SUMMARY
The ability to inhibit the activity of maternally stored gene products in Xenopus has led to numerous insights into early developmental mechanisms. Oocytes can be cultured and manipulated in vitro and then implanted into the body cavity of a host female to make them competent for fertilization. Here, we summarize the methods for obtaining, culturing and fertilizing Xenopus oocytes, with the goal of inhibiting maternal gene function through antisense oligonucleotide-mediated mRNA knockdown. We describe a simplified technique for implanting donor oocytes into host females using intraperitoneal injection. Also, we present optimized methods for performing the host-transfer procedure with X. tropicalis oocytes.
Keywords: Xenopus laevis, Xenopus tropicalis, maternal genes, antisense, host-transfer, oocyte, oligonucleotides
1. Introduction
Maternal stores of mRNAs and proteins accumulated during oogenesis control many early developmental events in Xenopus. In some cases, these gene products are localized to different regions of the egg and can be differentially inherited during cleavage to influence early cell fate decisions. Zygotic transcription in Xenopus does not begin until the mid-blastula stage, about 8 hours after fertilization (1, 2), and therefore maternal factors are particularly important for events occurring prior to this mid-blastula transition (MBT). These processes include dorsoventral axis specification, germ layer induction and specification of germline fate (reviewed in Ref. 3). Because these processes are initiated soon after fertilization, it can be problematic to interfere with the underlying molecular pathways by microinjecting reagents into fertilized eggs, as is commonly done in Xenopus. Maternal effect mutants have proven useful for studying early events in invertebrates, but these mutants are more difficult to generate in vertebrates, owing to longer life cycles and more complex genomes. Recently, several maternal effect mutants have been identified in zebrafish (4, 5), and screens are underway for maternal mutants in Xenopus tropicalis, a diploid relative of the allotetraploid X. laevis. However, these methods are labor and cost intensive, precluding a routine assessment of maternal gene function in most vertebrates.
Fortunately, it is possible to readily study the activity of maternally stored gene products in Xenopus using the injection of antisense oligodeoxynucleotides (oligos) into oocytes to deplete maternally stored mRNAs (6-8). Antisense is a particularly successful strategy to assess maternal function in Xenopus because of several main advantages. First, oocytes can be easily obtained and manipulated in culture, something not readily done with oocytes from most species. Second, the biochemical makeup of oocytes favors the degradation of mRNAs in response to antisense oligo injection, as opposed to steric blockage of translation or stimulation of innate immune responses. Stage VI oocytes have endogenous RNase H-like activity (9, 10), therefore injection of DNA oligos results in cleavage and destruction of target mRNA strands of the resulting DNA:RNA hybrids. This leaves no doubt as to the mechanism of antisense action, which is not always clear in other antisense experiments. Third, and most important, the embryological outcome of maternal mRNA depletion can be assessed, since oocytes can be cultured through meiosis in vitro and then fertilized to initiate development (11-13). Last, the effects of maternal depletion persist at least until MBT, since zygotic mRNAs will not be resynthesized until then.
The main obstacle to using oligo-injected oocytes for studies of embryonic development is that ovarian oocytes are not directly competent for fertilization. Normally, ovulated eggs require proteolytic processing of the vitelline membrane and investment by jelly coats to attract and activate sperm and to facilitate sperm-egg binding. Methods exist to mimic the sperm activation process in vitro with jelly coat extracts (14, 15), and to bypass fertilization entirely using nuclear transfer or intracytoplasmic sperm injection (16, 17). Although these methods can successfully trigger cleavage and normal development from oocytes, their use in conjunction with antisense experiments has not been widely reported in the literature.
More commonly, oligo-injected oocytes are transferred into the body cavity of a host female to facilitate fertilization. In the coelomic cavity, the transferred eggs (along with normally ovulated host eggs) are moved by the action of peritoneal cilia into the oviducts (18), whereupon they acquire the necessary modifications for fertilization prior to egg laying. After the experimental eggs are recovered, they can be fertilized by normal methods to generate embryos lacking the mRNA of interest. The unusual robustness of Xenopus oocytes and adult animals to manipulation allows this procedure to be performed with a high rate of success. Thus, these methods allow maternal gene function in Xenopus to be analyzed without generating maternal effect mutations, something not easily done in other vertebrates.
Here we describe methods for obtaining, culturing and injecting Xenopus oocytes with antisense DNA oligos to deplete maternal mRNAs, and for fertilizing these oocytes to obtain viable embryos. We detail a method for transferring in vitro cultured oocytes into host females by intraperitoneal injection. We also outline adaptations to the well-characterized host-transfer methods for Xenopus laevis for use with X. tropicalis oocytes.
2. Materials
Prepare all solutions using deionized, ultrapure water and clean glassware, free from detergent residue. Also, use high quality and recently ordered reagents.
2.1 Surgery and oocyte collection
Buffered MS222 (Ethyl 3-aminobenzoate methanesulfonate salt): 1.0 g/L MS222, 0.7 g/L sodium bicarbonate, adjust final pH to ~7.0.
OCM (Oocyte Culture Media): 70% Liebovitz L-15, 0.004% BSA, 1 × Pen-Strep, 0.5 μg/ml gentimicin): adjust pH to 7.6-7.8 with NaOH, make fresh and store at 14-18°C for up to one week.
Surgical and dissection instruments (Fine Science Tools). Scalpel handle (#3) and blade (#10 or 11), several pairs of Dumont forceps (#4 or #5, Biologie), Bonn iris scissors (curved or straight), Halsey or Olsen-Hegar microneedle holders.
4-0 PDS II sutures: Violet monofilament with 17mm ½ circle needle, RB-1 taper (Ethicon). 5-0 sutures can be used for Xenopus tropicalis surgeries (Ethicon).
Frog water: tap water treated with Amquel (chloramine remover).
Povidone-iodine USP (10% solution; Betadine ® or equivalent): dilute 1:20 in distilled water before use.
2.2. Oocyte transfer and collection of embryos
Progesterone: 10 mM stock in 100% ethanol, dilute to 1 mM in 100% EtOH to make a 500 × working stock solution, store at −20°C.
Human Chorionic Gonadotropin (hCG): 10,000 IU/vial, reconstitute with 10 ml sterile water, store at 4°C up to 30 days.
- Vital Dyes: Stocks of Blue, Red and Brown are made up in 50 ml deionized water, incubated for 20 minutes with rocking and spun in a clinical centrifuge. Aliquots (~1 ml) are taken from the supernatants and stored at −20°C.
- 0.1% Nile Blue A: 0.05 g/50 mL
- 0.25% Neutral Red: 0.125 g/50 mL.
- 1% Bismarck Brown: 1.0 g/50 mL.
2 ml glass syringe (Tomopal Inc.), with metal luer lock adaptor.
16 gauge, 1 inch, sterile syringe needle.
10 × Marc’s Modified Ringers (MMR): 1 M NaCl, 18 mM KCl, 20 mM CaCl2, 10 mM MgCl2, 150 mM Hepes, adjust to pH 7.8 with 5N NaOH, filter sterile and store at 4° C, readjust pH to 7.6-7.8 when making 1 x or 1.2 x (high-salt) solutions are made, dilutions of 0.3 x, 0.1 x and 0.05 x are made by diluting the 1 × stock.
2% cysteine in 0.1 × MMR, pH to ~7.8, check with pH paper.
Teflon pestles for 1.5 ml microfuge tubes (Kimble-Kontes).
3. Methods
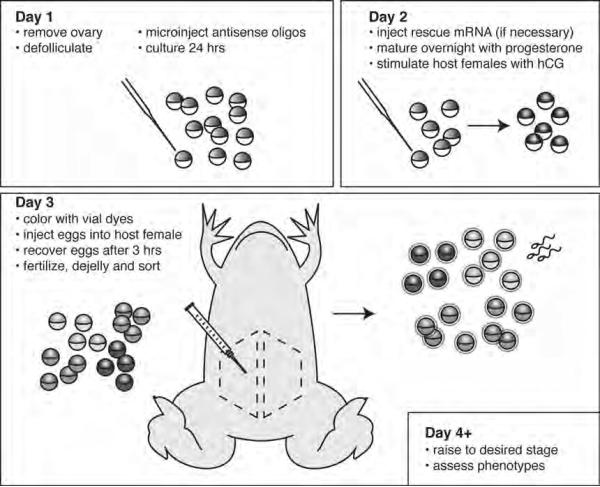
Carry out all procedures at room temperature unless otherwise specified. Be sure to follow all relevant animal care and use guidelines. See Fig. 1 for a graphical overview and general timeline of the procedure.
Fig. 1. Outline of the host-transfer procedure by intraperitoneal injection.
On day one, healthy ovary is removed and oocytes are defolliculated. Oocytes are then injected with mRNAs or with antisense oligonucleotides targeting the endogenous mRNA of the candidate gene. On the second day, rescue mRNA is injected (if necessary), the oocytes are matured with progesterone and prospective host females are induced with hCG. On the third day, the oocytes are colored with vital dyes, injected into an egg-laying host female. Once the eggs are released, they are fertilized, sorted from host eggs and analyzed as desired. The dotted areas indicate optimal regions in the abdomen for inserting the experimental oocytes.
3.1. Surgical isolation of ovarian tissue
Ovary is obtained surgically from anesthetized females. In most cases, survival surgery is performed so that ovary is removed from the same female multiple times. Survival surgeries should be done using aseptic technique (see Note 1).
Prepare fresh OCM media prior to obtaining ovary.
Prepare the surgical area and sterilize the instruments. Disinfect a dedicated surgical area using 70% EtOH. Place the tips of the instruments in a bead sterilizer at 250°C for 20 seconds and then place on a sterile surface, such as a Petri dish, sterile gauze or the inside of a suture package.
Select several females dedicated for ovary removal and place one in buffered MS222 anesthetic. A surgical plane of anesthesia will be reached in about 10 minutes. This can be checked by turning the frog onto her back or by pinching a toe. Anesthetized animals will be unresponsive. Frogs that are not fully anesthetized are placed back into MS222 and checked at five-minute intervals until anesthesia is complete. Females are operated one at a time until acceptable ovary is found.
Place the anesthetized female on her back on a damp wipe and cover the head and legs. Pour a small amount of diluted povidone-iodine (1:20 in distilled water) over the abdomen and gently blot with gauze (see Note 2).
Make a small (1 cm) incision in the skin in the lower part of the abdomen using small iris scissors. Incisions on the same frog should be performed on alternate sides, and can be made either parallel or perpendicular to the midline. The midline itself should be avoided, owing to the presence of the xxx artery. Use forceps to grasp the underlying muscle, pull upward and incise smartly to expose the body cavity. The ovary should be visible or may be found following careful exploration using blunt forceps.
The ovary has about 24 lobes, each containing hundreds of oocyte follicles (19). Pull several of the lobes through the incision and trim away at the level of the body wall. Place the tissue into a large (100 mm) Petri dish of OCM. Repeat until the desired amount is obtained. Six or eight large lobes are sufficient for a typical experiment (see Note 3).
Close the muscle and fascia layer first with simple interrupted suturing, using basic instrument ties to place surgeon’s square knots (also called reef knots) several millimeters apart. After each suture, trim the material to leave a short tail before proceeding to the next suture. Once the body wall layer is secure, proceed to close the skin in a similar manner (see Note 4).
Rinse the frog in water and place in a shallow recovery bucket. The container can be inclined so the water does not cover the frog’s nostrils. Cover the container and allow the animal to recover before moving her to a larger recovery bucket. Recovery is indicated by response to touch, eye bulging and eventually purposeful movements. Return to the colony after the frog is fully recovered.
Return to the dish of ovary. Cut the ovary into small pieces using sterile iris scissors. To subdivide the ovary, first cut open one side of an individual lobe, and flatten out the tissue. The ovary is then cut into small pieces, about 2 cm2, and pieces are transferred to a dish of clean OCM, keeping about six pieces per dish. Dividing the ovary in this way extends the life of the tissue in culture and makes defollicating a bit easier (see below). Culture up to 4-5 days in OCM at 18°C.
3.2. Isolation, culture and microinjection of oocytes
Oocytes for host-transfer must be manually defolliculated since collagenased oocytes cannot be fertilized (20). Select only healthy, fully grown stage VI oocytes (1.2-1.3 mm diameter). These oocytes will have a uniformly pigmented animal hemisphere and may show a distinct equatorial band of lighter pigmentation. An ideal ovary will have > 50 such oocytes per small ovary piece.
To begin manually defolliculating oocytes, select a piece of ovary and transfer to a separate dish of OCM. Grasp the connective tissue theca layer near a desired oocyte with forceps, using the non-dominant hand. Using a second pair of forceps, lightly grasp the ovary adjacent to the oocyte and tear apart the theca layer and continue pulling away from the ovary (see Note 5). The oocyte will be squeezed out between the follicle layers and can be teased gently away.
Successfully defolliculated oocytes will be somewhat flaccid and will lack blood vessels, which are part of the theca layer. Properly defolliculated oocytes can be easily microinjected, whereas oocytes remaining in their follicles are quite tough (see Note 6). Experienced operators can easily defolliculate 200-400 oocytes per hour. Transfer groups of defolliculated oocytes to medium (60 mm) Petri dishes using a sterile Pasteur pipette. Culture groups of 75-200 oocytes at 18°C (see Note 7).
Microinject oocytes with antisense oligos or RNAs as desired. The oocytes can be injected directly while in OCM. Typical injection volumes are 5-10 nl, delivering doses of 2-6 ng for DNA-based oligos, 5-100 ng for morpholinos or 20 pg- 1 ng for mRNA. To affect localized molecules it may be necessary to target injections to the appropriate region of the oocyte.
Injected oocytes are transferred to dishes containing 8 ml of fresh OCM and cultured at 18°C. Because of limitations on the number of colors that can be made using vital dyes (see below), only five-to-six groups (~ 75-200 oocytes each) can be transferred per female, so experiments must be planned with this in mind. Oocytes injected with DNA-based oligos should be cultured for at least 24 hours to allow for degradation of the oligo before proceeding with the host-transfer. Culture can be extended to 72 hours without affecting oocyte viability or developmental capacity of the resulting embryos.
If rescue experiments are being performed, inject the desired mRNA after the oligo has had time to degrade (~24 hours). It is important for correctly interpreting the specificity of the oligo that the mRNA does not compete for binding to the oligo, but rather replace the function of the knocked-down protein. RNAs are typically injected the evening before the transfer is to be performed, irrespective of the length of oligo incubation.
3.3. Preparation of donor oocytes and host females for transfer
On the evening before the transfer, usually the day after oligos are injected, oocytes are stimulated to undergo maturation by addition of progesterone to a final concentration of 2 μM (16 μl of 1 mM stock/8 ml OCM). The dishes are swirled briefly and returned to 18°C overnight.
Around the same time, inject 3-5 females with hCG (1000 U) to induce ovulation. Females are placed in 18°C water and left at room temperature overnight. Host transfer experiments work best in our hands if oocyte maturation and hCG injection are done about 10-12 hours prior to oocyte implantation. Multiple females are injected to ensure that at least one will have suitable egg quality to serve as the host (see step 5 below).
The next morning, check that the oocytes have undergone maturation (look for germinal vesicle breakdown (GVBD)) and that the females have begun to lay eggs. Matured oocytes can be frozen on dry ice at this point for verification of mRNA or protein knockdown.
Vital dye stocks are thawed and spun for 2-3 minutes (see Note 8). Oocytes are stained with vital dyes by adding 80 μl of stock to each dish, followed by gentle swirling. When adding multiple dyes, add 80 μl of each and swirl to mix in the dish. Oocytes are incubated with rocking for 15 minutes, transferred to a large dish of OCM to wash until implantation.
While the oocytes are staining, choose a host female and place in buffered MS222 anesthetic. An ideal host female will have just started laying healthy eggs, and can be induced to release more eggs upon gentle squeezing. Females that are laying stringy eggs or that crush eggs upon squeezing should be avoided.
Place the anesthetized female on her back on a damp wipe. Cover the head and legs as above and gently swab with diluted povidone iodine.
3.4. Oocyte transfer by intraperitoneal injection
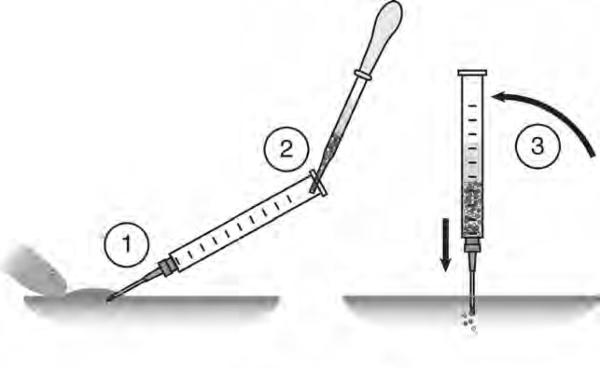
Oocytes can be transferred into the body cavity of the host by surgical implantation. Recently, our lab has devised a method to transfer oocytes by intraperitoneal (IP) injection, using a large-bore hypodermic needle. Since the surgical method has been described previously (7, 21), the IP injection method will be described here (see Note 9). An overview of the method is shown in Fig. 2.
Attach a new 16-gauge, 1” disposable needle (16G1) onto a 2 ml glass syringe fitted with a metal luer lock tip. Remove the plunger and set aside (it will not be used for injection). Rinse the inside of the syringe with OCM to coat the surfaces with BSA (in the OCM), reducing sticking of oocytes.
Holding the syringe at a 45° angle to the frog (vertically), with the beveled edge of the needle up, insert the tip into the lower abdomen of the host female, passing through both skin and muscle. Orient the needle toward the anterior. Placing a finger just above the injection site may be necessary to provide some support, allowing the needle to insert smoothly. The needle will have passed through both layers when resistance against the needle can no longer be felt. Do not penetrate too deeply into the body cavity.
Once the needle is inserted, hold the syringe steady at a 45° angle with one hand. Slowly introduce the colored oocytes into the upper part of the syringe using a Pasteur pipette. Add the oocytes against the side and let them drop to the bottom. They should collect in a ~1 ml volume of OCM, but should not enter the needle while the syringe is still inclined.
When all the oocytes have been added to the syringe, tilt the syringe upright, keeping the needle in the frog. Tap gently on the side of the syringe and the oocytes will begin draining into the body cavity. Adjust the needle depth up and down if oocytes do not flow right away. Flush with OCM if some oocytes become stuck around the edge of the syringe port. Excess OCM, up to 2 ml or so does not seem to be detrimental.
Return the syringe to a 45° angle and withdraw the needle. Oocytes should not spill out of the insertion site, although some OCM may drain out. Very little bleeding should occur. The female is rinsed in distilled water to wash off MS222 and placed immediately into a recovery bucket of water at 18°C. Suturing or other means of closing the wound are not necessary.
Monitor the host’s recovery from anesthesia as above. She should resume laying eggs soon after the procedure. Body cavity eggs and the implanted oocytes will be translocated by peritoneal cilia to the openings to the oviducts and the host should begin laying colored eggs 2-3 hours after implantation. The female can be gently squeezed if colored eggs do not appear by 3-4 hours. Rarely, the experiment can be lost if the host stops laying altogether, since it is problematic to recover the transferred eggs from the oviducts.
Fig. 2. Details of the intraperitoneal injection technique.
(1) A 16-gauge needle is inserted into the body cavity at a 45° angle. The skin is braced by a finger. (2) The colored oocytes are back-loaded into the syringe, adding a minimal amount of excess OCM. (3) The oocytes will drain into the body cavity when the needle is brought vertical. Some gentle tapping or needle depth readjustment may be necessary to get the oocytes to flow. Figure shows a lateral view of the ventral surface of the abdomen, anterior is to the left.
3.5. Recovery of experimental oocytes and in vitro fertilization
About one hour after implantation, place the host female in a container of 1L high salt MMR (1.2 x MMR, pH 7.6). A 4 L Nalgene beaker is a convenient vessel for this purpose. Eggs released into high salt will remain competent for fertilization for many hours.
In the meantime (or beforehand) obtain testes from a male frog through non-survival surgery. Keep testes in OCM at room temperature throughout the day, and at 4°C for longer-term storage.
Once colored eggs appear in the bucket, the female can be squeezed if desired. Eggs are squeezed into a dry Petri dish and the female is returned to the high salt MMR. Eggs are fertilized with a sperm suspension made in 1 × MMR (or L15) for 4 minutes. The eggs are then flooded and rinsed with 0.1 × MMR and left to develop. It is often convenient to dejelly and sort cleaving colored eggs at the 4-cell stage (see Note 10).
After the female has stopped laying (or after about 5 hours), the eggs in high salt can be fertilized. Gently squeeze out any remaining eggs and carefully drain most of the high salt buffer. Rinse the eggs once in 0.3 × MMR and carefully drain again. The eggs are transferred to a Petri dish and as much of the remaining buffer as possible is removed with a transfer pipette.
Fertilize the eggs by homogenizing half a testis in a microfuge tube using a Teflon pestle, in a volume of 500 μl 0.3 × MMR. Add the homogenate to the eggs and swirl to mix thoroughly. After 10 minutes, flood and rinse with 0.1 × MMR. Colored eggs are dejellied and sorted as above and left to develop to the desired stages for analysis (Fig. 3).
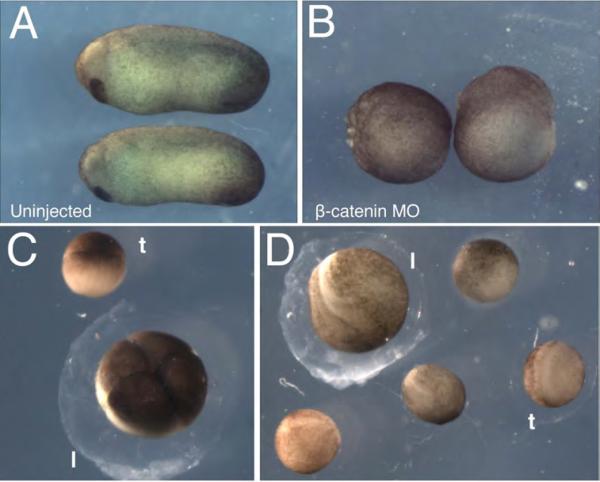
Fig. 3. Representative results of host transfers using X. laevis and X. tropicalis oocytes.
(A-B) Maternal knockdown of β-catenin. Oocytes injected with 10 ng of β-catenin MO were matured and fertilized via the host-transfer method, as in ref. (23). (A) Uninjected and (B) MO-injected embryos at stage 25 demonstrate the knockdown phenotype. Uninjected embryos develop normally while knockdown embryos are ventralized (100%, n=28). (C-D) Representative results of X. tropicalis oocytes fertilized following host-transfer into X. laevis females. (C) X. tropicalis embryo at the 4-cell stage (t) shown next to a host X. laevis embryo (l). (D) X. tropicalis embryos at the neurula stage (t) shown next to a host X. laevis embryo (l).
3.6. Modifications for fertilization of cultured Xenopus tropicalis oocytes
Oocyte transfers using X. tropicalis oocytes can be performed either by implanting oocytes back into X. tropicalis hosts or by heterotopically transferring into X. laevis females. The cross-species transfers are possible because X. tropicalis sperm can penetrate X. laevis jelly coats (22). This cross-species method is preferable since transfers are easier to perform on the larger X. laevis females. Additionally, X. tropicalis females lay eggs in larger bursts with many more host eggs, and often stop laying before many experimental eggs are recovered. The basic methods of obtaining ovarian tissue and isolating oocytes are similar for Xenopus tropicalis, although the “trop” oocytes are somewhat more difficult to defolliculate. We have optimized the following changes in the timing of the procedure to facilitate transfer of X. tropicalis oocytes into X. laevis females.
Isolate ovary from X. tropicalis females using the general methods described above. Manually defolliculate fully-grown stage VI oocytes (these will be about 1/3 the size of X. laevis oocytes) and culture in OCM at 25°C. Microinject as desired.
On the evening prior to transfer, 3-5 X. laevis females are injected with hCG (1000 U) to induce ovulation. Females are placed in 18°C water and left at room temperature overnight. X. tropicalis oocytes do not need to be treated with progesterone at this time; this will be done the following morning.
The next morning, progesterone is added to the X. tropicalis oocytes to a final concentration of 2 μM (16 μl of 1 mM stock/8 ml OCM). The dishes are swirled briefly and cultured at 25°C for 2-4 hours. X. tropicalis oocytes mature faster than X. laevis oocytes, therefore this treatment can be done the same day as the transfer.
Once the oocytes have matured, vital dye stain them and anesthetize a suitable X. laevis host female, as described above.
Rinse the donor oocytes in fresh OCM and transfer into the X. laevis female by intraperitoneal injection as described above.
Squeeze or recover the oocytes in high salt MMR. The much smaller X. tropicalis eggs can be easily distinguished from the large X. laevis eggs. These are sorted into a separate Petri dish for fertilization using a wide-bore Pasteur pipette.
Rinse X. tropicalis eggs in 0.3 × MMR, decant well and fertilize with a sperm suspension, made by homogenizing a X. tropicalis testis in L15. Incubate for 10 minutes and then flood with 0.05 × MMR. Dejelly and sort dividing embryos and culture as desired (Fig. 3; Note 11). A variable number of fertilized host embryos, most likely interspecific hybrids, will also be generated by this method.
3.7. Use of antisense oligonucleotides in Xenopus oocytes
Among the uses for this procedure, one of the most powerful is the depletion of maternally stored gene products in the oocyte followed by analysis of resulting embryos. This is often necessary because interference with maternal gene function after fertilization is frequently ineffective. This section describes the basic procedure for designing and testing for suitable antisense oligonucleotides.
Antisense oligos against a given mRNA are designed by trial and error. Paste the 5’ half of the gene of interest into primer or oligo design software (IDT, Primer3, MacVector; see Note 12). Set the parameters to identify 18-20 mer antisense oligos, having a moderate GC content (40-60%) and a Tm of about 50-60°C. Exclude oligos with GGGG motifs or multiple GG or CC dimers, since these can form tetraplex structures linked to drastic non-specific effects.
Order 3-4 DNA oligos modified with three phosphorothioate linkages on the 5’ and 3’ termini (chimeric oligos). The modified bonds on the ends will provide a suitable amount of nuclease resistance, while minimizing the potential side effects of a fully thioate-modified oligo (see Note 13). Obtain the smallest scale possible from your preferred oligo provider (50-100 nmol) and have the oligos HPLC purified.
To hedge against possible targeting of undesired mRNA, the oligo sequences are screened against X. laevis or X. tropicalis databases using BLAST (http://www.xenbase.org/genomes/blast.do). Sequences that show large stretches (> 13 bases) of complementarity to other genes should be discarded and more oligos designed.
Oligos are dissolved in deionized water to a final concentration of 1 mM (~ 5-6 μg/μl), from which working stocks of 1μg/μl are made. Aliquots of the concentrated stocks and working stocks are stored at −80°C.
Oocytes for oligo screening are isolated as above. Test oligos are injected at several doses (e.g., 2 ng, 4 ng and 6 ng) and the oocytes are cultured overnight (~16 hours) at 18°C. Remember to centrifuge oligos prior to injection; especially since oligo preps are prone to clogging the needle.
Levels of mRNA depletion are assessed by RT-PCR (preferred) or other method of RNA analysis. Oocytes are placed into microfuge tubes in minimal liquid, frozen on dry ice and either stored at −80°C or processed immediately. Total RNA is isolated and single strand cDNA is prepared (23). Primers amplifying the target gene and a housekeeping gene are used and the degree of reduction in target gene expression relative to the control is determined. Realtime PCR is useful to obtain more quantitative data, but is not necessary to find usable oligos. Analysis of target protein levels by immunoblotting blotting can also be done, if a suitable antibody is available (23).
Oligos that are effective (≥ 80% reduction in target mRNA levels) can then be used right away in pilot experiments and a larger synthesis is ordered. Usually 1-2 suitable oligos can be identified after a round or two of this exercise (see Note 14).
Acknowledgements
This work was supported by NIH grant GM083999 (DWH) and The University of Iowa (DWH).
Footnotes
4. Notes
Guidelines for Xenopus survival surgery allow for five surgeries, followed by a sixth terminal procedure. Other surgical guidelines, such as the requirement for masks or maintenance of records, are regulated differently at different institutions, so be sure to check the guidelines established by your local animal regulatory committee.
Surgical scrubs containing detergents or ethanol are not appropriate for disinfecting the incision, as these will damage the sensitive skin of amphibians. Diluted iodine or chlorhexidine are acceptable.
Although the majority of females will have ovary suitable for these experiments, it is a good practice, however, to briefly inspect the ovary quality before proceeding. A small piece is removed initially and quickly examined under a dissecting microscope. Unhealthy ovary typically has many yellowish, speckled or dying/atretic oocytes, or has very few fully-grown stage VI oocytes. The presence of many oocytes undergoing resorption, which are heavily covered in blood vessels, is another indication of poor ovary quality. If the ovary is deemed unsuitable, the female is sutured right away and another is tried.
The suturing technique is documented in Schneider et al. (21), and numerous internet-based videos are also available to demonstrating proper suturing. Additionally, institutional animal care offices should provide instruction in surgical methods if requested.
Manual defolliculating is labor intensive, but is a skill in which most students can become skilled with some practice. It is necessary to use polished and sharpened watchmakers’ forceps. The tips should be properly shaped and must meet precisely at the tips. Forceps are first slightly blunted and sanded on a whetstone, followed by polishing with 800-grit sandpaper. When defolliculating, it is best to use extremely light pressure (just enough to close the tips) when tearing the theca; gripping too tightly generally results in pulling the oocyte away without actually removing the follicle layer.
Defolliculated oocytes should be free of marks and scratches, since even slightly damaged ones will not survive the host-transfer procedure. Also, perfectly manually defolliculated oocytes retain a single cell inner epithelial layer of follicle cells, which are intimately associated with the vitelline envelope. Although not important for the host-transfer procedure, this fact should be kept in mind when interpreting RT-PCR or imaging results using manually defolliculated oocytes.
Since the maturation response rate varies with oocytes from different females, it is good practice to perform a test of the maturation rate prior to microinjection. Early in the day, treat 20 or so oocytes with 2 μM progesterone and assess the number that undergo germinal vesicle breakdown (GVBD), about six hours later. If more than half the oocytes fail to mature, it may be wise to start again with new ovary.
The final vital dye concentrations are: Blue= 0.001% Nile Blue A, Red= 0.0025% Neutral Red, Brown= 0.01% Bismarck Brown. Five main colors are possible; each of the single colors plus Mauve (80μl Blue + 80 μl Red) and Green (80 μl Blue + 80 μl Brown). A sixth color can also be made, Orange (80 μl Brown + 80 μl Red), but this can be very difficult to distinguish from the either Brown or Red alone and is only used as a last resort.
IP injection is less invasive than typical abdominal surgery and the female is under anesthesia for much less time. Also, IP injection is not usually considered a “survival surgery” for the purposes of animal use protocols and thus may be used without incurring additional regulatory burden. For conventional surgical implantation, females are incised as for ovary removal, oocytes are introduced using a Pasture pipette and the incision is sutured. In this method, it is essential that the operator maintain a grip on one side of the muscle layer at all times after introducing the oocytes, up to the completion of the first suture. Failure to do so will result in oocytes leaking out of the incision.
The vital dye colors are most easily distinguishable when viewed from the animal pole at the 2-8-cell stage. Afterwards it is necessary to roll the embryos over to see the vegetal pole. This can be laborious if the colors are faint or if there are numerous embryos.
A variable number of host X. laevis eggs will fertilize and develop normally, apparently as interspecific hybrids.
Oligos can also be chosen by eye, but it is useful to run these through primer design programs to screen out oligos with undesirable motifs, self-dimerization, hairpin formation or unbalanced GC content.
It is also possible to test unmodified oligos, and then order modified version of successful ones. For this method, 10 or more oligos can be tested at 10 ng and 5 ng doses. Unmodified oligos that deplete more than 50% of the target mRNA are reordered in modified and purified form. Oligos should be retested, since phosphorothioate modification can alter the binding of the oligo.
For the best interpretation of experimental results, it is desirable to identify two oligos targeting independent sequences in the target (non-overlapping sites). Finding more than one oligo that produces the same phenotype at similar levels of target mRNA depletion is one way to show specificity of the knock-down. The other, preferred method is to rescue the phenotype by injecting doses of mRNA that do not elicit overexpression effects.
References
- 1.Bachvarova R, Davidson E. Nuclear activation at the onset of amphibian gastrulation. Journal of experimental zoology. 1966;163:285–295. [Google Scholar]
- 2.Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982;30:687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- 3.Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- 4.Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC. Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell. 2004;6:771–780. doi: 10.1016/j.devcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Wagner DS, Dosch R, Mintzer KA, Wiemelt AP, Mullins MC. Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell. 2004;6:781–790. doi: 10.1016/j.devcel.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Hulstrand AM, Schneider PN, Houston DW. The use of antisense oligonucleotides in Xenopus oocytes. Methods. 2010 doi: 10.1016/j.ymeth.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mir A, Heasman J. How the mother can help: studying maternal Wnt signaling by anti-sense-mediated depletion of maternal mRNAs and the host transfer technique. Methods Mol Biol. 2008;469:417–429. doi: 10.1007/978-1-60327-469-2_26. [DOI] [PubMed] [Google Scholar]
- 8.Torpey N, Wylie CC, Heasman J. Function of maternal cytokeratin in Xenopus development. Nature. 1992;357:413–415. doi: 10.1038/357413a0. [DOI] [PubMed] [Google Scholar]
- 9.Cazenave C, Chevrier M, Nguyen TT, Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987;15:10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dash P, Lotan I, Knapp M, Kandel ER, Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun R. Oocyte maturation in vitro: contribution of the oviduct to total maturation in Xenopus laevis. Experientia. 1975;31:1275–1276. doi: 10.1007/BF01945777. [DOI] [PubMed] [Google Scholar]
- 12.Holwill S, Heasman J, Crawley C, Wylie CC. Axis and germ line deficiencies caused by u.v irradiation of Xenopus oocytes cultured in vitro. Development. 1987;100:735–743. [Google Scholar]
- 13.Smith LD, Ecker RE, Subtelny S. In vitro induction of physiological maturation in Rana pipiens oocytes removed from their ovarian follicles. Dev Biol. 1968;17:627–643. doi: 10.1016/0012-1606(68)90010-9. [DOI] [PubMed] [Google Scholar]
- 14.Kloc M, Miller M, Carrasco AE, Eastman E, Etkin L. The maternal store of the xlgv7 mRNA in full-grown oocytes is not required for normal development in Xenopus. Development. 1989;107:899–907. doi: 10.1242/dev.107.4.899. [DOI] [PubMed] [Google Scholar]
- 15.Elinson R. Fertilization of frog body cavity eggs enhanced by treatments affecting the vitelline coat. J Exp Zool. 1973;183:291–302. [Google Scholar]
- 16.Amaya E, Kroll KL. A method for generating transgenic frog embryos. Methods Mol Biol. 1999;97:393–414. doi: 10.1385/1-59259-270-8:393. [DOI] [PubMed] [Google Scholar]
- 17.Schorderet-Slatkine S, Drury KC. Progesterone induced maturation in oocytes of Xenopus laevis. Appearance of a 'maturation promoting factor' in enucleated oocytes. Cell Differ. 1973;2:247–254. doi: 10.1016/0045-6039(73)90013-4. [DOI] [PubMed] [Google Scholar]
- 18.Rugh R. Ovulation in the frog. II. Follicular rupture to fertilization. J Exp Zool. 1935;71:163–194. [Google Scholar]
- 19.Dumont J. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972;136:153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- 20.Heasman J, Holwill S, Wylie CC. Fertilization of cultured Xenopus oocytes and use in studies of maternally inherited molecules. Methods Cell Biol. 1991;36:213–230. doi: 10.1016/s0091-679x(08)60279-4. [DOI] [PubMed] [Google Scholar]
- 21.Schneider PN, Hulstrand AM, Houston DW. Fertilization of Xenopus oocytes using the host-transfer method. J Vis Exp. 2010:e1864. doi: 10.3791/1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsay L, Peavy T, Lejano R, Hedrick J. Cross-fertilization and structural comparison of egg extracellular matrix glycoproteins from Xenopus laevis and Xenopus tropicalis. Comparative Biochemistry and Physiology-Part A: Molecular & Integrative Physiology. 2003;136:343–352. doi: 10.1016/s1095-6433(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 23.Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–34. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]