Short abstract
The polychlorinated aromatic antimicrobials triclosan and triclocarban are in widespread use for killing microorganisms indiscriminately, rapidly, and by nonspecific action. While their utility in healthcare settings is undisputed, benefits to users of antimicrobial personal care products are few to none. Yet, these latter, high-volume uses have caused widespread contamination of the environment, wildlife, and human populations. This feature article presents a timeline of scientific evidence and regulatory actions in the U.S. concerning persistent polychlorinated biocides, showing a potential path forward to judicious and sustainable uses of synthetic antimicrobials, including the design of greener and safer next-generation alternatives.
Abstract

The polychlorinated aromatic antimicrobials triclosan and triclocarban are in widespread use for killing microorganisms indiscriminately, rapidly, and by nonspecific action. While their utility in healthcare settings is undisputed, benefits to users of antimicrobial personal care products are few to none. Yet, these latter, high-volume uses have caused widespread contamination of the environment, wildlife, and human populations. This feature article presents a timeline of scientific evidence and regulatory actions in the U.S. concerning persistent polychlorinated biocides, showing a potential path forward to judicious and sustainable uses of synthetic antimicrobials, including the design of greener and safer next-generation alternatives.
Introduction
Antimicrobial agents are both a boon and threat to human health, with questions about their proper design, useful application, disposal and regulatory framework looming large for scientists, the medical community, regulators and consumers of antiseptic personal care products.
In the late 1930s and early 1940s, it was discovered that the substitution on aromatic rings of hydrogen atoms with chlorine, yielded a novel chemistry of powerful biocides, including antimicrobials.1 The resultant synthetic organohalides, which are either absent or rare in natural environments,2,3 immediately were put to large volume, worldwide use as biocides. However, within a few years, many of these compounds and formulations showed adverse effects, including human toxicity, ecotoxicity, and unwanted environmental persistence and bioaccumulation, quickly leading to regulatory bans and phase-outs.1,4 For example, hexachlorophene, introduced in 1948 as a binuclear aromatic organohalide carrying six chlorine substituents,5 was banned from most uses by the 1970s.6,7 Curiously, triclocarban (TCC) and triclosan (TCS), two persistent antimicrobials first introduced to commerce in 1957 and 1964, respectively,8 feature a very similar chemistry (i.e., two benzene rings carrying multiple chlorines) yet continue to be produced and consumed to this day at high volume.9,10
Indeed, the consumption of TCS and TCC and the abundance of antimicrobial products have increased in the U.S. and abroad over the past two decades, due to relaxed regulation, aggressive and widespread advertising, and media reports driving fears of potent and sometimes lethal microbial infections acquired in everyday-life by unsuspecting victims. This multibillion dollar market has saturated supermarkets worldwide and vastly accelerated the consumption of antimicrobial products; today, TCC and more so TCS can be found in soaps, detergents, clothing, carpets, paints, plastics, toys, school supplies, and even in pacifiers, with over 2000 antimicrobial products available in 2014‘s $1.4 billion U.S. market alone.9,11 Despite labeling requirements, consumer awareness of harmful active ingredients in household products remains low.12 By contrast, TCC sees far more limited applications, mostly in bar soap formulated to concentrations of about 2% by weight, higher than the 0.1–0.5% content of TCS-enabled antimicrobial products. Consumers reaching for a random soap on U.S. supermarket shelves, likely bring home a product containing either TCS or TCC. In 1999/2000, TCS or TCC were present in 75% of liquid soaps and 29% of bar soaps in the U.S. market.13 Today, these numbers may be even higher.
More than a decade into the accelerated use of polychlorinated aromatic antimicrobials, there now are unmistakable signs of these chemicals taking a toll on the health of the environment14,15 and possibly on susceptible human populations.16 This situation has drawn an increased scrutiny by agencies in the U.S., Canada17 and abroad, including the U.S. Environmental Protection Agency (EPA),18,19 Food and Drug Administration (FDA),20 as well as the Centers for Disease Control and Prevention,21 and the European Union.10 On the state-level, efforts have begun to curtail the use of antimicrobials22 after the discovery of TCS, TCC, and their dioxin-like chemical progeny in Minnesota’s treasured water resources.23,24
In parallel to the discovery of environmental pollution and new health risks of antimicrobials,25,26 concerns about the emergence of microbial pathogens resistant to multiple groups of antibiotics of medical import27 have triggered the need for reassessing the status quo of antimicrobial usage.28 The present feature article takes a look at the knowledge timeline concerning TCC and TCS, starting with their mid 20th century introduction into commerce and culminating with an assessment of today’s information gaps as well as a glimpse of what the future may hold for the age-old chemical war on microbes.29
How Environmental Contamination with Antimicrobials Was Discovered
Enabled by advances in analytical chemistry detection methods, most notably gas and liquid chromatography/mass spectrometry (GC-MS and LC-MS, respectively), TCS and TCC emerged as important environmental pollutants in disparate times and ways.
Triclosan—a broadspectrum bacteriostat and fungicide30—garnered the attention of environmental chemists soon after its large volume use in the early 1970s. After its patenting in 1964 and worldwide production, TCS was detected within 14 years as an environmental contaminant, first in U.S. wastewater, river water, and sediment,31,32 and shortly thereafter in its methylated form, methyl-TCS, in fish from Tokyo Bay.33 These early and subsequent environmental detections of TCS were enabled by its amenability to GC-MS analysis.33,34 Initially, these detections went without much notice. This changed in 2002, however, when the United States Geological Survey (USGS) reported TCS as one of the top 10 contaminants of American rivers in its first national reconnaissance of 95 pharmaceuticals, hormones, and organic wastewater contaminants.35,36
Triclocarban—a fungicide and bacteriostat with activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE)37—emerged as a contaminant of emerging concern (CEC) much later, enabled by LC-MS rather than GC-MS detection techniques. Contrary to TCS, TCC cannot be analyzed by standard GC methods, thereby concealing for decades TCC’s presence in environmental samples acquired, extracted and analyzed for the occurrence of anthropogenic pollutants. For TCC to travel through a standard GC column and be detected, its reactive groups first need to be derivatized.38 Relief from this conundrum arrived in 2004 with a simple LC-MS technique allowing direct detection of underivatized TCC.39 Use of this tool on samples from Baltimore, Maryland, showed the presence of TCC in every urban stream monitored.39 When applied to the city’s groundwater, drinking water, wastewater, and sewage sludge, TCC was detected in many of these matrices and consistently in samples also containing TCS.8 Significant co-occurrence of TCC and TCS (R2 = 0.988) can be easily understood from their similar uses, chemical structures, and down-the-drain disposal mode. Upon entering USGS national data on TCS35 into the forecasting algorithm, TCC emerged in 2005 as a previously unrecognized CEC that had been overlooked by environmental analysts for almost half a century; it was predicted to rank in the top 10 CECs in occurrence rate and in the top 20 in maximum concentration among 96 water pollutants.8 Follow-up research using tandem mass spectrometry (LC-MS/MS) confirmed these predictions40 and adoption of LC-based analytical tools by laboratories around the world quickly accelerated the discovery of TCC pollution in the environment and in humans.41−45
Today, TCS and TCC rank in the list of top contaminants of concern worldwide.36 For example, U.S. streams have a 60–100% likelihood of containing detectable quantities of TCS and TCC.8,23 TCS has been detected in drinking water resources,14,46 75% of urine samples representative of the U.S. population,47 97% of representative U.S. breast milk samples,48 and combined TCS and TCC constitute over 60% of the total mass of 96 pharmaceuticals detectable in municipal sludge using EPA Method 1694.49−51 Indeed, the environmental ubiquity of both chemicals has escalated such that TCS, TCC or both compounds are now detectable in house dust worldwide,52−54 in ocean water,55 and locations as remote as the water loop of spacecraft.56 To understand this phenomenon of ubiquitous pollution, it is important to examine their production rates, distribution mechanisms, and long-term persistence upon environmental release. This behavior may be understood best when viewed through the lens of green chemistry57 and engineering.58
Are TCS and TCC Sustainable Chemicals?
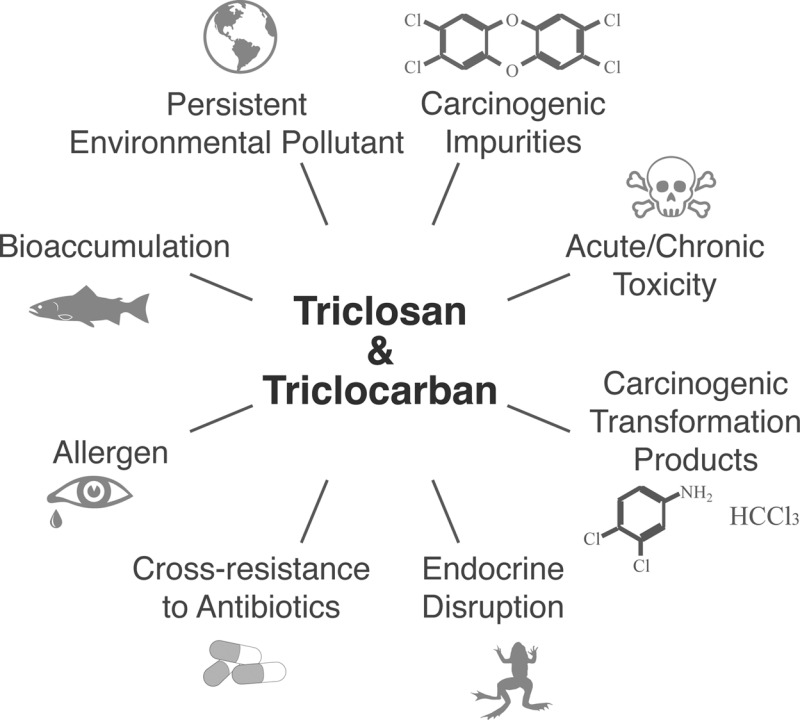
Sustainably produced green chemicals serve their intended purpose without creating hazardous conditions for either people or the planet during chemical production, use, and following disposal.1,57 Of particular concern for the EPA are chemicals featuring one or multiple of the following characteristics: (i) Persistence in the environment, (ii) Bioaccumulation in animals and humans; and (iii) Toxicity to humans and ecosystems.59,60 As with other problematic chemicals,61 early warnings existed for decades concerning PBT properties of TCS and TCC, and the unsustainability of their large-volume uses.33,62,63
Life Cycle of TCS and TCC
The cradle-to-grave life cycle of TCS and TCC can be characterized as an open loop that violates multiple principles of green chemistry and engineering.1,57,58 Structurally related to highly toxic and carcinogenic dioxins, TCS had been labeled a predioxin as early as 1993 by the U.S. EPA. Technical grade TCS contains traces of the most toxic member of the dioxin family, 2,3,7,8-tetrachlorodibenzo-p-dioxin (17.2 – 1,712 ng/kg), and 2,3,7,8-tetrachlorodibenzofuran (0.7 – 207.3 ng/kg).64 Concerns over dioxins in TCS have motivated U.S. producers of antimicrobial products to source TCS from tightly monitored European chemical suppliers as opposed to lower-cost competitors in the Asian markets.65 Furthermore, mixing of TCS with chlorinated drinking water can result in the formation of carcinogenic chloroform66 and, upon release into surface water and irradiation with sunlight, of additional toxic polychlorinated dioxins67 and less toxic dichlorinated dioxins, for example, 2,8-dichlorodibenzo-p-dioxin.67 Similarly, TCC also contains toxic, carcinogenic manufacturing byproducts, such as 4-chloroaniline and 3,4-dichloroaniline, and can release more of these carcinogens upon chemical, physical, and biological attack.68,69
Durations of utility, i.e., useful lifespans, of TCS and TCC in personal care products are short, on the order of seconds,70 but their environmental after-lives are much longer, measured at time-scales of up to several decades.71−73 Upon disposal by consumers, both compounds are washed down the drain and typically are conveyed to municipal wastewater treatment plants (WWTPs). These facilities remove both TCS and TCC from raw sewage at a high efficiency of 97–98%,74,75 leading to low ng/L levels in effluent discharged to surface waters.76−83 However, removal from sewage does not necessarily equal degradation. During wastewater treatment, both antimicrobials distribute themselves preferentially into carbon- and lipid-rich sewage sludge, thereby accumulating in this abundant byproduct of biological sewage treatment.74,75 During anaerobic sludge digestion, losses can occur as a result of biodegradation of TCS and TCC but concentrations also may increase due to a reduction in volume by gasification of natural organics to methane.76,84 Levels of TCS and TCC in digested sewage sludge as high as 133 and 441 mg/kg dry weight, respectively, have been reported by the EPA; however, mean concentrations are closer to 16 ± 65 and 39 ± 59 mg/kg dry weight (±standard deviation), respectively.85 Antimicrobials arriving at U.S. WWTPs in substantial quantities (227 000–454 000 kg/y for TCC and 170 000–970 000 kg/yr for TCS)8 are known to break through WWTPs and subsequently can harm algae in surface waters at ng/L concentrations.86 Detected concentrations have been observed to exceed an acute-based predicted no-effect concentration (PNEC) of 4.7 ng/L in the River Elbe at 75% of monitoring locations,36 and can accumulate in sediments to mg/kg levels,14,71,87,88 where they may persist for several decades.71 In the U.S., sewage sludge is either incinerated (∼15% of total volume) which can release more carcinogenic dioxins from TCS,24,89 or deposited in landfills (∼30%) and on land (∼55%), from where antimicrobials and their carcinogenic transformation products may leach into adjacent surface water to impact the composition of microbial communities.90,91 Antimicrobials applied as sewer sludge on land constitute a pathway for transfer of these chemicals into animal feed and crops destined for human consumption.92−94 The volume of antimicrobials reentering the environment in sewage sludge after initial successful capture from wastewater is substantial; 57 000 ± 233 000 and 140 000 ± 211 000 kg/yr of TCS and TCC, respectively, are applied on U.S. land annually; for TCC, this is equivalent to a staggering 4.8–48.2% of its total U.S. consumption volume.95 Crops shown to take up antimicrobials from soil include barley, meadow fescue, carrots, and pinto beans.94,96,97
Human Exposure to TCS and TCC
Human exposure to antimicrobials occurs mostly as a result of elective topical application to the human body. Showering for 15 min with a 0.6% TCC containing antimicrobial soaps was demonstrated to lead to concentrations in the blood of volunteers sufficiently high to potentially cause local inhibition of enzyme soluble epoxide hydrolase.42,98 Use of TCS-containing toothpaste, typically formulated to 0.3% by weight, is another important source of human exposure.99 Other known or suspected human exposure routes of lesser importance include the inhalation of antimicrobial-laden house dust,52−54 consumption of contaminated drinking water,100 and ingestion of food contaminated with antimicrobials either during the growing season93,94 or postharvesting from antimicrobial-containing packaging materials.101,102 Unsuspected environmental exposures to TCS and TCC have attracted attention by news media and the general public but the magnitude of these exposures is easily eclipsed by elective, topical use of antimicrobial personal care products.42,98
Toxicity of TCS and TCC to Humans
TCS and TCC are known toxicants but there still is a paucity of data on adverse effects in humans from elective and incidental environmental exposures.48,99,103 Isolated early reports of infant deaths in the U.S. and Europe emphasized the need for caution but remain an anomaly, caused by misuse of antimicrobials in conditions not applicable to present day uses.104−106 Acute and chronic health effects of TCS and TCC observed in humans and animals following exposure include irritation of eyes and skin,30,107 sensitization to aeroallergens and food,108 immunologic reactions such as allergies,16,108−110 developmental and reproductive toxicity,111−113 inhibition of muscle function,114 as well as in vivo genotoxicity.115 While limited, the number of studies involving human subjects is increasing.98,104−106,108−110,116
TCS and TCC as Endocrine Disruptors
An emerging additional toxic outcome of concern is endocrine disruption,117 meaning an interfering of TCS and TCC with essential signaling systems in animals and humans, thereby adversely affecting development, sexual maturation, metabolism, and behavior.118,119 Endocrine disruption was observed after exposure of male rats to TCC,120 of rats to TCS,121−124 and of frogs to TCS.26 Of particular human health concern are the adverse effects of TCS on thyroid homeostasis and of TCC on reproductive health.111,121,123,125
TCS and TCC as Protagonists of Antibiotic Drug Resistance
A long recognized potential human health threat of antimicrobials is their ability to induce cross-resistance to medically important antibiotics in human pathogens and commensal microbes, thereby turning environmental microbial communities into a reservoir of antibiotic drug resistance.27,63,126−128 Concerns about TCS-induced cross-resistance to antibiotics used in human medicine were voiced as early as 2001129 and have since been substantiated by scientists worldwide.130 Whereas TCS resistance can decrease susceptibility to as many as seven antibiotics simultaneously,131 the applicability of such data to environmental settings and the actual risk remain uncertain.132 Available studies concentrated on household settings133 rather than on environmental locales, where the development and proliferation of drug resistance is more likely. One such unexplored locale is sewage sludge,28 where an abundance of pathogens, multiple antimicrobials and extended contact times creates a large and risky setting for the emergence of drug resistance.
Ecotoxicity of TCS and TCC
Ecotoxicological risks also result for other biota enduring antimicrobial contact times that are infinitely longer than the few seconds these persistent antimicrobials reside on consumers’ hands during their intended use. These unwanted long-term exposures of biota to high concentrations of antimicrobials take place in environments not targeted for disinfection.36 In the built water environment, for example, inputs and accumulation of antimicrobials in activated sludge units during wastewater treatment are of potential concern, as it may diminish treatment efficacy and microbial diversity while also potentially creating reservoirs of drug resistance.28 Similar risks also exist in soil environments subject to the application of biocide-laden sewage sludge. Here, as mentioned earlier, the proximity of large quantities of commensal and pathogenic bacteria with extremely high levels of antimicrobials is of particular concern, as is the uptake of the compounds into higher organisms, such as plants and animals.
Natural environments also feature multiple compartments where unwanted antimicrobial residues come in immediate and long-term contact with fauna and flora.36 Here, the native, multicellular biota are known to be orders of magnitude more susceptible to the killing power of antimicrobials than are microorganisms.14 Contrary to the situation described for hand washing (exposure times of a few seconds), these environmental toxic exposures are not temporal, but rather extend over the entire lifespan of aquatic and terrestrial organisms and across multiple generations. TCS and TCC are 100–1000 times more effective in inhibiting and killing algae, crustaceans and fish than they are in killing microbes.14 Shallow sediments in surface waters receiving treated wastewater inputs are known to contain high μg/kg to low mg/kg quantities of TCS and TCC, levels that make impossible the survival and activity of many different species. Sediments also represent a latent source of antimicrobials and can release the compounds back into the water column upon disturbance. Application of sewage sludge in forestry and nonagricultural settings also can lead to decade long exposure of plants, soil-dwelling biota and their predators over multiple generations.14,72
Bioconcentration, bioaccumulation and biomagnification of antimicrobials have been observed in multiple organisms, including algae,14,86 aquatic blackworms,134 fish,33 and even dolphins,135 whereas affected terrestrial organisms include earth worms72,136,137 and higher species up the food chain.138 Documented accumulation of antimicrobials in worms and plant material and subsequent uptake by higher organisms is a known pathway for ecological risks from exposure of vertebrae, including songbirds.15
Bioaccumulation of antimicrobials also occurs in humans48 but to a much lesser extent, because well-known detoxification reactions result in the rapid elimination of parental TCS and TCC.42,98 Despite this, lipid adjusted steady-state levels of TCS in U.S. breast milk as high as 2.1 mg/kg have been reported.48 The need for continuous elimination of antimicrobials by the human detoxification machinery has been speculated to potentially prevent expulsion of more harmful agents, such as dioxins, but scientific data are lacking.139
How Effective Are Antimicrobials?
Although TCS and TCC are effective in killing microorganisms when applied judiciously by professionals in health care settings,140 their proliferating use by the general population, which accounts for the vast majority of the chemicals’ production volume, lacks convincing data on health benefits, according to epidemiological studies.128,141
These seemingly contradictory findings between antimicrobials’ efficacy in clinical settings and their failure to perform in household settings can be understood easily when considering the contact time between the chemicals and their microbial targets. Thoroughly designed clinical studies reproducibly yield favorable results from hand washing times of 30 s to several minutes.140 However, hand-washing routines of the general population differ significantly from this optimal standard. In real-world settings, the application of soaps on the hands of consumers is followed immediately by rinsing away of the active antimicrobial ingredients. Thus, for the majority of household consumers, effective contact times amount to an average of six seconds,70 too short to provide a measurable impact on antimicrobial efficacy.
In 2005, an expert panel convened by the FDA had concluded by a vote of 11-to-1 that use of antiseptics does not provide a measurable benefit to consumers.142 This assessment apparently has not changed in years since, as the FDA has issued in late 2013 a notice to industry of its intent to institute tighter regulations in the near future.20
Regulatory Framework of Antimicrobials
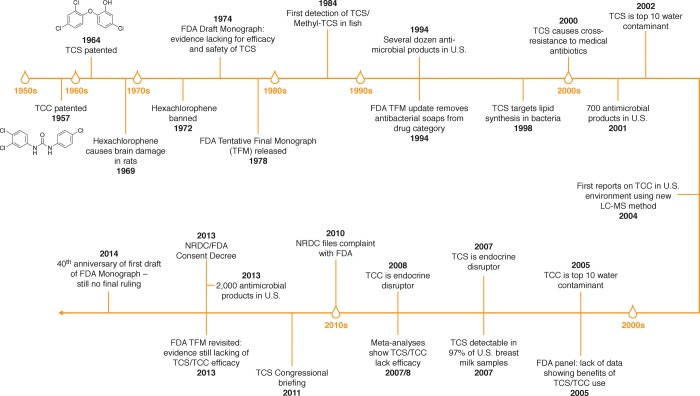
In the U.S., regulating TCS and TCC has been challenging over the course of the past half century, due in part to the desire to cover multiple uses and multiple compounds under a single umbrella guidance document, namely the topical antimicrobial drug products Over-the-Counter (OTC) Drug Monograph of the FDA20,142 (Figure 1). This regulation was first drafted in 1974, tentatively finalized in 1978, and updated in 1994 but never finalized. In 2010, the Natural Resources Defense Council (NRDC) filed a complaint against the FDA in an effort to force the agency to act.143 This legal action culminated in a consent decree, with the FDA agreeing in 2013 to finalize the monograph, at least with respect to TCS.144 The year 2014 marks the 40th anniversary of issuance of the yet to be finalized initial draft legislation (Figure 1). In 1972, in contrast, the FDA had acted much more swiftly, by banning the antimicrobial hexachlorophene6 over concerns of its neurotoxicity.145 At the time, hexachlorophene-containing personal care products had multiplied in the market similar to TCS-containing formulations today and adverse effects including accumulation in breast milk also had been reported for hexachlorophene.146 Technically, the FDA could regulate TCS and TCC over environmental concerns alone but such action would be without precedence; instead, the FDA has deferred to the EPA, which regulates TCS but not TCC as registered pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).18
Figure 1.
Timeline of scientific and regulatory events concerning the use and occurrence of triclosan (TCS) and triclocarban (TCC) in the United States, with particular emphasis on the Tentative Final Monograph (TFM) of the Food and Drug Administration (FDA).
Open Questions
So who should use antimicrobials? For what purpose? And what is the acceptable extent of collateral damage to ecological health and human populations? Answering these questions should best be left to public health experts, physicians, risk assessors, and sustainability scientists. Sixty years into the use of polychlorinated binuclear aromatic antimicrobials, multiple lessons can be learned from the past. Hexachlorophene was responsible for the first bloom of antimicrobial products, giving rise to over 400 hexachlorophene-containing personal care products; this episode lasted only a few years, though, before this active ingredient was banned over concerns of its neurotoxicity.6 The second bloom in U.S. antimicrobial products from a few dozens to the current count of >2000 was triggered by the FDA’s removal of antimicrobial soaps from the drug category of the Tentative Final Monograph (TFM) in 1994 (Figure 1). This history suggests that regulatory boundaries are critical in preventing imprudent uses of potentially harmful substances in personal care products.7 Restricting nonmedical uses of TCS and TCC is an approach championed by diverse scholars and health care professionals, including the American Medical Association (AMA), the Alliance for the Prudent Use of Antibiotics (APUA), an expert group of the American Academy for Microbiology,147 and members of the American Public Health Association (APHA).148
Any known and potential adverse effects of the usage of antimicrobials should be balanced with immediate and measurable benefits reaped. With respect to TCS and TCC, scientific evidence points to known benefits from their application in health care settings by health care professionals, and possibly from TCS-containing toothpaste used by individuals diagnosed with gingivitis.149 Exclusive sale of TCS/TCC-containing soaps in pharmacies and prescription requirements for TCS in toothpaste may aid in effecting the desirable reduction in unsustainable consumption patterns and with it associated adverse effects. This tiered approach worked well for the now restricted hexachlorophene, whose allowable and prudent applications continue to this date, as a preservative at concentrations of up to 0.1% by weight. Regulations proved effective in throttling back hexachlorophene production; today, the compound is present at levels below the detection limit in U.S. wastewaters, detectable only at low concentrations (0.18–0.37 mg/kg dry weight) in raw and treated sewage sludge, where it accumulates similarly to TCS and TCC.40
The question of what collateral damage to people and the planet is acceptable will be informed not only by cost-benefit analyses but also by broader sustainability considerations.1,57,58 Evidence abounds for TCS and TCC to represent nongreen chemicals whose current usage volumes are unsustainable, as indicated by large-scale pollution that needlessly places stress on the environment, animals and human populations.36,48 These findings suggest the need for next-generation antimicrobials to overcome some of the identified shortcomings of TCS and TCC, while preserving their essential benefits.
The Future
So what will greener, more sustainable antimicrobials of the future look like? Desirable properties of next-generation antimicrobial include broad-spectrum action and high efficacy toward pathogens but low toxicity to nontarget, multicellular organisms, including aquatic and terrestrial biota and humans. Furthermore, future-use antimicrobials should have no or very low potential for fostering antimicrobial drug resistance, should undergo rapid biodegradation in conventional wastewater treatment plants, and pose no risk of bioaccumulation. Ideally, the compounds also should be sourced from renewable feedstock and lack occupational hazards during production, storage, and use. Upon disposal they should return their benign elemental building blocks to the environment, to complete a more environmentally friendly cradle-to-cradle life-cycle.150 Studying the behavior of chemicals in WWTPs can provide helpful design clues.95 Sustainability considerations already are informing the design of green pharmaceuticals,151,152 and adopting this approach for antimicrobials promises to yield important benefits to people and the planet.
Acknowledgments
This study was supported in part by the Johns Hopkins Center for a Livable Future and by National Institute of Environmental Health Sciences (NIEHS) awards 1R01ES015445, 1R01ES020889 and their respective supplements. The content of this work is solely the responsibility of the author and does not necessarily represent the official views of the FDA, the NIEHS, or the National Institutes of Health (NIH).
Biography
Rolf Halden is the Founding Director of the Center for Environmental Security at the Biodesign Institute and professor in the School of Sustainable Engineering and the Built Environment at Arizona State University, as well as cofounding member and adjunct faculty at the Center for Water and Health of the Bloomberg School of Public Health at Johns Hopkins University. Trained as a biologist and engineer, Rolf’s primary interests are in identifying environmental stressors of human and ecological concern and devising engineering and regulatory solutions. Rolf is a special government employee of the FDA, served as a voting member on its 2005 expert panel and presented on antimicrobials at the National Academies and on Capitol Hill.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Manahan S. E.Green Chemistry and the Ten Commandments of Sustainability; ChemChar Research, Inc. Publishers: Columbia, MO, 2005. International Standard Book Number: 0-9749522-4-9. [Google Scholar]
- EuroChlor, Risk Assessment and the Cycle of Natural Organochlorines—Part A. In Euro Chlor Focus on Chlorine Science. 2013; 03A, 1–4. [Google Scholar]
- Adams D. E. C.; Halden R. U., Fluorinated Chemicals and the Impacts of Anthropogenic Use. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations, Halden R. U., Ed. 2010; Vol. 1048, pp 539–560. [Google Scholar]
- Rodricks J. V. In pursuit of safety: One-hundred years of toxicological risk assessment. Hum Ecol Risk Assess 2014, 2013–28. [Google Scholar]
- Fahlberg W. J.; Swan J. C.; Seastone C. V. CV, Studies on the retention of hexachlorophene (G-11) in human skin. J. Bacteriol. 1948, 563323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexachlorophene curbed. Science 1972, 177, (4055), 1175. [DOI] [PubMed] [Google Scholar]
- Bruch M. K.; Larson E. Regulation of topical antimicrobials: history, status and future perspective. Infect. Control Hosp. Epidemiol. 1989, 1011505–8. [DOI] [PubMed] [Google Scholar]
- Halden R. U.; Paull D. H. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 3961420–1426. [DOI] [PubMed] [Google Scholar]
- Young S., FDA examining antibacterial soaps, body washes. CNN Health 2013. http://www.cnn.com/2013/12/16/health/fda-antibacterial/ (accessed January 20, 2014). [Google Scholar]
- Scientific Committee on Consumer Safety (SCCS). Triclosan and Antibiotics Resistance. European Union, Ed. 2010. http://ec.europa.eu/health/scientific_committees/opinions_layman/triclosan/en/about-triclosan.htm#29 (accessed January 20, 2014).
- Smith S., U.S. Disinfectant & Antimicrobial Chemicals Market. PRWeb 2013. http://www.prweb.com/releases/2013/9/prweb11150188.htm (accessed February 28, 2014).
- Glegg G.; Richards J.; Heard J.; Dawson J.. Barriers to Green Buying: Household Chemicals; Marine and Coastal Policy Research Group, University of Plymouth: 2005. http://www.sas.org.uk/wp-content/uploads/sas-barriers-to-green-buying.pdf (accessed January 20, 2014).
- Perencevich E. N.; Wong M. T.; Harris A. D. National and regional assessment of the antibacterial soap market: A step toward determining the impact of prevalent antibacterial soaps. Am. J. Infect. Control. 2001, 295281–3. [DOI] [PubMed] [Google Scholar]
- Chalew T. E. A.; Halden R. U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Resour. Assoc. 2009, 4514–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. H.; O’Connor G. A. Risk assessment of land-applied biosolids-borne triclocarban (TCC). Sci. Total Environ. 2013, 442, 437–444. [DOI] [PubMed] [Google Scholar]
- Clayton E. M. R.; Todd M.; Dowd J. B.; Aiello A. E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ. Health Perspect. 2011, 1193390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B. Canada calls for triclosan action. Chem. Eng. News 2012, 901510–10. [Google Scholar]
- U.S. Environmental Protection Agency. Triclosan Facts. http://www.epa.gov/oppsrrd1/REDs/factsheets/triclosan_fs.htm (accessed January 20, 2014).
- U.S. Environmental Protection Agency. Frequent Questions Associated with the Reregistration Eligibility Decision (RED). http://www.epa.gov/pesticides/reregistration/triclosan/triclosan-questions.htm (accessed January 20, 2014).
- U.S. Food and Drug Administration. FDA Taking Closer Look at ‘Antibacterial’ Soap. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm378393.htm (accessed January 20, 2014).
- CDC National Biomonitoring Program, Factsheet Triclosan. 2013. http://www.cdc.gov/biomonitoring/Triclosan_FactSheet.html (accessed January 20, 2014).
- Dunbar E., Lawmakers consider banning triclosan, other chemicals. MPR News 2013. http://www.mprnews.org/story/2013/03/12/environment/lawmakers-ban-triclosan (accessed January 20, 2014). [Google Scholar]
- Venkatesan A. K.; Pycke B. F. G.; Barber L. B.; Lee K. E.; Halden R. U. Occurrence of triclosan, triclocarban, and its lesser chlorinated congeners in Minnesota freshwater sediments collected near wastewater treatment plants. J. Hazard. Mater. 2012, 229, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger C. T.; Sueper C.; Blumentrit D. J.; McNeill K.; Engstrom D. R.; Arnold W. A. Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ. Sci. Technol. 2013, 4741833–1843. [DOI] [PubMed] [Google Scholar]
- Ahn K. C.; Zhao B.; Chen J.; Cherednichenko G.; Sanmarti E.; Denison M. S.; Lasley B.; Pessah I. N.; Kultz D.; Chang D. P. Y.; Gee S. J.; Hammock B. D. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 11691203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen N.; Skirrow R. C.; Osachoff H.; Wigmore H.; Clapson D. J.; Gunderson M. P.; Van Aggelen G.; Helbing C. C. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat. Toxicol 2006, 803217–227. [DOI] [PubMed] [Google Scholar]
- Aiello A. E.; Marshall B.; Levy S. B.; Della-Latta P.; Larson E. Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob. Agents Chemother. 2004, 4882973–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruden A. Balancing water sustainability adn public health goals in the face of growing concerns about antibiotic resistance. Environ. Sci. Technol. 2013, 48, 5–14. [DOI] [PubMed] [Google Scholar]
- Blancou J. History of disinfection from early times until the end of the 18th century. Revue scientifique et technique 1995, 14121–39. [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Reregistration Eligibility Decision for Triclosan.″ Reregistration Eligibility Decision (RED) Document; EPA 939-RO-8009; 2008. http://www.epa.gov/oppsrrd1/REDs/2340red.pdf (accessed January 20, 2014).
- Jungclaus G.; Avila V.; Hites R. Organic compounds in an industrial wastewater: A case study of their environmental impact. Environ. Sci. Technol. 1978, 12, 88–96. [Google Scholar]
- Hites R. A.; Lopez-Avila V. Identification of organic compounds in an industrial wastewater. Anal. Chem. 1979, 51141452A–1456A. [Google Scholar]
- Miyazaki T.; Yamagishi T.; Matsumoto M. Residues of 4-chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene(triclosan methyl) in aquatic biota. Bull. Environ. Contam. Toxicol. 1984, 322227–32. [DOI] [PubMed] [Google Scholar]
- Lindstrom A.; Buerge I. J.; Poiger T.; Bergqvist P. A.; Muller M. D.; Buser H. R. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ. Sci. Technol. 2002, 36112322–2329. [DOI] [PubMed] [Google Scholar]
- Kolpin D. W.; Furlong E. T.; Meyer M. T.; Thurman E. M.; Zaugg S. D.; Barber L. B.; Buxton H. T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 3661202–11. [DOI] [PubMed] [Google Scholar]
- von der Ohe P. C.; Schmitt-Jansen M.; Slobodnik J.; Brack W. Triclosan-the forgotten priority substance?. Environ. Sci. Pollut. Res. 2012, 192585–591. [DOI] [PubMed] [Google Scholar]
- Walsh S. E.; Maillard J. Y.; Russell A. D.; Catrenich C. E.; Charbonneau D. L.; Bartolo R. G. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J. Hosp. Infect. 2003, 55298–107. [DOI] [PubMed] [Google Scholar]
- Gruenke L. D.; Craig J. C.; Wester R. C.; Maibach H. I.; North-Root H.; Corbin N. C. A selected ion monitoring GC/MS assay for 3,4,4′-trichlorocarbanilide and its metabolites in biological fluids. J. Anal. Toxicol. 1987, 11275–80. [DOI] [PubMed] [Google Scholar]
- Halden R. U.; Paull D. H. Analysis of triclocarban in aquatic samples by liquid chromatography electrospray ionization mass spectrometry. Environ. Sci. Technol. 2004, 38184849–4855. [DOI] [PubMed] [Google Scholar]
- Heidler J.; Halden R. U. Fate of organohalogens in US wastewater treatment plants and estimated chemical releases to soils nationwide from biosolids recycling. J. Environ. Monit. 2009, 11122207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb N. H.; Flores I.; Kurobe T.; Franze B.; Ranganathan A.; Hammock B. D.; Teh S. J. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes). Aquat. Toxicol. 2011, 1053–4448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb N. H.; Inceoglu B.; Ahn K. C.; Morisseau C.; Gee S. J.; Hammock B. D. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011, 4573109–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X. Y.; Zhou X. L.; Furr J.; Ahn K. C.; Hammock B. D.; Gray E. L.; Calafat A. M. Biomarkers of exposure to triclocarban in urine and serum. Toxicology 2011, 2861–369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan M. A.; Edziyie R. E.; La Point T. W.; Venables B. J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater, treatment plant receiving stream. Chemosphere 2007, 67101911–1918. [DOI] [PubMed] [Google Scholar]
- Coogan M. A.; La Point T. W. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ. Toxicol. Chem. 2008, 2781788–1793. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Health. Triclosan and Drinking Water. 2013. http://www.health.state.mn.us/divs/eh/risk/guidance/dwec/triclosaninfo.pdf (accessed January 20, 2014).
- Calafat A. M.; Ye X.; Wong L. Y.; Reidy J. A.; Needham L. L. Urinary concentrations of Triclosan in the US population: 2003–2004. Environ. Health Perspect. 2008, 1163303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan A. D. Risk assessment of triclosan [Irgasan (R)] in human breast milk. Food Chem. Toxicol. 2007, 451125–129. [DOI] [PubMed] [Google Scholar]
- Walters E.; McClellan K.; Halden R. U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44206011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan K.; Halden R. U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010, 442658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS 2007. http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2008_01_03_methods_method_1694.pdf (accessed January 20, 2014).
- Liao C.; Liu F.; Guo Y.; Moon H. B.; Nakata H.; Wu Q.; Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol. 2012, 46169138–45. [DOI] [PubMed] [Google Scholar]
- Geens T.; Roosens L.; Neels H.; Covaci A. Assessment of human exposure to bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 2009, 766755–60. [DOI] [PubMed] [Google Scholar]
- Fan X.; Kubwabo C.; Rasmussen P.; Jones-Otazo H. Simultaneous quantitation of parabens, triclosan, and methyl triclosan in indoor house dust using solid phase extraction and gas chromatography-mass spectrometry. J. Environ. Monit. 2010, 12101891–7. [DOI] [PubMed] [Google Scholar]
- Xie Z. Y.; Ebinghaus R.; Floser G.; Caba A.; Ruck W. Occurrence and distribution of triclosan in the German Bight (North Sea). Environ. Pollut. 2008, 15631190–1195. [DOI] [PubMed] [Google Scholar]
- Pycke B. F. G.; Halden R. U.. Personal Communication of Unpublished Data. 2014.
- Anastas P. T.; Kirchhoff M. M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 359686–94. [DOI] [PubMed] [Google Scholar]
- Anastas P. T.; Zimmerman J. B. Design through the 12 principles of green engineering. Environ. Sci. Technol. 2003, 37594A–101A. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Persistent, Bioaccumulative and Toxic (PBT) Chemical Program. http://www.epa.gov/pbt/ (accessed January 20, 2014).
- Consensus Panel. Scientific and Policy Analysis of Persistent, Bioaccumulative, and Toxic Chemicals: A Comparison of Practices in Asia, Europe, and North America; Indiana University: 2013. http://www.indiana.edu/∼spea/faculty/pdf/scientific_policy_analysis_of_persistent_bioaccumulative_and_toxic_chemicals_PBT_.pdf (accessed February 28, 2014).
- European Environment Agency. Late lessons from early warnings: science, precaution, innovation EEA Report No 1/2013; 2013. http://www.eea.europa.eu/publications/late-lessons-2 (accessed January 20, 2014).
- Gledhill W. E. Biodegradation of 3,4,4¢-trichlorocarbanilide, TCC, in sewage and activated sludge. Water Res. 1975, 97649–654. [Google Scholar]
- McMurry L. M.; Oethinger M.; Levy S. B. Triclosan targets lipid synthesis. Nature 1998, 3946693531–532. [DOI] [PubMed] [Google Scholar]
- Ni Y.; Zhang Z.; Zhang Q.; Chen J.; Wu Y.; Liang X. Distribution patterns of PCDD/Fs in chlorinated chemicals. Chemosphere 2005, 606779–84. [DOI] [PubMed] [Google Scholar]
- Menoutis J.; Parisi A. I. Testing for dioxin and furan contamination in triclosan. Cosmet. Toiletries Magazine 2002, 1171075–78. [Google Scholar]
- Rule K. L.; Ebbett V. R.; Vikesland P. J. Formation of chloroform and chlorinated organics by free-chlorine-mediated oxidation of triclosan. Environ. Sci. Technol. 2005, 3993176–3185. [DOI] [PubMed] [Google Scholar]
- Latch D. E.; Packer J. L.; Arnold W. A.; McNeill K. Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solution. J. Photochem. Photobiol. A 2003, 158163–66. [Google Scholar]
- Miller T. R.; Colquhoun D. R.; Halden R. U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater 2010, 1831–3766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J. W.; Xia K. Fate of triclosan and triclocarban in soil columns with and without biosolids surface application. Environ. Toxicol. Chem. 2012, 312262–269. [DOI] [PubMed] [Google Scholar]
- Borchgrevink C. P.; Cha J.; Kim S. Hand washing practices in a college town environment. J. Environ. Health 2013, 75818–24. [PubMed] [Google Scholar]
- Miller T. R.; Heidler J.; Chillrud S. N.; Delaquil A.; Ritchie J. C.; Mihalic J. N.; Bopp R.; Halden R. U. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ. Sci. Technol. 2008, 42124570–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. P.; Paesani Z. J.; Chalew T. E. A.; Halden R. U.; Hundal L. S. Persistence of triclocarban and triclosan in soils after land application of biosolids and bioaccumulation in Eisenia Foetida. Environ. Toxicol. Chem. 2011, 303556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. H.; O’Connor G. A.; McAvoy D. C. Fate of C-14-triclocarban in biosolids-amended soils. Sci. Total Environ. 2010, 408132726–2732. [DOI] [PubMed] [Google Scholar]
- Heidler J.; Sapkota A.; Halden R. U. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol. 2006, 40113634–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler J.; Halden R. U. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 662362–369. [DOI] [PubMed] [Google Scholar]
- McAvoy D. C.; Schatowitz B.; Jacob M.; Hauk A.; Eckhoff W. S. Measurement of triclosan in wastewater treatment systems. Environ. Toxicol. Chem. 2002, 2171323–1329. [PubMed] [Google Scholar]
- Singer H.; Muller S.; Tixier C.; Pillonel L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36234998–5004. [DOI] [PubMed] [Google Scholar]
- Bester K. Triclosan in a sewage treatment process - balances and monitoring data. Water Res. 2003, 37163891–3896. [DOI] [PubMed] [Google Scholar]
- Bester K. Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch. Environ. Contam. Toxicol. 2005, 4919–17. [DOI] [PubMed] [Google Scholar]
- Waltman E. L.; Venables B. J.; Waller W. Z. Triclosan in a North Texas wastewater treatment plant and the influent and effluent of an experimental constructed wetland. Environ. Toxicol. Chem. 2006, 252367–372. [DOI] [PubMed] [Google Scholar]
- Fiss E. M.; Rule K. L.; Vikesland P. J. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environ. Sci. Technol. 2007, 4172387–2394. [DOI] [PubMed] [Google Scholar]
- Bedoux G.; Roig B.; Thomas O.; Dupont V.; Le Bot B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 1941044–1065. [DOI] [PubMed] [Google Scholar]
- Buth J. M.; Ross M. R.; McNeill K.; Arnold W. A. Removal and formation of chlorinated triclosan derivatives in wastewater treatment plants using chlorine and UV disinfection. Chemosphere 2011, 8491238–1243. [DOI] [PubMed] [Google Scholar]
- Narumiya M.; Nakada N.; Yamashita N.; Tanaka H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard. Mater. 2013, 260, 305–312. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report. 2009. http://water.epa.gov/scitech/wastetech/biosolids/upload/2009_01_15_biosolids_tnsss-tech.pdf (accessed January 20, 2014).
- Tamura I.; Kagota K.; Yasuda Y.; Yoneda S.; Morita J.; Nakada N.; Kameda Y.; Kimura K.; Tatarazako N.; Yamamoto H. Ecotoxicity and screening level ecotoxicological risk assessment of five antimicrobial agents: Triclosan, triclocarban, resorcinol, phenoxyethanol and p-thymol. J Appl Toxicol 2013, 33111222–1229. [DOI] [PubMed] [Google Scholar]
- Lin H.; Hu Y. Y.; Zhang X. Y.; Guo Y. P.; Chen G. R. Sorption of triclosan onto sediments and its distribution behavior in sediment-water-rhamnolipid systems. Environ. Toxicol. Chem. 2011, 30112416–2422. [DOI] [PubMed] [Google Scholar]
- Cantwell M. G.; Wilson B. A.; Zhu J.; Wallace G. T.; King J. W.; Olsen C. R.; Burgess R. M.; Smith J. P. Temporal trends of triclosan contamination in dated sediment cores from four urbanized estuaries: Evidence of preservation and accumulation. Chemosphere 2010, 784347–352. [DOI] [PubMed] [Google Scholar]
- Doudrick K. D.; Jones D. B.; Kalinowski T.; Hartmann E. M.; Halden R. U., Assessment of the contribution of triclosan to dioxin emissions from sludge incineration in the US using a mathematical model. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; Halden R. U., Ed.; American Chemical Society: WA. 2010; Vol. 1048, pp 469–481. [Google Scholar]
- Al-Rajab A. J.; Sabourin L.; Scott A.; Lapen D. R.; Topp E. Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarban in an agricultural soil. Sci. Total Environ. 2009, 407235978–5985. [DOI] [PubMed] [Google Scholar]
- Lawrence J. R.; Zhu B.; Swerhone G. D. W.; Roy J.; Wassenaar L. I.; Topp E.; Korber D. R. Comparative microscale analysis of the effects of triclosan and triclocarban on the structure and function of river biofilm communities. Sci. Total Environ. 2009, 407103307–3316. [DOI] [PubMed] [Google Scholar]
- North East Biosolids Residuals Association (NEBRA). A national biosolids regulation, quality, end use & disposal survey 2007. http://www.nebiosolids.org/uploads/pdf/NtlBiosolidsReport-20July07.pdf. (accessed January 20, 2014).
- Schostarez S. E.; Schultz M. M. Plant uptake of triclosan. Abstr. Pap., Am. Chem. Soc. 2007, 233, 455–455. [Google Scholar]
- Karnjanapiboonwong A.; Chase D. A.; Canas J. E.; Jackson W. A.; Maul J. D.; Morse A. N.; Anderson T. A. Uptake of 17 alpha-ethynylestradiol and triclosan in pinto bean, Phaseolus vulgaris. Ecotoxicol. Environ. Saf. 2011, 7451336–1342. [DOI] [PubMed] [Google Scholar]
- Venkatesan A. K.; Halden R. U., Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Sci. Rep. 2014, 4, (Article Number 3731). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherius A.; Eggen T.; Lorenz W.; Moeder M.; Ondruschka J.; Reemtsma T. Metabolization of the bacteriostatic agent triclosan in edible plants and its consequences for plant uptake assessment. Environ. Sci. Technol. 2012, 461910797–10804. [DOI] [PubMed] [Google Scholar]
- Macherius A.; Eggen T.; Lorenz W. G.; Reemtsma T.; Winkler U.; Moeder M. Uptake of galaxolide, tonalide, and triclosan by carrot, barley, and meadow fescue plants. J. Agric. Food Chem. 2012, 60327785–7791. [DOI] [PubMed] [Google Scholar]
- Schebb N. H.; Ahn K. C.; Dong H.; Gee S. J.; Hammock B. D. Whole blood is the sample matrix of choice for monitoring systemic triclocarban levels. Chemosphere 2012, 877825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan M. P.; Palmer J. E.; Carle A. D.; West M. J.; Seymour G. J. Long term use of triclosan toothpaste and thyroid function. Sci. Total Environ. 2012, 416, 75–79. [DOI] [PubMed] [Google Scholar]
- Servos M. R.; Smith M.; McInnis R.; Burnison B. K.; Lee B. H.; Seto P.; Backus S. The presence of selected pharmaceuticals and the antimicrobial triclosan in drinking water in Ontario, Canada. Water Qual. Res. J. Can. 2007, 422130–137. [Google Scholar]
- Sanches-Silva A.; Sendon-Garcia R.; Lopez-Hernandez J.; Paseiro-Losada P. Determination of triclosan in foodstuffs. J. Sep. Sci. 2005, 28165–72. [DOI] [PubMed] [Google Scholar]
- Chung D. W.; Papadakis S. E.; Yam K. L. Evaluation of a polymer coating containing triclosan as the antimicrobial layer for packaging materials. Int. J. Food Sci. Technol. 2003, 382165–169. [Google Scholar]
- Cowan D. M.; Kingsbury T.; Perez A. L.; Woods T. A.; Kovochich M.; Hill D. S.; Madl A. K.; Paustenbach D. J. Evaluation of the California Safer Consumer Products Regulation and the impact on consumers and product manufacturers. Regul. Toxicol. Pharmacol. 2014, 68123–40. [DOI] [PubMed] [Google Scholar]
- Johnson R. R.; Navone R.; Larson E. L. An unusual epidemic of methemoglobinemia. Pediatrics 1963, 31, 222–225. [PubMed] [Google Scholar]
- Ponte C.; Richard J.; Bonte C.; Lequien P.; Lacombe A. Methemoglobinemia in newborn—Discussion of etiological role of trichlorocarbanilide. Sem. Hop. 1974, 5016359–365. [Google Scholar]
- Hazards of laundry products used in the newborn nursery. Pediatrics 1971, 48, (6), 988-989. [PubMed] [Google Scholar]
- Barbaud A.; Vigan M.; Delrous J. L.; Assier H.; Avenel-Audran M.; Collet E.; Dehlemmes A.; Dutartre H.; Geraut C.; Girardin P.; Le Coz C.; Milpied-Homsi B.; Nassif A.; Pons-Guiraud A.; Raison-Peyron N.; Membres du Groupe du R. [Contact allergy to antiseptics: 75 cases analyzed by the dermato-allergovigilance network (Revidal)]. Ann. Dermatol. Venereol. 2005, 13212 Pt 1962–5. [DOI] [PubMed] [Google Scholar]
- Savage J. H.; Matsui E. C.; Wood R. A.; Keet C. A. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J. Allergy Clin. Immunol. 2012, 1302453–60 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen R. J.; Longnecker M. P.; Lovik M.; Calafat A. M.; Carlsen K. H.; London S. J.; Lodrup Carlsen K. C. Triclosan exposure and allergic sensitization in Norwegian children. Allergy 2013, 68184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicherer S. H.; Leung D. Y. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2012. J. Allergy Clin. Immunol. 2013, 131155–66. [DOI] [PubMed] [Google Scholar]
- Dann A. B.; Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 314285–311. [DOI] [PubMed] [Google Scholar]
- Consortium T. High Production Volume (HPV) Chemical Challenge Program Data Availability and Screening Level Assessment for Triclocarban CAS # 2002, 101–20–2, 201–14186A. [Google Scholar]
- U.S. Environmental Protection Agengy. Screening-Level Hazard Characterization of High Production Volume Chemicals 2008. http://www.epa.gov/hpvis/hazchar/101202_Triclocarban_HC_INTERIM_March%202008.pdf (accessed January 20, 2014).
- Cherednichenko G.; Zhang R.; Bannister R. A.; Timofeyev V.; Li N.; Fritsch E. B.; Feng W.; Barrientos G. C.; Schebb N. H.; Hammock B. D.; Beam K. G.; Chiamvimonvat N.; Pessah I. N. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc. Natl. Acad. Sci. U. S. A. 2012, 1093514158–14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli A.; Cogni D.; Parolini M.; Riva C.; Provini A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in zebra mussel hemocytes. Aquat. Toxicol. 2009, 913238–44. [DOI] [PubMed] [Google Scholar]
- Schebb N. H.; Buchholz B. A.; Hammock B. D.; Rice R. H. Metabolism of the antibacterial triclocarban by human epidermal keratinocytes to yield protein adducts. J. Biochem. Mol. Toxicol. 2012, 266230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. G.; Ternes T. A. Pharmaceuticals and personal care products in the environment: agents of subtle change?. Environ. Health Perspect. 1999, 107Suppl 6907–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A.; Heindel J.; Jobling S.; Kidd K.; Zoeller R. T. State-of-the-science of endocrine disrupting chemicals, 2012. Toxicol. Lett. 2012, 211, S3–S3. [Google Scholar]
- Diamanti-Kandarakis E.; Bourguignon J. P.; Giudice L. C.; Hauser R.; Prins G. S.; Soto A. M.; Zoeller R. T.; Gore A. C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrin. Rev. 2009, 304293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. G.; Ahn K. C.; Gee N. A.; Ahmed M. I.; Duleba A. J.; Zhao L.; Gee S. J.; Hammock B. D.; Lasley B. L. Triclocarban enhances testosterone action: A new type of endocrine disruptor?. Endocrinology 2008, 14931173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton K. M.; Paul K. B.; De Vito M. J.; Hedge J. M. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 2007, 242194–197. [DOI] [PubMed] [Google Scholar]
- Zorrilla L. M.; Gibson E. K.; Jeffay S. C.; Crofton K. M.; Setzer W. R.; Cooper R. L.; Stoker T. E. The Effects of triclosan on puberty and thyroid hormones in male wistar rats. Toxicol. Sci. 2009, 107156–64. [DOI] [PubMed] [Google Scholar]
- Paul K. B.; Hedge J. M.; DeVito M. J.; Crofton K. M. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young long-evans rats. Toxicol. Sci. 2010, 1132367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K. B.; Hedge J. M.; Bansal R.; Zoeller R. T.; Peter R.; DeVito M. J.; Crofton K. M. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: A dynamic and kinetic evaluation of a putative mode-of-action. Toxicology 2012, 3001–231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P. E. A.; Sanchez M. S. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in wistar rat offspring. J. Toxicol. Environ. Health A 2010, 73241678–1688. [DOI] [PubMed] [Google Scholar]
- McMurry L. M.; Oethinger M.; Levy S. B. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS. Microbiol. Lett. 1998, 1662305–309. [DOI] [PubMed] [Google Scholar]
- McMurry L. M.; McDermott P. F.; Levy S. B. Genetic evidence that InhA of Mycobacterium smegmatis is a target for triclosan. Antimicrob. Agents Chem. 1999, 433711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A. E.; Larson E. L.; Levy S. B. Consumer antibacterial soaps: effective or just risky?. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2007, 45Suppl 2S137–47. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Antibacterial household products: Cause for concern. Emerging Infect. Dis. 2001, 73 Suppl512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdankhah S. P.; Scheie A. A.; Hoiby E. A.; Lunestad B. T.; Heir E.; Fotland T. O.; Naterstad K.; Kruse H. Triclosan and antimicrobial resistance in bacteria: An overview. Microb. Drug Resist. 2006, 12283–90. [DOI] [PubMed] [Google Scholar]
- Braoudaki M.; Hilton A. C. Low level of cross-resistance between triclosan and antibiotics in Escherichia coli K-12 and E. coli O55 compared to E-coli O157. FEMS Microbiol. Lett. 2004, 2352305–309. [DOI] [PubMed] [Google Scholar]
- Pycke B. F. G.; Crabbe A.; Verstraete W.; Leys N. Characterization of Triclosan-resistant mutants reveals multiple antimicrobial resistance mechanisms in Rhodospirillum rubrum S1H. Appl. Environ. Microbiol. 2010, 76103116–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain A. J.; Bartolo R. G.; Catrenich C. E.; Charbonneau D.; Ledder R. G.; Price B. B.; Gilbert P. Exposure of sink drain microcosms to triclosan: Population dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 2003, 6995433–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. P.; Paesani Z. J.; Chalew T. E. A.; Halden R. U. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ. Toxicol. Chem. 2009, 28122580–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair P. A.; Lee H. B.; Adams J.; Darling C.; Pacepavicius G.; Alaee M.; Bossart G. D.; Henry N.; Muir D. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ. Pollut. 2009, 1578–92248–2254. [DOI] [PubMed] [Google Scholar]
- Pannu M. W.; O’Connor G. A.; Toor G. S. Toxicity and bioaccumulation of biosolids-borne triclosan in terrestrial organisms. Environ. Toxicol. Chem. 2012, 313646–653. [DOI] [PubMed] [Google Scholar]
- Snyder E. H.; O’Connor G. A.; McAvoy D. C. Toxicity and bioaccumulation of biosolids-borne triclocarban (TCC) in terrestrial organisms. Chemosphere 2011, 823460–467. [DOI] [PubMed] [Google Scholar]
- Reiss R.; Lewis G.; Griffin J. An ecological risk assessment for triclosan in the terrestrial environment. Environ. Toxicol. Chem. 2009, 2871546–1556. [DOI] [PubMed] [Google Scholar]
- Tarnow P.; Tralau T.; Hunecke D.; Luch A. Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in human breast cancer cells. Toxicol. In Vitro 2013, 2751467–1475. [DOI] [PubMed] [Google Scholar]
- Jones R. D.; Jampani H. B.; Newman J. L.; Lee A. S. Triclosan: A review of effectiveness and safety in health care settings. Am. J. Infect. Control 2000, 282184–196. [PubMed] [Google Scholar]
- Aiello A. E.; Coulborn R. M.; Perez V.; Larson E. L. Effect of hand hygiene on infectious disease risk in the community setting: A meta-analysis. Am. J. Public Health 2008, 9881372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Meeting of the Nonprescription Drugs Advisory Committee. October 20, 2005 - Final Report. In FDA, U. S., Ed. 2005. http://www.fda.gov/ohrms/dockets/ac/05/minutes/2005-4184M1.pdf (accessed January 20, 2014).
- Karst K. R., NRDC Sues FDA for Failing to Take Action on Triclosan and Triclocarban. In FDA Law Blog, 2010; Vol. 2014. http://www.fdalawblog.net/fda_law_blog_hyman_phelps/2010/08/nrdc-sues-fda-for-failing-to-take-action-on-triclosan-and-triclocarban.html (accessed February 28, 2014). [Google Scholar]
- Karst K. R., FDA Enters Into Consent Decree; Agrees to Timely Complete Triclosan OTC Drug Antiseptic Monographs. In FDA Law Blog, 2013; Vol. 2014. http://www.fdalawblog.net/fda_law_blog_hyman_phelps/2010/08/nrdc-sues-fda-for-failing-to-take-action-on-triclosan-and-triclocarban.html (accessed February 28, 2014). [Google Scholar]
- Kimbroug M. D.Review of Toxicity of Hexachlorophene, Including Its Neurotoxicity. J Clin. Pharmacol. 1973, 13, (11–1), 439-444. [DOI] [PubMed] [Google Scholar]
- Hexachlorophene today. Lancet 1982, 1, (8263), 87-88. [PubMed] [Google Scholar]
- American Academy of Microbiology. Antibiotic Resistance: An Ecological Perspective on an Old Problem; 2009. http://academy.asm.org/images/stories/documents/antibioticresistance.pdf (accessed January 20, 2014).
- Alliance for the Prudent Use of Antibiotics (APUA). Triclosan; 2011. http://www.tufts.edu/med/apua/consumers/personal_home_21_4240495089.pdf (accessed January 20, 2014).
- Peter S.; Nayak D. G.; Philip P.; Bijlani N. S. Antiplaque and antigingivitis efficacy of toothpastes containing Triclosan and fluoride. Int. Dent. J. 2004, 545299–303. [DOI] [PubMed] [Google Scholar]
- Daughton C. G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ. Health Perspect. 2003, 1115775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable D. J. C.; Dunn P. J.; Hayler J. D.; Humphrey G. R.; Leazer J. L.; Linderman R. J.; Lorenz K.; Manley J.; Pearlman B. A.; Wells A.; Zaks A.; Zhang T. Y. Key green chemistry research areas—A perspective from pharmaceutical manufacturers. Green Chem. 2007, 95411–420. [Google Scholar]
- Khetan S. K.; Collins T. J. Human pharmaceuticals in the aquatic environment: A challenge to Green Chemistry. Chem. Rev. 2007, 10762319–64. [DOI] [PubMed] [Google Scholar]