Abstract
Background
Postinfectious autoimmunity has been implicated in Tourette’s syndrome and obsessive-compulsive disorder (TS/OCD), whereas increased frequency of upper respiratory tract infections (URTI) in TS/OCD patients suggests immune deficiency. We hypothesized that antineuronal antibodies may be elevated in patients (reflecting autoimmune processes), and levels of total immunoglobulins (Igs) may be decreased (reflecting immune deficiency).
Methods
We analyzed plasma of TS/OCD patients (n =24) and healthy age- and sex-matched control subjects (n =22) by enzyme-linked immunosorbent assay (ELISA) for the levels of total and specific IgG, IgM, and IgA against antigens previously identified in multiple sclerosis (myelin basic protein and myelin-associated glycoprotein) and Sydenham’s chorea (ganglioside-GM1, lysoganglioside, and tubulin).
Results
Total IgA was decreased in TS/OCD patients (median 115 mg/100 mL) compared with control subjects (141 mg/100 mL; p = .02). Specific IgA against all antigens, except tubulin were also decreased in the patients (MPB 0 vs. 13 [ELISA units [EU]; myelin-associated glycoprotein 29 vs. 44 EU, p = .04; ganglioside GM1 21 vs. 35 EU, p = .01; lysoganglioside 44 vs. 56 EU, p = .03; tubulin 44 vs. 44 EU, p = .8). The levels of total IgA and anti-myelin basic protein (MBP) IgA were significantly lower in the subgroup of pediatric autoimmune neuropsychiatric disorder associated with Streptococcus (PANDAS) cases (n =10) than in non-PANDAS cases (n =9; total IgA 98 mg/100 mL vs. 133 mg/mL, p = .03; anti-MBP IgA 1 vs. 6 EU, p = .03) or healthy control subjects (total IgA 141 mg/100 mL, p = .02; anti-MBP IgA 13 EU, p = .005).
Conclusions
At least some TS/OCD patients may suffer IgA dysgammaglobulinemia, possibly rendering the children more prone to URTI.
Keywords: Autoimmunity, immune deficiency, immunoglobulins, Group A β-hemolytic streptococcus, PANDAS, Tourette’s syndrome
Tourette’s syndrome (TS) is a chronic, relapsing disorder characterized by involuntary motor and phonic tics, and obsessive-compulsive disorder (OCD) with unknown etiology.
Postinfectious autoimmunity was implied in TS/OCD because of its clinical similarity to Sydenham’s chorea (SC), a component of autoimmune rheumatic fever occurring after Group A β-hemolytic Streptococcus (GABHS) infection (1). The concept of pediatric autoimmune neuropsychiatric disorder associated with Streptococcus (PANDAS) has been supported by temporary relief of symptoms in severe patients after plasmapheresis (1), the presence of antibasal ganglia antibodies in serum of TS/OCD patients (2), the cross-reactivity of antistreptococcal antibodies with neuronal epitopes (3–6), enhanced activity of T cell and NK cells in peripheral blood (7–9), and decreased numbers of regulatory T lymphocytes, the function of which is to suppress immune responses and prevent autoimmunity (10). This suggests enhanced activity of the immune system in TS/OCD patients, which is consistent with autoimmune processes. Other studies have demonstrated increased frequency of streptococcal infections and sinusitis in the patients, implying some form of immune deficiency (11,12). Simultaneous occurrence of autoimmunity and immune deficiency is not an uncommon scenario.
Neuronal circuits affected in TS/OCD involve both gray and white matter (striatum, associated limbic system, frontal cortex, and corpus callosum) (13). We hypothesized that TS/OCD patients may have increased levels of anti–basal ganglia antibodies previously shown to be elevated in SC (antibodies against ganglioside GM1, lysoganglioside, and tubulin) (6), as well as anti-myelin autoantibodies typically increased in multiple sclerosis, a white matter disorder (anti-myelin basic protein [MBP] and anti-myelin-associated glycoprotein [MAG] antibodies). We also hypothesized that the putative immune deficiency may be reflected by decreased levels of total immunoglobulins (Igs).
Methods and Materials
Subjects
Blood samples of TS/OCD (n = 24, Table 1) and healthy age-matched control subjects (n = 22, Table 1) were collected as part of three clinical studies to perform pilot investigations of immune system in TS/OCD. The Human Investigation Committee at Yale University approved these studies; all parents signed a permission statement, and each child signed a statement of informed assent. Clinical evaluation was performed as described previously using ordinal severity scales of the Yale Global Tic Severity Scale and Children’s Yale–Brown Obsessive Compulsive Scale (7,10).
Table 1.
Demographic and Clinical Characteristics
| Variable | n | TS/OCD Patients (n = 24) | Unaffected Control Subjects (n = 22) |
|---|---|---|---|
| Age in years (SD), range | 12.9 (2.8), 7–17 | 13.6 (2.2), 9–17 | |
| Sex (% Female) | 22% | 43% | |
| Ethnicity (% Caucasian) | 96% | 71% | |
| Baseline Symptom Severity by Diagnosis | |||
| TS (including chronic tics)a | 20c | 27.8 (14.1) | NA |
| OCDb | 16b | 9.5 (9.5) | |
| PANDAS subjects | 39% | NA | |
OCD, obsessive-compulsive disorder; TS, Tourette’s syndrome.
Total tic severity score on the Yale Global Tic Severity Scale.
Total score on the Children’s Yale-Brown Obsessive Compulsive Scale.
Number of subjects out of 24 patients who had TS (including chronic tics).
Blood Drawing and Analysis
Blood was drawn into heparinized vacutainer tubes (BD Biosciences, Bedford, Massachusetts) and placed on ice. Within 1 hour, blood was loaded on column of lymphocyte separation medium and spun at 400 g for 30 min to separate peripheral blood mononuclear cells and plasma. The upper layer containing plasma was collected into Eppendorf tubes and stored at −80°C.
Analysis of Plasma Samples
The plasma samples were analyzed for total IgG, IgM, and IgA by nephelometry using the Immulite system (DPC, Los Angeles, California) and for specific antibodies to MBP, MAG, lysoganglioside, ganglioside GM1, and tubulin using the enzyme-linked immunosorbent assay (ELISA) technique as previously described (14). Coefficient of intraassay variation for IgG, IgM and IgA against all antigens was less than 6%, and coefficient of interassay variation was less than 15%.
Data Analysis
The Mann–Whitney U test was used to compare patients and healthy control subjects because the data did not follow normal distribution. The results are reported as medians with inter-quartile ranges (IQR). Multivariant comparison of PANDAS, non-PANDAS and healthy control groups was performed by Kruskal–Wallis test, and where relevant, subsequent analysis of differences between individual groups was performed by Mann Whitney U test. Values of p < .05 were considered significant.
Results
Plasma Levels of Total Ig Isotypes
TS/OCD patients had significantly lower levels of total plasma IgA (median 115 mg/100 mL, IQR 86–151) than the age-matched control subjects (141 mg/100 mL, IQR 121–170 in control subjects; U = 145; n1 = 24, n2 = 22, p = .02), although there were no differences in total IgG (935 mg/100 mL, IQR 746–1064 in patients vs. 977 mg/mL, IQR 803–1332 in control subjects, U = 200; p = .32) or total IgM levels (199 mg/mL, IQR 152–259 in patients vs. 209 mg/100 mL, IQR 148–240 in control subjects, U = 232; p = .81; Figure 1).
Figure 1.
Plasma levels of total immunoglobulin (Ig)G (A), IgM (B), and IgA (C) in Tourette’s syndrome/obsessive-compulsive (TS/OCD) patients (n = 24) and age-matched healthy control subjects (n = 22). There were no differences in the levels of IgG and IgM between the patients and control children, but the levels of total IgA are significantly lower in the patients (*p = .02).
Plasma Levels of Specific Ig Isotypes
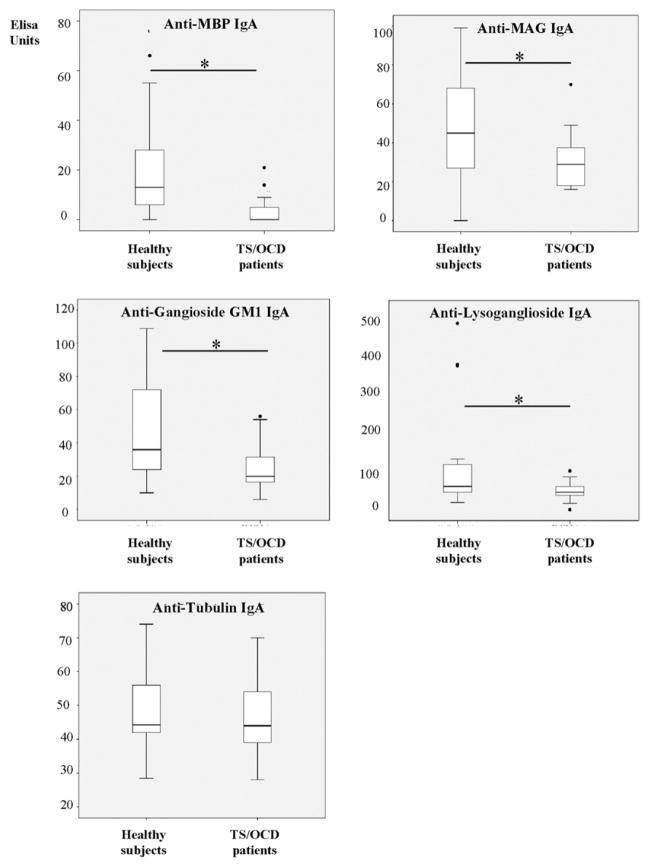
TS/OCD patients had significantly lower levels of plasma anti-MBP, MAG, ganglioside GM1, or lysoganglioside IgA than their age-matched control subjects (MPB 0 ELISA units [EU], IQR 0–5 in patients vs. 13 EU, IQR 4–28 in control subjects, U = 99, p = .001; MAG 29 vs. 44 EU, U = 153; p = .04; ganglioside GM1 21 EU, IQR 17–30 vs. 35 EU, IQR 16 – 45, U = 134, p = .01; lysoganglioside 44 EU, IQR 37–58 vs. 56 EU, IQR 43–105, U = 140 p = .03; Figure 2A–2D). There was no difference in antitubulin IgA between patients and control subjects (44 EU, IQR 39–54 vs. 44 EU, IQR 42–55, U = 231, p = .79; Figure 2E). Also, no differences were observed in specific IgG (MBP 18 EU, IQR 5–27 vs. 16 EU, IQR 3–41, U = 216; p = .54; MAG 30 EU, IQR 24–38 vs. 39 EU, IQR 15–46, U = 204, p = .37; ganglioside GM1 55 EU, IQR 41–63 vs. 51 EU, IQR 44–73, U = 232, p = .83; lysoganglioside 49 EU, IQR 37–58 vs. 46 EU, IQR 43–105, U = 227, p = .72; tubulin 54 EU, IQR 65–98 vs. 50 EU, IQR 76–112, U = 220, p = .6) or specific IgM (MBP 7 EU, IQR 0–26 vs. 9 EU, IQR 0–31, U = .9, p = .9; MAG 28, IQR 24–38 vs. 23 EU, IQR 15–46, U = 234, p = .86; ganglioside GM1 28 EU, IQR 18–38 vs. 27 EU, IQR 6–52, U = 231, p = .81; lysoganglioside 44, IQR 33–67 vs. 47 EU, IQR 33–83, U = 220, p = .61; tubulin 85, IQR 65–98 vs. 99 EU, IQR 76–112, U = 205, p = .38) in patients versus control subjects, respectively.
Figure 2.
Plasma levels of immunoglobulin (Ig)A against myelin basic protein (MBP) (A), myelin-associated glycoprotein (MAG) (B), ganglioside GM1 (C), and lysoganglioside (D) are significantly lower in Tourette’s syndrome/obsessive-compulsive (TS/OCD) patients than in healthy age-matched control subjects (*p < .05), whereas the levels of anti-tubulin IgA do not differ (E).
Plasma Levels of PANDAS and Non-PANDAS Cases
Among our patients, there were 19 subjects with defined PANDAS status according to a history of association between TS/OCD symptoms and GABHS infection as defined by Swedo’s recommendations (15). Multivariant comparison of PANDAS cases (n = 9), non-PANDAS cases (n = 10), and healthy subjects by Kruskal–Wallis test revealed a significant differences in total IgA (χ2 = 6, df = 2P, = .05) and anti-MBP IgA levels (χ2 = 14, df = 2, p = .001). We then compared the differences between the individual groups using Mann–Whitney test. Total IgA levels were significantly lower in PANDAS versus non-PANDAS cases (98 mg/mL, IQR 82–103 vs. 133 mg/m; IQR 118–222, U = 16; p = .02) or PANDAS versus healthy subjects (98 mg/mL, IQR 82–103 vs. 141 mg/mL, IQR 121–170; U = 47; p = .02; Figure 3A). Anti-MBP IgA was decreased in PANDAS versus non-PANDAS cases (1 EU, IQR 0 – 0 vs 6 EU, IQR 0–10; U = 16 p = .02) and PANDAS versus healthy subjects (1 EU, IQR 0 – 0 vs. 13 EU, IQR 4–28, U = 35; p = .005; Figure 3B). Levels of other Igs did not significantly differ.
Figure 3.

Plasma levels of total immunoglobulin (Ig)A (A) and anti-myelin basic protein (MBP) IgA (B) in patients with history of pediatric autoimmune neuropsychiatric disorder associated with Streptococcus (PANDAS; n = 9) compared with non-PANDAS cases (n = 10) and healthy children (n = 22) shown as values in individual subjects, as well as mean ± SD (*p < .05). No differences were observed in anti-myelin-associated glycoprotein, ganglisoside, lysoganglioside, or tubulin IgA levels.
Discussion
TS/OCD patients have decreased total and specific IgA plasma levels in comparison with healthy age-matched children (Figures 1 and 2). This could contribute to deviation of immune responses in TS/OCD patients by at least two mechanisms. First, inhibitory functions of IgA in plasma on immune responses may be reduced (16), which could increase the vulnerability of TS/OCD patients for developing autoimmune disorders (17). Second, IgA secretion on mucosal surfaces may be affected (18,19), and in this case, the very first steps of immune defense against mucosal pathogens would be affected. This could then explain increased frequency of streptococcal infections and sinusitis in TS/OCD patients (11,12).
Decreased levels of IgA in some TS/OCD patients were previously reported in a letter to the editor (20). The authors described two families, each with 1 TS/OCD child who had IgA levels below laboratory reference values (a 2-year-old boy with IgA below 7 mg/100 mL vs. the laboratory range for normal individuals of 33–236 mg/100 mL, and a 9-year-old boy with 11 mg IgA/100 mL vs. laboratory range 14–159 mg IgA/100 mL). Other family members had a history of symptoms that were consistent with IgA immunodeficiency. Further, about 10% of TS/OCD patients whom the authors followed for immune disturbances exhibited IgA dysgammaglobulinemia, suggesting a frequency 57 to 76 times higher than in the healthy population (20). This observation corresponds to our findings of significant overlap in IgA levels with values 56–198 mg/100 mL in the patients (115 mg/100 mL, IQR 86–151) and 66 mg–276 mg/100 mL in age-matched control children (median 141 mg/100 mL, IQR 121–170). A tendency to lower IgA in TS/OCD patients was also observed during evaluation before administration of intravenous immunoglobulin (Sue Swedo, M.D., National Institute of Mental Health, oral communication, November 20, 2009.). These differences do not correspond to fully expressed selective IgA deficiency (<7 mg IgA/100 mL). However, the association between IgA deficiency and chronic immune disorders (autoimmune disorders occur in 19% and atopic disorders in 48% of IgA deficient patients) warrants our attention to the role of IgA in TS/OCD pathogenesis (16,17).
IgA is the most abundant immunoglobulin in the body. In serum, IgA is the second most prevalent antibody after IgG, it is produced mainly by bone marrow B cells and occurs predominantly in monomeric form as IgA1 subclass (16). Monomeric IgA is a weak activator of complement and rarely mediates secondary immune responses; IgA downregulates cell-mediated immune responses involving IgG-mediated phagocytosis (16,17). The inhibitory effects are elicited through binding of Fc portion of IgA to receptors FcαRI on immune cells (CD89), including neutrophils, monocytes, macrophages, dendritic cells (16,17). Increased immune responsiveness has previously been observed in TS/OCD patients (7,8), and the elevated levels of tumor necrosis factor-α in the patients might be related to the suppressed IgA levels because serum IgA downregulates tumor necrosis factor-α release from human monocytes (21).
The highest amounts of IgA are present at mucosal surfaces where mainly IgA2 subclass occurs. The secretory IgA is generated by B lymphocytes in germinal centers of mucosa-associated lymphoid tissue at the basolateral epithelial compartment of lamina propria. Two IgA molecules are joined by polypeptide called J-chain forming dimers, which then bind covalently to polymeric Ig receptor and are transported through the epithelial cells to the apical/luminal site of mucosal surface. There it is cleaved from the polymeric Ig receptor. The function of IgA is to prevent the attachment of microorganisms at mucosal surfaces and to neutralize the effects of exotoxins, as well as to induce effector functions to destroy microorganisms and mammalian cells, such as activation of lectin pathway of complement (16). We observed that PANDAS cases have significantly lower IgA levels than non-PANDAS cases or healthy subjects. If the decrease of plasma IgA also reflects a decrease of secretory IgA, the inhibition of GABHS attachment to epithelial cells could be compromised (16). This could lead to prolongation of GABHS presence on the mucosa, allowing release of more exotoxins, the effects of which are also less opposed in this situation. Streptococcal exotoxins were shown to alter immune responses and worsen the course of inflammatory disease in experimental model of multiple sclerosis (22). Little is known about the effects of exotoxins in TS/OCD patients. However, our previous study implied that a decreased number of Vbeta18+ CD8+ T cells in the blood of patients may be caused by exotoxin SPE-1 derived from common M1 serotype of GABHS (10).
Why the levels of total IgA are reduced in TS/OCD patients is not understood. IgA is produced at higher rates per day (66 mg/kg/day) than all other classes combined, and it is catabolized with a half-life of 3 to 6 days. A deviation in any of these two branches of IgA metabolism could alter IgA levels. Interestingly, among critical factors controlling generation of IgA is tumor growth factor-β, the major source of which are regulatory T cells (23,24). In relation to TS/OCD, we showed that patients have 40% lower numbers of regulatory T cells in their peripheral blood than healthy, age-matched control subjects (10). Whether these two phenomena are causally related remains to be addressed in future studies.
An alternative explanation for the decrease in serum IgA could be an increased binding of IgA to its receptors on immune cells, such as FcαRI or Fcα/μ receptors (16), leading to a form of consumptive IgA-opathy. In relation to TS/OCD, increased expression of Fcα/μ receptors on B cells of TS patients, due to increased binding of IgM to the surface of the lymphocytes, was proposed (25). However, a direct evidence for increased expression of Fcα/μ receptors in immune cells of TS/OCD patients is not yet available.
What the decrease of IgA levels against MBP, MAG, ganglioside, or lysoganglioside mean in pathophysiology of TS/OCD is not clear at present. Interestingly, levels of IgA against tubulin (an antigen previously shown to be important in SC) were not altered in TS/OCD patients (Figure 2) and antimycoplasma IgA was increased in TS/OCD patients (26). This suggests that a repertoire of specificities of IgA could be altered in TS/OCD patients, possibly reflecting responses to more frequent antigen stimulation.
No changes in total and specific IgG and IgM levels were found in TS/OCD patients. Similarly, Libbey et al. (27) also found no changes in total and anti-MBP IgG and IgM in TS/OCD patients, arguing against the importance of these antibodies in the pathogenesis of TS/OCD.
In conclusion, we report that IgA dysgammaglobulinemia may alter GABHS-driven immune responses in a subgroup of TS/OCD patients. Future studies should evaluate whether IgA levels are altered also in saliva and tears of TS/OCD patients, as well as address the rate of IgA metabolism and overall function of B cells in these children.
Acknowledgments
The study was supported by a Tourette’s Syndrome Association Research Grant (IK), National Institutes of Health grants (JFL), and additional funds to Dr. Leckman from Brian and Linda Richmand (B. Richmand is one of the coauthors), Betsey Henley-Cohn, Scott D. and Amy I. Horwitz, Marty and Susan Kravet, Samuel Gejdenson, and the Echlin Foundation. We thank Mrs. Heidi Grantz for coordination of the clinical studies.
Footnotes
AV is an owner of Immunosciences Laboratory, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Snider LA, Swedo SE. PANDAS: Current status and directions for research. Mol Psychiatry. 2004;9:900–907. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- 2.Church AJ, Dale RC, Giovannoni G. Anti-basal ganglia antibodies: A possible diagnostic utility in idiopathic movement disorders? Arch Dis Child. 2004;89:611–614. doi: 10.1136/adc.2003.031880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 4.Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2006;172:187–197. doi: 10.1016/j.jneuroim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Kansy JW, Katsovich L, McIver KS, Pick J, Zabriskie JB, Lombroso PJ, et al. Identification of pyruvate kinase as an antigen associated with Tourette’s syndrome. J Neuroimmunol. 2006;181:165–176. doi: 10.1016/j.jneuroim.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Leckman JF, Katsovich L, Kawikova I, Lin H, Zhang H, Kronig H, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol Psychiatry. 2005;57:667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Moller JC, Tackenberg B, Heinzel-Gutenbrunner M, Burmester R, Oertel WH, Bandmann O, et al. Immunophenotyping in Tourette’s syndrome—A pilot study. Eur J Neurol. 2008;15:749–753. doi: 10.1111/j.1468-1331.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 9.Lit L, Gilbert DL, Walker W, Sharp FR. A subgroup of Tourette’s patients overexpress specific natural killer cell genes in blood: A preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:958–963. doi: 10.1002/ajmg.b.30550. [DOI] [PubMed] [Google Scholar]
- 10.Kawikova I, Leckman JF, Kronig H, Katsovich L, Bessen DE, Ghebremichael M, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with Tourette’s syndrome: A preliminary study. Biol Psychiatry. 2007;61:273–278. doi: 10.1016/j.biopsych.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics. 2005;116:56– 60. doi: 10.1542/peds.2004-2058. [DOI] [PubMed] [Google Scholar]
- 12.Leslie DL, Kozma L, Martin A, Landeros A, Katsovich L, King RA, et al. Neuropsychiatric disorders associated with streptococcal infection: A Case-control study among privately insured children. J Am Acad Child Adolesc Psychiatry. 2008;47:1166–1172. doi: 10.1097/CHI.0b013e3181825a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey KA, Albin RL. Neuroimaging of Tourette’s syndrome. J Child Neurol. 2006;21:672–677. doi: 10.1177/08830738060210080501. [DOI] [PubMed] [Google Scholar]
- 14.Vojdani A, Campbell AW, Anyanwu E, Kashanian A, Bock K, Vojdani E. Antibodies to neuron-specific antigens in children with autism: Possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J Neuroimmunol. 2002;129:168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 15.Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 16.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 17.Jacob CM, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC. Autoimmunity in IgA deficiency: Revisiting the role of IgA as a silent housekeeper. J Clin Immunol. 2008;28(suppl 1):S56–S61. doi: 10.1007/s10875-007-9163-2. [DOI] [PubMed] [Google Scholar]
- 18.Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Russell MW, Moldoveanu Z, et al. IgA antibody-producing cells in peripheral blood after antigen ingestion: Evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987;84:2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norhagen G, Engstrom PE, Hammarstrom L, Soder PO, Smith CI. Immunoglobulin levels in saliva in individuals with selective IgA deficiency: Compensatory IgM secretion and its correlation with HLA and susceptibility to infections. J Clin Immunol. 1989;9:279–286. doi: 10.1007/BF00918659. [DOI] [PubMed] [Google Scholar]
- 20.Hansen CR, Jr, Bershow SA. Immunology of TS/OCD. J Am Acad Child Adolesc Psychiatry. 1997;36:1648–1649. doi: 10.1097/00004583-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83:1278–1288. [PubMed] [Google Scholar]
- 22.Racke MK, Quigley L, Cannella B, Raine CS, McFarlin DE, Scott DE. Superantigen modulation of experimental allergic encephalomyelitis: Activation of anergy determines outcome. J Immunol. 1994;152:2051–2059. [PubMed] [Google Scholar]
- 23.Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989;170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 25.Hoekstra PJ, Bijzet J, Limburg PC, Steenhuis MP, Troost PW, Oosterhoff MD, et al. Elevated D8/17 expression on B lymphocytes, a marker of rheumatic fever, measured with flow cytometry in tic disorder patients. Am J Psychiatry. 2001;158:605–610. doi: 10.1176/appi.ajp.158.4.605. [DOI] [PubMed] [Google Scholar]
- 26.Muller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele-Horn M. Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 2004;129:119–125. doi: 10.1016/j.psychres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Libbey JE, Coon HH, Kirkman NJ, Sweeten TL, Miller JN, Stevenson EK, et al. Are there enhanced MBP autoantibodies in autism? J Autism Dev Disord. 2008;38:324–332. doi: 10.1007/s10803-007-0400-6. [DOI] [PubMed] [Google Scholar]