The finding that N400 effects were correlated with the Psychotic but not the Disorganized factor was somewhat unexpected, since the hypotheses we examined aimed to explain disorganized speech and thought disorder. Moreover, decreased N400 relatedness effects have been reported by Kostova et al. (2005) to correlate with a measure of disorganization, the Thought, Language and Communication Disorders Scale (Andreasen, 1979). The present study may have been limited in its ability to detect N400 differences associated with disorganized speech because the severity of this symptom in our patient sample was relatively low, or because the SAPS may not have been as sensitive in detecting disorganized speech as more specialized instruments.
Some researchers have proposed that semantic processing abnormalities in schizophrenia could be caused by abnormal organization of concepts within the semantic network (Aloia et al., 1996; Leeson et al., 2005; Rossell and David, 2006). This disorganized storage could exist instead of, or along with, deficient access to semantic memory. Our results cannot definitively distinguish between these possibilities, as the N400 is modulated both by factors affecting ease of lexical access (including activation), and by the organization of concepts in semantic memory (see Kutas and Federmeier (2000) for a review of how these different factors influence N400 amplitude). Thus, the trend toward smaller N400 low-typicality category effects in patients could be due either to reduced access to low-typicality exemplars following a category prime; or to degradation of the representations of low-typicality exemplars, or of their links to their categories, within semantic memory. It is possible, however, that future N400 priming studies could be specifically designed to distinguish between abnormalities of semantic memory access and organization. For example, if N400 priming were normal in schizophrenia patients at one SOA but abnormal at another, this might suggest normal organization of the semantic network, with abnormal activation over a specific time window.
Further studies are necessary to confirm whether our observed correlation of psychosis with decreased differences in N400 priming for strongly versus weakly related category members generalizes to other schizophrenia patient samples. If replicated, this finding would suggest that delusions are associated with a smaller than normal difference in processing of typical versus atypical members of a category, at least at a semantic level. In other words, high and low typicality members are treated more similarly by psychotic patients than by normal individuals.
If this finding generalizes to other semantic stimuli, it would suggest that development of delusions may be associated with one type of “conceptual disorganization” – namely, a reduced difference in the degree to which context facilitates processing of other stimuli strongly versus weakly meaningfully related to it. This abnormality could lead to the subjective experience that stimuli weakly related to their context are unusually meaningful. In turn, this could prompt patients to search for an explanation connecting the stimulus and context, even when these are in reality unrelated.
This sequence of events would be consistent with Hemsley's (2005) proposal that “an abnormal view of relationships between events is among the most prominent features of delusional thinking.” In common with some previous hypotheses about delusion formation (Kapur, 2003; Maher, 1988; Roberts, 1992), it postulates that delusions reflect an attempt to explain puzzling subjective experiences that stem from pathological neurophysiological processes. Thus, unusual subjective experiences “produced endogenously by various neuropathologies” (Maher, 1988) give rise to a feeling of significance and tension, or “delusional mood.” Since “this stage is deeply perplexing and uncomfortable, promoting a powerful drive to understand what is being experienced” (Roberts, 1992), the development of an explanation, even if erroneous, causes relief. Of relevance to our results, the delusional mood has been described as “a fracturing and disintegration of previous meaning patterns” (Roberts, 1992). In particular, psychotic patients commonly report that they have experienced a strong meaningful relationship between stimuli that normal individuals would regard as minimally related, and that their delusional belief explains these experiences. This is seen in the following patient's account, as described by Schneider (1959):
A dog lay in wait for me as he sat on the steps of a Catholic convent. He got up on his hind legs and looked at me seriously. He then saluted with his front paw as I approached him. Another man was a little way in front of me. I caught up to him hurriedly and asked if the dog had saluted him too. An astonished ‘no’ told me I had to deal with a revelation addressed to me.
Thus, an alteration in the relative degree to which meaningful stimuli affect processing of concepts strongly versus weakly related to them may be one instance of an aberrant neurophysiological process that predisposes to psychotic symptoms.
Acknowledgments
This study was supported by grant HD22614 to M. Kutas from the National Institutes of Health, a grant from the Department of Veteran Affairs (VISN 22 Mental Illness Research, Education and Clinical Center), and grants MH042228 and MH065571 from the National Institute of Mental Health. M. Kiang is supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. M. Kutas was a Lady Davis Fellow at Hebrew University during the writing of this manuscript. We thank Barbara Haugeland, Sheldrick Holmes, Paul Krewski, Katrin Meyer-Gomes, Jocelyn Prugh, Pete Sharp, Joyce Sprock, Kari Tweedale and Thomas Urbach for technical assistance.
Role of the Funding Source: Funding for this study was provided by NIH grants HD22614, MH042228 and MH065571, and by a grant from the Department of Veteran Affairs (VISN 22 Mental Illness Research, Education and Clinical Center). These funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors: All authors participated in designing the study and analyzing the data. M. Kiang wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloia MS, Gourovitch ML, Weinberger DR, Goldberg TE. An investigation of semantic space in patients with schizophrenia. J Int Neuropsychol Soc. 1996;2(4):267–73. doi: 10.1017/s1355617700001272. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Pirolli PL. Spread of activation. J Exp Psychol Learn Mem Cogn. 1984;10(4):791–8. [Google Scholar]
- Andreasen NC. Thought, language, and communication disorders. I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315–21. doi: 10.1001/archpsyc.1979.01780120045006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- Andrews S, Shelley AM, Ward PB, Fox A, Catts SV, McConaghy N. Event-related potential indices of semantic processing in schizophrenia. Biol Psychiatry. 1993;34(7):443–58. doi: 10.1016/0006-3223(93)90235-6. [DOI] [PubMed] [Google Scholar]
- Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer SS, van Kammen DP. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105(4):592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: a replication and extension of the Connecticut category norms. J Exp Psychol Monogr. 1969;80(3):1–45. [Google Scholar]
- Bleuler E. Dementia Praecox, or the Group of Schizophrenias. New York: International Universities Press; 1911/1950. [Google Scholar]
- Carreiras M, Vergara M, Barber H. Early event-related potential effects of syllabic processing during visual word recognition. J Cogn Neurosci. 2005;17(11):1803–17. doi: 10.1162/089892905774589217. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82(6):407–28. [Google Scholar]
- Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol Psychiatry. 2003;54(11):1134–48. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, Cohen JD, van Kammen DP, Kasparek A. Modulation of language processing in schizophrenia: effects of context and haloperidol on the event-related potential. Biol Psychiatry. 1999;45(10):1336–55. doi: 10.1016/s0006-3223(98)00317-5. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Federmeier, K.D., Kutas, M., Manuscript in preparation.
- Federmeier KD, Kutas M. A rose by any other name: long-term memory structure and sentence processing. J Mem Lang. 1999;41:469–95. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Grillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biol Psychiatry. 1991;29(5):467–80. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Hampton JA. Polymorphous concepts in semantic memory. J Verbal Learning Verbal Beh. 1979;19:485–502. [Google Scholar]
- Hauser RM, Warren JR. A Socioeconomic Index for Occupations in the 1990 Census, Working Paper #96-01. Madison, WI: Center for Demography and Ecology, University of Wisconsin; 1996. [Google Scholar]
- Heinze HJ, Muente TF, Kutas M. Context effects in a category verification task as assessed by event-related brain potential (ERP) measures. Biol Psychol. 1998;47(2):121–35. doi: 10.1016/s0301-0511(97)00024-0. [DOI] [PubMed] [Google Scholar]
- Hemsley DR. The schizophrenic experience: taken out of context? Schizophr Bull. 2005;31(1):43–53. doi: 10.1093/schbul/sbi003. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Neville HJ. Auditory and visual semantic priming in lexical decision: a comparison using event-related brain potentials. Lang Cognitive Processes. 1990;5:281–312. [Google Scholar]
- Holcomb PJ, Neville HJ. Natural speech processing: an analysis using event-related brain potentials. Psychobiol. 1991;19:286–300. [Google Scholar]
- Hunt KP, Hodge MH. Category-item frequency and category-name meaningfulness (m′): taxonomic norms for 84 categories. Psychon Monogr Suppl. 1971;4(6):97–121. [Google Scholar]
- Iragui V, Kutas M, Salmon DP. Event-related brain potentials during semantic categorization in normal aging and senile dementia of the Alzheimer's type. Electroencephalogr Clin Neurophysiol. 1996;100(5):392–406. [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O'Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78(2-3):269–84. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kiang M, Kutas M. Association of schizotypy with semantic processing differences: an event-related brain potential study. Schizophr Res. 2005;77:329–342. doi: 10.1016/j.schres.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Kimble M, Lyons M, O'Donnell B, Nestor P, Niznikiewicz M, Toomey R. The effect of family status and schizotypy on electrophysiologic measures of attention and semantic processing. Biol Psychiatry. 2000;47(5):402–12. doi: 10.1016/s0006-3223(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Kostova M, Passerieux C, Laurent JP, Hardy-Bayle MC. N400 anomalies in schizophrenia are correlated with the severity of formal thought disorder. Schizophr Res. 2005;78(2-3):285–91. doi: 10.1016/j.schres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kostova M, Passerieux C, Laurent JP, Saint-Georges C, Hardy-Bayle MC. Functional analysis of the deficit in semantic context processes in schizophrenic patients: an event-related potentials study. Neurophysiol Clin. 2003;33(1):11–22. doi: 10.1016/s0987-7053(03)00006-6. [DOI] [PubMed] [Google Scholar]
- Koyama S, Hokama H, Miyatani M, Ogura C, Nageishi Y, Shimokochi M. ERPs in schizophrenic patients during word recognition task and reaction times. Electroencephalogr Clin Neurophysiol. 1994;92(6):546–54. doi: 10.1016/0168-5597(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, David AS. Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from on-line monitoring for words in linguistically anomalous sentences. J Abnorm Psychol. 1998;107(3):423–34. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- Kutas M. ERP comparisons of the effects of single word and sentence contexts on word processing. Psychophysiology. 1985;22:575–6. [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–5. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Iragui V. The N400 in a semantic categorization task across 6 decades. Electroencephalogr Clin Neurophysiol. 1998;108(5):456–71. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten CK. Psycholinguistics electrified: event-related brain potential investigations. In: Gernsbacher MA, editor. Handbook of Psycholinguistics. San Diego: Academic Press; 1994. [Google Scholar]
- Leeson VC, McKenna PJ, Laws KR. Storage and access procedures in schizophrenia: evidence for a two phase model of lexical impairment. J Clin Exp Neuropsychol. 2005;27(6):700–10. doi: 10.1080/13803390490918507. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Braff DL. The value of referring to recently introduced antipsychotics as “second generation”. Am J Psychiatry. 2003;160(8):1371–2. doi: 10.1176/appi.ajp.160.8.1371. [DOI] [PubMed] [Google Scholar]
- Maher BA. Anomalous experience and delusional thinking: the logic of explanations. In: Oltmanns TF, Maher BA, editors. Delusional Beliefs. New York: John Wiley & Sons; 1988. pp. 15–33. [Google Scholar]
- Mathalon DH, Faustman WO, Ford JM. N400 and automatic semantic processing abnormalities in patients with schizophrenia. Arch Gen Psychiatry. 2002;59(7):641–8. doi: 10.1001/archpsyc.59.7.641. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Niznikiewicz MA, Salisbury DF, Nestor PG, O'Donnell BF, Hirayasu Y, Grunze H, Greene RW, Shenton ME. Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. Eur Arch Psychiatry Clin Neurosci. 1999;249(4):69–82. doi: 10.1007/pl00014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey ME, Glucksberg S. Natural categories: well defined or fuzzy sets. Mem Cognit. 1978;6:462–72. [Google Scholar]
- McEvoy CL, Nelson DL. Category name and instance norms for 106 categories of various sizes. Am J Psychol. 1982;95:581–634. [Google Scholar]
- Miller DD, Arndt S, Andreasen NC. Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry. 1993;34(4):221–6. doi: 10.1016/0010-440x(93)90002-l. [DOI] [PubMed] [Google Scholar]
- Mitchell PF, Andrews S, Fox AM, Catts SV, Ward PB, McConaghy N. Active and passive attention in schizophrenia: an ERP study of information processing in a linguistic task. Biol Psychol. 1991;32(2-3):101–24. doi: 10.1016/0301-0511(91)90004-z. [DOI] [PubMed] [Google Scholar]
- Moritz S, Mersmann K, Kloss M, Jacobsen D, Wilke U, Andresen B, Naber D, Pawlik K. ‘Hyper-priming’ in thought-disordered schizophrenic patients. Psychol Med. 2001;31(2):221–9. doi: 10.1017/s0033291701003105. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophr Res. 2003;59(2-3):181–6. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- Neely J. Semantic priming and retrieval from lexical memory: roles of inhibitionless spreading activation and limited-capacity attention. J Exp Psychol. 1977;106(3):226–254. [Google Scholar]
- Nestor PG, Akdag SJ, O'Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: a connectionist model. Am J Psychiatry. 1998;155(12):1685–90. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Shenton ME, Voglmaier M, Nestor PG, Dickey CC, Frumin M, Seidman LJ, Allen CG, McCarley RW. Semantic dysfunction in women with schizotypal personality disorder. Am J Psychiatry. 2002;159(10):1767–74. doi: 10.1176/appi.ajp.159.10.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Uchiyama M, Matsushima E, Toru M. An event-related potential study in schizophrenia using Japanese sentences. Schizophr Res. 1999;40(2):159–70. doi: 10.1016/s0920-9964(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Kutas M, Nowacki R, Jeste DV. N400 abnormalities in late life schizophrenia and related psychoses. Biol Psychiatry. 1997;42(1):13–23. doi: 10.1016/S0006-3223(96)00242-9. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72(2):184–7. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Roberts G. The origins of delusion. Br J Psychiatry. 1992;161:298–308. doi: 10.1192/bjp.161.3.298. [DOI] [PubMed] [Google Scholar]
- Rossell SL, David AS. Are semantic deficits in schizophrenia due to problems with access or storage? Schizophr Res. 2006;82(2-3):121–34. doi: 10.1016/j.schres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Schneider K. Clinical Psychopathology. New York: Grune and Stratton; 1959. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shapiro SI, Palermo DS. Conceptual organization and class membership: normative data for representatives of 100 categories. Psychon Monogr Suppl. 1970;3(11):107–27. [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Spitzer M. A cognitive neuroscience view of schizophrenic thought disorder. Schizophr Bull. 1997;23(1):29–50. doi: 10.1093/schbul/23.1.29. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34(12):864–77. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- Stelmack RM, Miles J. The effect of picture priming on event-related potentials of normal and disabled readers during a word recognition memory task. J Clin Exp Neuropsychol. 1990;12(6):887–903. doi: 10.1080/01688639008401029. [DOI] [PubMed] [Google Scholar]
- Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Guthrie D, Harper R, Yee CM, Nuechterlein KH. Event-related potential correlates of linguistic information processing in schizophrenics. Biol Psychiatry. 1997;42(7):596–608. doi: 10.1016/S0006-3223(96)00410-6. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Picton TW, Cerri AM. Electrophysiological manifestations of typicality judgment. Brain Lang. 1988;33(2):260–72. doi: 10.1016/0093-934x(88)90068-5. [DOI] [PubMed] [Google Scholar]