Abstract
Background
Obsessive–compulsive disorder (OCD) is clinically heterogeneous. Previous studies have reported different patterns of treatment response to serotonin reuptake inhibitors (SRI) based on symptom dimension. Our objective was to replicate these results in OCD patients who participated in one of four randomized, placebo-controlled, clinical trials (RCT).
Methods
A total of 165 adult OCD subjects participated in one or more eight-week RCT with clomipramine, fluvoxamine, or fluoxetine. All subjects were classified as having major or minor symptoms in four specific OC symptom dimensions that were derived in a previous factor analytic study involving many of these same patients. Ordinal logistic regression was used to test the association between OC symptom dimensions and SRI response.
Results
We found a significant association between the symptom dimension involving sexual, religious and harm-related obsessions as well as checking compulsions (AGG/SR) and improved SRI response. This increased rate of SRI response was experienced primarily by individuals with harm-related obsessions. Over 60% of patients with AGG/SR OCD symptoms were rated as very much improved after SRI treatment.
Limitations
As some of the RCTs included were conducted prior to the development of the Yale-Brown Obsessive–compulsive Scale (Y–BOCS), improvement in OCD severity was assessed using the Clinical Global Improvement (CGI) Scale. Data from the double-blind and open-label continuation phases of these trials was collapsed together to increase statistical power.
Conclusions
Patients with OCD vary in their response to SRIs. The presence of AGG/SR symptoms is associated with an initial positive response to SRIs. These data add to the growing body of work linking central serotonin systems with aggressive behavior.
Keywords: Obsessive–compulsive disorder, Symptom dimensions, Serotonin reuptake inhibitors
1. Introduction
Obsessive–compulsive disorder (OCD) is a neuropsychiatric disorder characterized by obsessions (unwanted, recurrent and distressing thoughts) and compulsions (repetitive and ritual-like behaviors typically done in response to unwanted obsession thoughts). Since it was recognized as a psychiatric disorder, there have been many attempts to split the heterogeneous symptomatology of OCD into more homogenous subtypes. For example in 1869, Falret made the distinction between “Folie du doute” (madness of doubt) and “Delire du toucher” (delusions of touch) (Hantouche and Lancrenon, 1996). Despite these earlier efforts, mainstream diagnostic systems, such as DSM and ICD, have persisted in describing OCD as a unitary category characterized by heterogeneous clinical manifestations (Berrios, 2003).
Planning for DSM-V in 2012 has led to the desire to incorporate a more dimensional approach to diagnostic entities. These dimensions should be consistent and replicable. They should have validity and utility in predicting treatment responses and outcome. These dimensions may also serve as more precise phenotypic markers for genetic and brain imaging studies. This approach may be particularly useful in studying OCD, where over a dozen factor analytic studies have identified four to five fairly consistent symptom dimensions of the disorder (Baer, 1994; Cavallini et al., 2002; Delorme et al., 2006; Denys et al., 2004; Feinstein et al., 2003; Foa et al., 2002; Leckman et al., 1997, 2003; Mataix-Cols et al., 2002, 1999, 2005; McKay et al., 2006; Rufer et al., 2005; Summerfeldt et al., 1999; Tek and Ulug, 2001). The symptom dimensions in OCD that have been consistently replicated across studies include: aggressive obsessions and checking behavior (AGG), sexual/religious obsessions and compulsions (SR), contamination obsessions and related washing obsessions (CW), obsessions with symmetry and exactness and ordering compulsions (SYM), and hoarding obsessions and compulsions (HRD). The main disagreement between studies involving four and five factor solutions for OCD symptom dimensions is whether aggressive obsessions and checking behavior (AGG) and sexual/religious obsessions and compulsions (SR) should be combined into a single dimension (AGG/SR) or two separate dimensions. We used a four factor solution involving a combined AGG/SR dimensions as this factor analytic solution was derived from data involving many of the same subjects included in this present sample (Bloch et al., 2008; Leckman et al., 1997).
OCD symptom dimensions are temporally stable and have been associated with distinct patterns of comorbidity (Holzer et al., 1994; Pitman et al., 1987). Preliminary data suggest that these quantitative traits may be useful phenotypic markers for genetic, neuroimaging, and treatment-outcome studies (Baer, 1994; Mataix-Cols et al., 2005). Specifically, HRD symptoms also have been reported to be associated with poor Serotonin Reuptake Inhibitor (SRI) response in most (Mataix-Cols et al., 1999; Samuels et al., 2002; Stein et al., 2007) but not all studies (Saxena et al., 2007). In contrast, AGG/SR symptoms have been associated with improved long-term outcome (Eisen et al., 2006) and with improved SSRI response at trend levels (Samuels et al., 2002); on the other hand, when parsing out the SR dimension Alonso et al. found a worse outcome(Alonso et al., 2001). In this study we sought to clarify the association between OCD these two symptom dimensions and response to SRI pharmacotherapy.
2. Methods
2.1. Subjects
Subjects were originally seen at the Yale OCD Clinic from 1982 to 1996 and diagnosed with OCD by an expert clinician. Each subject received a trial of at least 1 of 3 SRI medications (fluoxetine, fluvoxamine, clomipramine) for at least 8 weeks at the maximum tolerated dose as part of 4 RCTs (Clomipramine_Collaborative_Study_Group, 1991; Goodman et al., 1996; Goodman et al., 1989; Zohar et al., 1992). Since some patients participated in more than one of these trials, only data from their initial SRI clinical trial was included in this study. Data from each of these trials was collected with permission from the Yale IRB.
2.2. Clinical measures
Subjects were assessed using the Clinical Global Improvement Scale (CGI) (Guy, 1976), the Yale–Brown Obsessive Compulsive Scale (Y–BOCS) and the Hamilton Depression Rating Scale.
2.3. Data analysis
Each subject was asked to describe their major and minor OC symptoms based on the 15 categories that came to be the headings of the Y–BOCS Symptom Checklist. Based on these answers, subjects were rated in the four previously described OC symptom dimensions (Leckman et al., 1997) as having either no symptoms present in a particular dimension (coded as a 0), any symptoms present in a particular dimension (coded as 1) or predominant symptoms in a particular dimension (the most impairing of the 4 dimensions, coded as 2). In post-hoc analysis the AGG/SR dimension was divided into individuals with either harm-related or sexual/religious obsessions. The subjects who were much or very much improved after the medication trial (CGI = 1–2) were classified as responders (coded as 2), subjects who were minimally improved (CGI = 3) were considered partial responders (coded as 1), and subjects who showed no improvement or were worse (CGI=4–7) were considered nonresponders (coded as 0). Medication response was evaluated during the period where an individual got active medication in RCT, i.e. randomized placebo controlled period, patients where assigned to active medication or in open trial of medication following placebo assignment.
Association between SRI response and presenting OC symptom dimensions was assessed using ordinal logistic regression. The subject’s response to SRI medication was the dependent variable and each symptom dimension was entered into separate logistic regression models as the independent variable.
In exploratory analyses we examined the association between OC symptom dimensions or the presence of lifetime psychiatric conditions [Major Depression, Anxiety Disorders, Substance Abuse, Eating Disorders, and Tic Disorders] and gender using forward stepwise binomial regression analysis with the disorders as the dependent variable and the OC dimensions as the independent variables. We analyzed the association between age of onset of OCD symptoms and symptom dimensions using a stepwise linear regression analysis with age of onset as the dependent variable and OC symptoms as the independent variable. For all exploratory stepwise models a two-tailed significance level of 0.05 was set as the threshold for entry of terms and 0.10 for exclusion of terms.
3. Results
3.1. Subjects
There were 165 subjects eligible for analysis that completed the trials. Sixty-two subjects were on clomipramine, 79 on fluvoxamine and 24 on fluoxetine. Baseline demographics of the sample are presented in Table 1.
Table 1.
Baseline demographics of 165 subjects whowere treated with 1 of 3 Serotonin Reuptake Inhibitors medications during double-blind clinical trials or open-label extension period of those studies at the Yale Obsessive Compulsive Disorder Clinic.
| Baseline demographical and clinical characteristics | ||||
|---|---|---|---|---|
| Total | Clomipramine | Fluvoxamine | Fluoxetine | |
| Number | 165 | 62 | 79 | 24 |
| Age | 35.9±11.0 | 35.1±10.8 | 37.2±11.7 | 34.0±8.9 |
| Gender | 69 M | 27 M | 32 M | 10 M |
| Comorbid diagnosis | ||||
| Tics | 23 (14%) | 4 (7%) | 14 (18%) | 5 (21%) |
| Major depression disorder |
67 (34%) | 15 (24%) | 44 (56%) | 8 (33%) |
| Anxiety disorders | 26 (16%) | 7(11%) | 19 (23%) | 1 (4%) |
| Substance abuse | 54 (32%) | 21 (34%) | 25 (32%) | 8 (33%) |
| Trichotillomania | 8 (5%) | 4 (7%) | 4 (5%) | 1 (4%) |
| Eating disorders | 13 (8%) | 4 (7%) | 6 (8%) | 3 (13%) |
| Age of Onset | 24.1±8.9 | 23.1±7.0 | 25.0±10.3 | 23.6±7.6 |
| Duration of illness |
14.3±11.7 | 15.1±10.9 | 13.5±12.3 | 14.8±11.6 |
| Y–BOCS score | 25.1±5.7 (N=91) |
25.1±6.3 (37) |
25.7±5.2 (40) |
23.9±5.9 (14) |
| HAM-D score | 23.8±12.8 (82) |
20.0±12.3 (19) |
24.4±12.7 (51) |
27.0± 13.4 (12) |
| Prominent OC symptom dimensions | ||||
| CW | 53 (33%) | 28 (45%) | 22 (28%) | 3 (13%) |
| AGG/SR | 43 (26%) | 12 (19%) | 24 (30%) | 7 (29%) |
| AGG | 29 (18%) | 6 (10%) | 18 (23%) | 5 (21%) |
| SR | 14 (9%) | 6 (10%) | 6 (8%) | 2 (8%) |
| HRD | 5 (3%) | 0 | 5 (6%) | 0 (0) |
| SYM | 32 (19%) | 13 (21%) | 14 (18%) | 5 (20.8) |
| MISC | 30 (18.4) | 9 (15%) | 13 (16%) | 8 (33.3) |
Y–BOCS: Yale–Brown Obsessive Compulsive Scale, HAM-D: Hamilton Depression Rating Scale, OCD symptom dimensions: CW: cleaning/contamination; AGG/SR=fear of harm, sexual and religious obsessions and checking compulsions (this dimension is subdivided into AGG: fear of harm obsessions and checking compulsions and SR: sexual and religious obsessions and compulsions in posthoc analysis); HRD: hoarding and SYM: ordering, symmetry and arranging obsessions and compulsions.
3.2. Dimensional predictors of SRI response
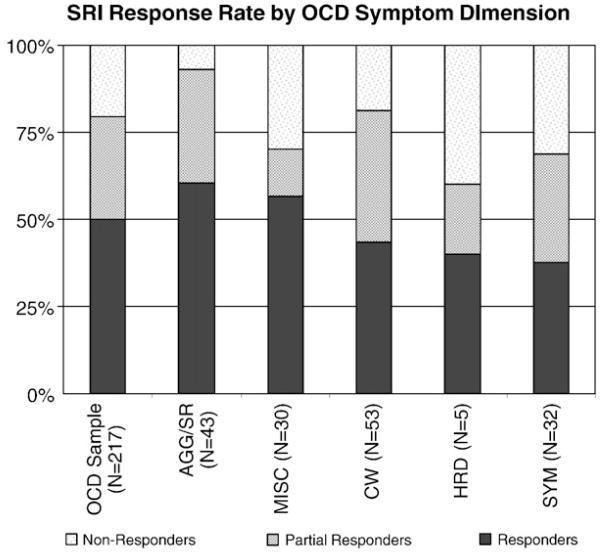
The proportion of responders to SRI medication stratified by symptom dimension is depicted in Fig. 1. The presence of AGG/SR OC symptoms was associated with a good response to SRIs (parameter estimate [PE]=0.42±0.18, Wald=5.6, df=1, p=.018). When the result was stratified by particular pharmacological agent utilized (clomipramine vs. a Selective Serotonin Reuptake Inhibitor), there was a significant association of good response for subjects with AGG/SR OC symptoms receiving SSRI pharmacotherapy (PE = 0.70 ±0.23, Wald = 9.6, df = 1, p=.002) but not clomipramine (PE=0.04±0.30, Wald=0.01, df=1, p=.91). When the results in this dimensionwere split into individuals with harm-related obsession and checking compulsions (AGG) and those with sexual and religious obsession and compulsions (SR), only AGG OC symptoms were associated with good SRI response (parameter estimate [PE]=0.68±0.21, Wald=10.1, df=1, p=.001). There was no evidence SR OC symptoms were associated with SRI treatment response SR (PE=0.14±0.24, Wald=0.3, df=1, p=. 56). There was an insufficient number of patients with prominent HRD symptoms to assess this dimension statistically, although the highest percentage of SRI non-responders occurred in those with primary symptoms in the HRD dimension (40% non-responders). There was a modest negative association between OC symptoms in the SYM dimension (PE=−0.40±0.20, Wald=4.1, df=1, p=.04). The association between SYM symptoms and poor medication response was most suggestive in subjects receiving clomipramine (PE=−0.64±0.33, Wald=3.7, df=1, p=.06) but not SSRI medication (PE=−0.28±0.25, Wald=1.3, df=1, p=.26). Neither CW (PE=0.01±0.170, Wald=0, df=1, p=.98) nor MISC (PE=−0.06±0.20, Wald=0.09, df=1, p=.76) symptoms were associated with a differential response to SRIs medication.
Fig. 1.
Proportion of response to SRI based on CGI score and divided according to symptom dimension. OCD symptom dimensions: CW: cleaning/contamination; AGG/SR=fear of harm, sexual and religious obsessions and checking compulsions; HRD: hoarding and SYM: ordering, symmetry and arranging obsessions and compulsions.
3.3. Dimensional association with subject demographics
We found that male gender was associated with having increased OC symptoms in the AGG/SR dimension (β=0.38± 0.18, Wald=4.3, df=1, p=0.039). When AGG/SR dimension was divided into two separate dimensions, only SR symptoms (β=0.97±0.28, Wald=12.2, df=1, p=0.001) and not AGG symptoms (β=−0.02±0.20, Wald=0.1, df=1, p=.91) were associated with male gender. Later age of onset of OCD symptoms was associated with CW symptoms (β=1.48±0.54, t=2.8, df=1, p=.007).
3.4. Dimensional associations with comorbid psychiatric illness
When analyzing the association between lifetime history of psychiatric disorders and OC symptom dimensions, no associations were found with Major Depression, Anxiety Disorders, Eating Disorders and Substance Abuse. However, the presence of a comorbid Tic Disorder was associated with increased symptoms in the SYM (β=0.61±0.31, Wald=3.9, df=1, p=0.05).
4. Discussion
We found that OC symptoms in the AGG/SR symptom dimension were associated with good response to SRIs in accordance to our a priori hypothesis. Sixty percent of OCD patients with predominant symptoms in the AGG/SR dimension were very much improved in response to SRI treatment. Although no previous studies have demonstrated a significant association between AGG/SR OC symptoms and response to pharmacotherapy, there has been some evidence suggesting that this might be the case. A recent factor analysis study in the OCD Consortium group showed there was a trend-level association between good response to SRI pharmacotherapy and symptoms in the AGG/SR dimension (Nestadt et al., 2000). AGG OC symptoms have also been associated with good long-term outcome in the Brown Longitudinal OCD study (Eisen et al., 2006). It should be noted that our results differed from Mataix-Cols et al. (1999), which used similar methodology, but failed to show an association between SRI response and symptoms in either the AGG or SR symptom dimensions (Mataix-Cols et al., 1999). When stratifying by type of pharmacological agent, we found a significant association between the AGG/SR OC symptom dimension and a good pharmacological response in patients treated with SSRIs (fluoxetine and fluvoxamine), but not clomipramine. There are two possible explanations for this finding — (1) there is a better response to SSRIs within the AGG/SR OC symptom dimension or (2) this finding is due to type I error.
There exists significant basic science and clinical evidence to suggest that the former explanation may be correct. Studies measuring serotonin metabolite levels (5-HIAA) in psychiatric patients have associated low serotonergic brain activity with hostile mood and aggressive behavior (Knutson et al., 1998). When SRI’s are given to individuals without psychiatric illness they have been demonstrated to decrease negative affect and hostile tendencies (Knutson et al., 1998). Patients with post-traumatic stress disorder (PTSD) not only have flashbacks and aggressive behavior, but they have a heightened sense of threat, similar to what may be seen in OCD patients with symptoms in the AGG dimension. SSRIs are currently the first-line pharmacological intervention for PTSD (Cassano and D’Mello, 2001; Walsh and Dinan, 2001). It is logical to hypothesize that SSRI medications may be particularly effective in reducing the symptoms of OCD patients with AGG/SR symptoms because they are additionally effective in reducing hostile tendencies and threat perception.
Given the small number of subjects reporting HRD symptoms we were unable to replicate previous studies that have associated HRD OC symptoms with poor pharmacological treatment response. The negative result obtained is likely attributable to our very limited power to detect this difference. We had only 5 subjects with hoarding as a main OC symptom and only 18 subjects who reported any hoarding symptoms.
It is important to note some other limitations of this study. First, given the relatively low power and high probability of type I error of existing studies in this area more studies involving larger number of subjects and future meta-analysis are needed to confirm the finding vis a vis AGG/SR and SRI treatment response. Also, we combined data from individuals receiving unknown (double-blind) or known (open label extension period) active treatment as part of this study to increase our statistical power. Lastly, we used CGI scores to measure clinical outcome rather than Y–BOCS Scale.
In our exploratory analyses, the SYM OCD symptoms were associated with a poor response to SRI treatment. Symptoms in the ordering/symmetry dimension were also associated with the presence of a comorbid tic disorder. This result is not surprising as several previous studies have demonstrated that tic disorders are associated with ordering and symmetry OCD symptoms (Leckman et al., 2001; Mataix-Cols et al., 1999). The presence of a tic disorders has been associated with poor response to SRI pharmacotherapy in previous studies(Leckman et al., 1994).
The development of OCD severity scales specific to these symptom dimensions should facilitate these efforts (Rosario-Campos et al., 2006). Further studies are needed to extend genetic, neuroanatomical understanding of these quantitative phenotypes so that a better understanding of the heterogeneous symptoms of OCD. Understanding further the differences in treatment response in OCD patients presenting with different symptoms may help us to provide better treatments and more accurate prognostic information to them.
Acknowledgements
We wish to acknowledge the support and mentorship from the APA/NIMH Psychiatry Minority Research Training Program (ALW). We also wish to acknowledge the support of the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (MHB), the National Institutes of Health Loan Repayment Program (MHB, VC), the support of the Tourette’s Syndrome Association Inc. (MHB), the support of the Obsessive Compulsive Foundation (VC), the National Alliance for Research on Schizophrenia and Depression Young Investigator Award 2005 (VC), and the support of the APA/Janssen Research Scholars Program (MHB), and the AACAP Pilot Research Award (MHB).
Role of funding source
No funding conflicts.
Footnotes
Conflict of interest The authors have no conflict of interests to report.
References
- Alonso P, Menchon JM, Pifarre J, Mataix-Cols D, Torres L, Salgado P, Vallejo J. Long-term follow-up and predictors of clinical outcome in obsessive–compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J. Clin. Psychiatry. 2001;62:535–540. doi: 10.4088/jcp.v62n07a06. [DOI] [PubMed] [Google Scholar]
- Baer L. Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J. Clin. Psychiatry. 1994;55(Suppl):18–23. [PubMed] [Google Scholar]
- Berrios GE. Our knowledge of anancasm (psychic compulsive states) Hist. Psychiatry. 2003;14:113–128. doi: 10.1177/0957154X03014001007. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive–compulsive disorder. Am. J. Psychiatry. 2008;165:1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano WJ, Jr., D Mello AP. Acute stress-induced facilitation of the hypothalamic–pituitary–adrenal axis: evidence for the roles of stressor duration and serotonin. Neuroendocrinology. 2001;74:167–177. doi: 10.1159/000054683. [DOI] [PubMed] [Google Scholar]
- Cavallini MC, Di Bella D, Siliprandi F, Malchiodi F, Bellodi L. Exploratory factor analysis of obsessive–compulsive patients and association with 5-HTTLPR polymorphism. Am. J. Med. Genet. 2002;114:347–353. doi: 10.1002/ajmg.1700. [DOI] [PubMed] [Google Scholar]
- Clomipramine_Collaborative_Study_Group. The clomipramine collaborative study group Clomipramine in the treatment of patients with obsessive–compulsive disorder. Arch. Gen. Psychiatry. 1991;48:730–738. doi: 10.1001/archpsyc.1991.01810320054008. [DOI] [PubMed] [Google Scholar]
- Delorme R, Bille A, Betancur C, Mathieu F, Chabane N, Mouren-Simeoni MC, Leboyer M. Exploratory analysis of obsessive compulsive symptom dimensions in children and adolescents: a prospective follow-up study. BMC Psychiatry. 2006;6:1. doi: 10.1186/1471-244X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, de Geus F, van Megen HJ, Westenberg HG. Symptom dimensions in obsessive–compulsive disorder: factor analysis on a clinician-rated scale and a self-report measure. Psychopathology. 2004;37:181–189. doi: 10.1159/000079509. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Greenberg BD, Mancebo M, Pinto A, Rasmussen S, Marsland R, Orphanides A, Dyck I, Stout R. OCD Subtypes. American Psychiatric Association; Toronto, CA: 2006. [Google Scholar]
- Feinstein SB, Fallon BA, Petkova E, Liebowitz MR. Item-by-item factor analysis of the Yale–Brown Obsessive Compulsive Scale Symptom Checklist. J. Neuropsychiatry. Clin. Neurosci. 2003;15:187–193. doi: 10.1176/jnp.15.2.187. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The Obsessive–Compulsive Inventory: development and validation of a short version. Psychol. Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Delgado PL, Heninger GR, Charney DS. Efficacy of fluvoxamine in obsessive–compulsive disorder. A double-blind comparison with placebo. Arch. Gen. Psychiatry. 1989;46:36–44. doi: 10.1001/archpsyc.1989.01810010038006. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Kozak MJ, Liebowitz M, White KL. Treatment of obsessive–compulsive disorder with fluvoxamine: a multicentre, double-blind, placebo-controlled trial. Int. Clin. Psychopharmacol. 1996;11:21–29. [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health; Rev. Rockville, Md.: 1976. pp. 76–338. DHEW publication no. (ADM. [Google Scholar]
- Hantouche EG, Lancrenon S. Modern typology of symptoms and obsessive–compulsive syndromes: results of a large French study of 615 patients. Encephale. 1996;22(1):9–21. Spec No. [PubMed] [Google Scholar]
- Holzer JC, Goodman WK, McDougle CJ, Baer L, Boyarsky BK, Leckman JF, Price LH. Obsessive–compulsive disorder with and without a chronic tic disorder. A comparison of symptoms in 70 patients. Br. J. Psychiatry. 1994;164:469–473. doi: 10.1192/bjp.164.4.469. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, Terpstra J, Turner RA, Reus VI. Selective alteration of personality and social behavior by serotonergic intervention. Am. J. Psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Grice DE, Barr LC, de Vries AL, Martin C, Cohen DJ, McDougle CJ, Goodman WK, Rasmussen SA. Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety. 1994;1:208–215. [PubMed] [Google Scholar]
- Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, Alsobrook J, Peterson BS, Cohen DJ, Rasmussen SA, Goodman WK, McDougle CJ, Pauls DL. Symptoms of obsessive-compulsive disorder. Am. J. Psychiatry. 1997;154:911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Alsobrook JP, Pauls DL. Symptom dimensions in obsessive–compulsive disorder: toward quantitative phenotypes. Am. J. Med. Genet. 2001;105:28–30. [PubMed] [Google Scholar]
- Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK, Pakstis AJ, Alsobrook JP, Robertson MM, McMahon WM, Walkup JT, van de Wetering BJ, King RA, Cohen DJ. Obsessive–compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;116:60–68. doi: 10.1002/ajmg.b.10001. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive–compulsive disorder. Am. J. Psychiatry. 1999;156:1409–10416. doi: 10.1176/ajp.156.9.1409. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Rauch SL, Baer L, Eisen JL, Shera DM, Goodman WK, Rasmussen SA, Jenike MA. Symptom stability in adult obsessive–compulsive disorder: data from a naturalistic two-year follow-up study. Am. J. Psychiatry. 2002;159:263–268. doi: 10.1176/appi.ajp.159.2.263. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive–compulsive disorder. Am. J. Psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- McKay D, Piacentini J, Greisberg S, Graae F, Jaffer M, Miller J. The structure of childhood obsessions and compulsions: dimensions in an outpatient sample. Behav. Res. Ther. 2006;44:137–146. doi: 10.1016/j.brat.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, III, Liang KY, Hoehn-Saric R, Cullen B, Grados M, Beaty TH, Shugart YY. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive–compulsive disorder and for heterogeneity by sex. Am. J. Hum. Genet. 2000;67:1611–1616. doi: 10.1086/316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Green RC, Jenike MA, Mesulam MM. Clinical comparison of Tourette’s disorder and obsessive–compulsive disorder. Am. J. Psychiatry. 1987;144:1166–1171. doi: 10.1176/ajp.144.9.1166. [DOI] [PubMed] [Google Scholar]
- Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, Katsovich L, Scahill L, King RA, Woody SR, Tolin D, Hollander E, Kano Y, Leckman JF. The Dimensional Yale–Brown Obsessive–Compulsive Scale (DY–0BOCS): an instrument for assessing obsessive–compulsive symptom dimensions. Mol. Psychiatry. 2006;11:495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- Rufer M, Grothusen A, Mass R, Peter H, Hand I. Temporal stability of symptom dimensions in adult patients with obsessive–compulsive disorder. J. Affect. Disord. 2005;88:99–102. doi: 10.1016/j.jad.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Samuels J, Bienvenu OJ, III, Riddle MA, Cullen BA, Grados MA, Liang KY, Hoehn-Saric R, Nestadt G. Hoarding in obsessive compulsive disorder: results from a case–control study. Behav. Res. Ther. 2002;40:517–528. doi: 10.1016/s0005-7967(01)00026-2. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Baxter LR., Jr. Paroxetine treatment of compulsive hoarding. J. Psychiatr. Res. 2007;41:481–487. doi: 10.1016/j.jpsychires.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Andersen EW, Overo KF. Response of symptom dimensions in obsessive–compulsive disorder to treatment with citalopram or placebo. Rev. Bras. Psiquiatr. 2007;29:303–307. doi: 10.1590/s1516-44462007000400003. [DOI] [PubMed] [Google Scholar]
- Summerfeldt LJ, Richter MA, Antony MM, Swinson RP. Symptom structure in obsessive–compulsive disorder: a confirmatory factor-analytic study. Behav. Res. Ther. 1999;37:297–311. doi: 10.1016/s0005-7967(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Tek C, Ulug B. Religiosity and religious obsessions in obsessive–compulsive disorder. Psychiatry Res. 2001;104:99–108. doi: 10.1016/s0165-1781(01)00310-9. [DOI] [PubMed] [Google Scholar]
- Walsh MT, Dinan TG. Selective serotonin reuptake inhibitors and violence: a review of the available evidence. Acta Psychiatr. Scand. 2001;104:84–91. doi: 10.1034/j.1600-0447.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Zohar AH, Ratzoni G, Pauls DL, Apter A, Bleich A, Kron S, Rappaport M, Weizman A, Cohen DJ. An epidemiological study of obsessive–compulsive disorder and related disorders in Israeli adolescents. J. Am. Acad. Child Adolesc. Psych. 1992;31:1057–1061. doi: 10.1097/00004583-199211000-00010. [DOI] [PubMed] [Google Scholar]