Summary
Chronic tuberculosis in an immunocompetent host is a consequence of the delicately balanced growth of Mycobacterium tuberculosis (Mtb) in the face of host defense mechanisms. We identify an Mtb enzyme (TdmhMtb) that hydrolyzes the mycobacterial glycolipid trehalose dimycolate and plays a critical role in balancing the intracellular growth of the pathogen. TdmhMtb is induced under nutrient limiting conditions and remodels the Mtb envelope to increase nutrient influx, but concomitantly sensitizes Mtb to stresses encountered in the host. Consistent with this, a ΔtdmhMtb mutant is more resilient to stress and grows to higher levels than wild-type in immunocompetent mice. By contrast, mutant growth is retarded in MyD88−/− mice indicating that TdmhMtb provides a growth advantage to intracellular Mtb in an immunocompromised host. Thus, the effects and counter-effects of TdmhMtb play an important role in balancing intracellular growth of Mtb in a manner that is directly responsive to host innate immunity.
Introduction
The global burden of tuberculosis (TB), which kills over a million people every year, is perpetuated by the vast majority of chronic and often asymptomatic infections with Mycobacterium tuberculosis (Mtb), estimated to be prevalent in about one third of the world’s population (WHO, 2012). Chronic infection with Mtb in an immunocompetent host is associated with controlled but persisting bacterial burden, established after an early phase of relatively rapid growth against the host-imposed antimicrobial activities (Cooper et al., 2011; Ernst, 2012; Lin et al., 2009; Stallings and Glickman, 2010). Furthermore, long-term infections of Mtb, even without clinical symptoms, are likely associated with a dynamic host-pathogen interaction. This is supported by evidence for active bacterial replication (Ford et al., 2011; Gill et al., 2009), continuous engagement of host immune system (Ulrichs et al., 2005), and the presence of drug-responsive lesions (Park et al., 2008) in chronic or latent TB. A question then arises as to what molecular mechanisms balance the interaction such that both the pathogen’s growth and the host inflammatory response are contained below a symptomatic threshold in an immunocompetent host. From the host perspective, a tightly regulated process of granuloma formation, involving optimum secretion of pro-inflammatory cytokines followed by recruitment of immune cells at the infection sites, has been implicated in containing the infection (Chan and Flynn, 2004; Ernst, 2012; Tobin et al., 2012; Yang et al., 2012a). It is further emerging that an optimum inflammatory response in macrophages that is high enough to trigger effective anti-bacterial activity, yet below the threshold of cellular necroptosis is most effective in restricting Mtb growth, implying that Mtb growth is most likely restricted in the intracellular environment (Roca and Ramakrishnan, 2013). Among the key antimicrobial intracellular factors are free radicals, low pH, antimicrobial peptides and digestive enzymes (Beutler, 2004).
From the pathogen’s perspective, long-term survival would involve successful adaptation to limiting nutrients in the intracellular environment while simultaneously resisting host-imposed antimicrobial activities. Nutrient acquisition and stress resistance are mechanistically distinct processes in bacteria. However, it is extremely likely that these pathways intersect at the cell envelope, which constitutes entry point for nutrients as well as antimicrobials in the environment. The mycobacterial envelope is stratified into a cytoplasmic membrane of phospholipids, a core cell wall of mycolylarabinogalactan-peptidoglycan (mAGP) complex, and a membrane-like outer layer called mycomembrane (MoM) (Brennan and Nikaido, 1995; Niederweis et al., 2010). This architecture is broadly similar to the gram-negative bacterial envelope, in which the lipid bilayers of inner and outer membranes are the two primary permeability barriers against environmental solutes (Nikaido, 2003). While solute-specific transporters facilitate the import of hydrophilic nutrients across the inner membrane, entry across the outer membrane is facilitated by either passive diffusion across lipid matrix or through the channel proteins, called porins (Niederweis, 2008; Nikaido, 2003). The charged amino-acids lining the water-filled channels of porins facilitate the entry of a broad spectrum of hydrophilic substrates from the environment (Nikaido, 2003). Four porins (MspA-D) have been identified in the non-pathogenic mycobacterial species, Mycobacterium smegmatis. (Stephan et al., 2005). Deletion of the primary porin, MspA, retards the influx of hydrophilic nutrients from the environment, and its overexpression in Mtb and M. bovis (BCG) increases nutrient influx (Mailaender et al., 2004; Stephan et al., 2005). However, induction of porin-dependent outer membrane permeability also increases sensitivity to a variety of chemical stresses and retards the intracellular growth of Mtb in cultured macrophages (Fabrino et al., 2009; Mailaender et al., 2004; Purdy et al., 2009). Taken together, these studies suggest that if intracellular Mtb were to adapt in a healthy host by inducing permeability of MoM for efficient nutrient uptake, the concomitant exposure to stress factors could self-limit its growth. Although porin-like proteins and associated non-selective permeability of MoM are likely to be present in Mtb (Molle et al., 2006; Siroy et al., 2008), the mechanism underlying the regulation of envelope permeability in intracellular Mtb remains unclear.
The lipid bilayer of MoM is considered to be made of mycolic acids of mAGP as the inner leaflet, and non-covalently associated glycolipids and phospholipids as the outer leaflet (Hoffmann et al., 2008; Niederweis et al., 2010). Among the non-covalently associated glycolipids, the mycolyl esters of trehalose – trehalose monomycolates (TMM) and trehalose dimycolates (TDM)– are the predominant species (Takayama et al., 2005). TMM is the common donor of mycolyl chain in the synthesis of mAGP and TDM (Belisle et al., 1997; Takayama et al., 2005). TDM has long been studied as a cord factor – conferring a cord-like appearance to Mtb in vitro – with strong immunomodulatory activities (Hunter et al., 2006; Ishikawa et al., 2009; Rao et al., 2006). However, recent studies suggest that TDM could be a structural component of MoM (Ojha et al., 2010; Yang et al., 2012b). An uncontrolled exogenous exposure of mycobacteria to a serine hydrolase of TDM from M. smegmatis, called TdmhMs, leads to a rapid depletion of trehalose mycolates and subsequent rupturing of the envelope (Ojha et al., 2010; Yang et al., 2012b). Interestingly, one of the products of TDM hydrolysis – free mycolic acid (FM) – is an abundant extracellular component of in vitro biofilms of both Mtb and M. smegmatis, although the physiological role of TdmhMs during mycobacterial growth in biofilms is unclear (Ojha et al., 2008; Ojha et al., 2010).
Because a TDM hydrolyzing activity in Mtb lysates was also detected during the discovery of TdmhMs (Ojha et al., 2010), we sought to identify the enzyme in Mtb and investigate its impact on TB pathogenesis. In this study we show that Rv3451 is the primary TDM hydrolase in Mtb and is induced under limiting nutrients. Its activity facilitates nutrient influx, but also substantially sensitizes the pathogen to the stresses prevalent in the host. Further, we provide evidence that effects and counter-effects of TdmhMtb plays an important role in balancing intracellular growth of Mtb in a manner that is directly responsive to host innate immunity.
Results
Identification of TDM hydrolases in Mtb
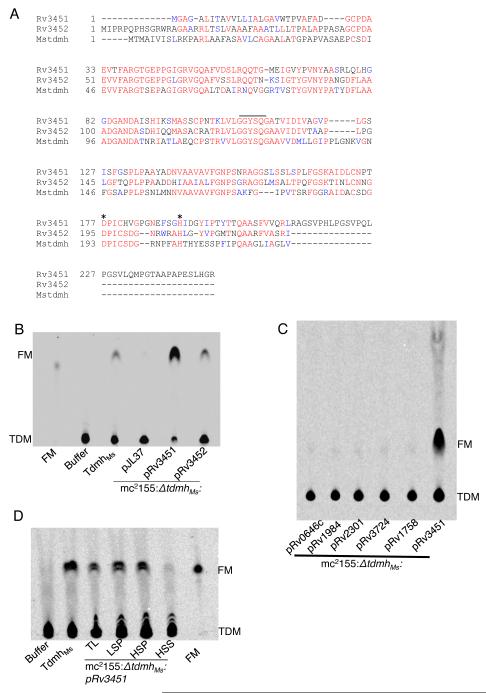
Two tandemly organized serine esterases in the Mtb genome, Rv3451 and Rv3452, have over 50% similarity to tdmhMs (Fig. 1A). Importantly, both contain the conserved catalytic triad of Ser (in GXSXG motif), Asp and His (Fig. 1A). We therefore addressed whether these two ORFs encode TDM hydrolases. Figures 1B and S1 show the release of 14C-FM when lysates of recombinant M. smegmatis lacking TdmhMs (Ojha et al., 2010) and expressing either Rv3451 (mc2155:ΔtdmhMs:pRv3451) or Rv3452 (mc2155:ΔtdmhMs:pRv3452), were mixed with purified 14C-TDM (Fig.1B and S1). The activity of Rv3452 lysate however was weaker that Rv3451 (Fig. 1B). Moreover, five other putative cutinase-like esterases of Mtb did not produce such activity (Fig. 1C). Therefore, we designate Rv3451 as tdmhMtb, and Rv3452 as tdmhMtb2. In addition, we also noted that the activity in mc2155:ΔtdmhMs:pRv3451 lysate was excluded from the cytosolic fraction (Fig. 1D), suggesting that TdmhMtb is exported to the envelope.
Figure 1.
Rv3451 and Rv3452 are TDM hydrolases in Mtb. (A) Alignment of Rv3451 and Rv3452 protein sequences with TdmhMs, originally marked as Mstdmh (Ojha et al., 2010). The catalytic triad of Ser, Asp and His are indicated. (B) Radio-TLC analysis of lipids in a mixture containing 14C TDM and lysates from recombinant M. smegmatis, mc2155:ΔtdmhMs, transformed with either empty plasmid (pJL37), pRv3451 or pRv3452 (also see figure S1). Reactions with purified TdmhMs, and storage buffer were positive and negative controls, respectively. Purified FM was marker. (C) Absence of TDM hydrolyzing activity in the lysates of mc2155:ΔtdmhMs with five other hydrolases cloned on the same plasmid backbone (pJL37), under constitutive hsp60 promoter, as Rv3451. These plasmids are indicated below each lane. mc2155:ΔtdmhMs:pRv3451 is positive control. (D) The hydrolase activity of TdmhMtb (Rv3451) in cellular factions of mc2155:ΔtdmhMs:pRv3451. TL- total lysate; LSP- low speed pellet, obtained from centrifugation of TL at 20,000g for 60 minutes; HSP- high-speed pellet, obtained from centrifugation of low-speed supernatant at 100,000g for 60 minutes; HSS- high-speed supernatant, obtained from the same centrifugation described for HSP. Purified FM was loaded as marker. (Figure 1, related to Figure S1)
The presence of TDM hydrolases in Mtb indicates that this glycolipid is turned over into FM under certain unknown physiological conditions. Because FM is highly abundant in the 4-week biofilms of Mtb, (Ojha et al., 2008), we examined TDM turnover under these conditions. An isogenic ΔtdmhMtb mutant of an attenuated Mtb strain, mc27000 (Ojha et al., 2008), accumulated ~3-fold more TDM than the parent wild-type in 4-week biofilms (Fig. 2A-D), suggesting a slower turnover of the glycolipid in the biofilms, although the appearance of the mutant biofilms was indistinguishable from the wild-type. Moreover, the hydrolase-dependent TDM turnover appeared to be specifically induced in the later growth phase, since TDM levels in the mutant were similar to wild-type at the 2-week stage (Fig. 2D). Furthermore, we did not notice any other significant change in the lipids in 4-week biofilms of ΔtdmhMtb (Fig. S2). TDM therefore is likely among the primary substrates of the esterase, although TDM hydrolysis only partly contributes to the FM pool since its level is reduced only by about 20% in ΔtdmhMtb (data not shown). Thus, FM accumulation likely occurs through additional unknown mechanisms.
Figure 2.
Accumulation of TDM in mc27000:ΔtdmhMtb. (A) Schematic representation of genomic organization of rv3451 (tdmhMtb) and rv3452 (tdmhMtb2), and the allelic exchange substrate including upstream (ups) and downstream (ds) regions used for replacement of tdmhMtb with hygr. (B). Southern blot of BsaAI digested genomic DNA from the parent wild-type (mc27000) and two hygr colonies (ΔtdmhMtb) probed with ups. (C) A representative radio-TLC of apolar lipids extractable from Mtb strains, mc27000 and mc27000:ΔtdmhMtb at 2, 3, and 4-week stages of biofilms. Purified TDM is the marker. (D) Quantitative analysis of the level of TDM as a percentage of total apolar lipids in mc27000 (wild-type) and mc27000:ΔtdmhMtb (mutant) in 2- and 4- week stages of biofilms (also see figure S2 for complete lipid profile). (Figure 2, related to Figure S2)
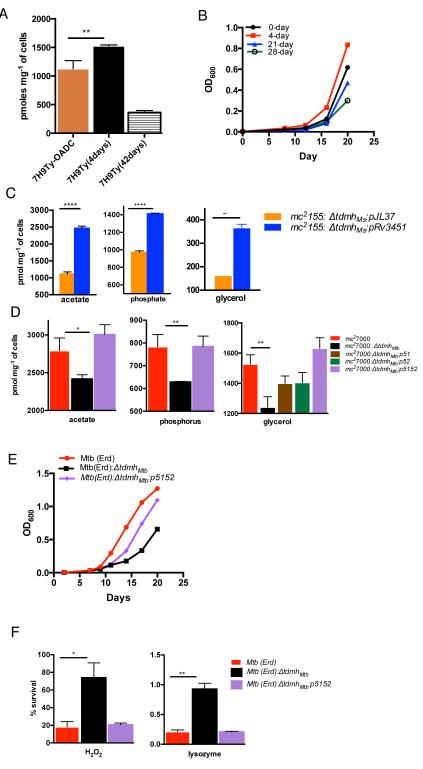
TdmhMtb increases nutrient influx under limiting condition but sensitizes Mtb to stresses We next addressed the physiological significance of TdmhMtb. An earlier microarray study observed induced transcription of TdmhMtb after 96-hour nutritional downshift of Mtb cultures in vitro (Betts et al., 2002). We confirmed a similar increase in mRNA abundance of TdmhMtb upon 96-hour exposure of normal planktonic mc27000 to a nutrient-limiting media, 7H9 base without supplements, and in 4-week biofilms (Fig S3A). This raises a possibility that TdmhMtb dependent envelope remodeling could facilitate appropriate adaptative response to nutrient limitation. To investigate this further, we first analyzed changes in nutrient influx upon exposure of wild-type Mtb to limiting condition. Interestingly, 96-hour incubation in 7H9 base medium increases glycerol influx in bacilli by ~40%, although prolonged (6-week) incubation led to decrease by ~4-fold (Fig. 3A). We further noted that under these conditions Mtb maintained slow but distinctively positive growth for about 2 weeks, and regenerated in fresh nutrients like a normal nutrient-rich culture, before assuming stationary phase (Fig. 3B and S3B). These data demonstrate a two-stage adaptation to limiting nutrients in Mtb; an early response that involves growth maintenance under moderate limitation of nutrients by inducing the influx, followed by a late response of slow nutrient influx and metabolic slowdown under extreme limitation. We next investigated whether TdmhMtb has any role in maintaining Mtb growth under limiting nutrients. This was preliminarily suggested by increase in the influx of nutrients upon constitutive expression of tdmhMtb in M. smegmatis through the hsp60 promoter (Fig. 3C). We further observed that the influx of acetate, phosphate and glycerol were retarded in ΔtdmhMtb, relative to the wild-type, when the cultures were pre-exposed to limiting nutrients for 96-hour (Fig. 3D). The mutant had no such defect in a normal culture (Fig. S3C). Interestingly, complementation of ΔtdmhMtb required a genomic fragment containing both tdmhMtb and tdmhMtb2 (Fig. 3D). It is noteworthy that tdmhMtb2 transcripts were reduced by 50% in ΔtdmhMtb, but restoration of expression of either tdmhMtb or tdmhMtb2 alone insufficient for complementing the mutant (Fig. 3D, S3D and S3E). The complementation analysis not only shows that tdmhMtb2 is transcriptionally linked to tdmhMtb but also suggests that the two hydrolases likely interact with each other. Reduced influx of nutrients in ΔtdmhMtb was further consistent with its retarded growth in media with limiting glycerol (Fig. 3E). The data together suggest that TDM hydrolases could comprise components of an adaptative response in Mtb to facilitate its growth under nutrient limiting conditions by inducing the non-selective permeability of the envelope, thereby increasing influx of multiple nutrients.
Figure 3.
Correlation between induced TdmhMtb activity and increased non-selective permeability of mycobacterial envelope under limiting nutrients. (A) Influx of glycerol over two hours in mc27000 incubated in normal (7H9TyOADC) and nutrient-limiting media (7H9Ty) for indicated time. (B) Regeneration of Mtb(Erd) in 7H9Ty with 0.1% v/v glycerol from a culture maintained in 7H9Ty for indicated time. (C) Accumulation of acetate, phosphorus and glycerol over 30 minutes by mc2155:ΔtdmhMs transformed with either pJL37, or pRv3451. (D) Accumulation of acetate, phosphorus and glycerol over two hours by cultures of mc27000, mc27000:ΔtdmhMtb, and mc27000:ΔtdmhMtb expressing Rv3451 (mc27000:ΔtdmhMtb:p51), Rv3452 (mc27000:ΔtdmhMtb:p52), or Rv3451-52 (mc27000:ΔtdmhMtb:p5152) in nutrient-limiting media (also see Fig. S3). (E) Growth of Mtb(Erd), ΔtdmhMtb mutant, and the complemented strain (mc27000:ΔtdmhMtb:p5152) in 7H9Ty with 0.1% v/v glycerol. (F) Sensitivity of 4-week biofilms of Mtb (Erd), Mtb (Erd):ΔtdmhMtb, and Mtb (Erd):ΔtdmhMtb:p5152 to H2O2 (40mM) and lysozyme (6μg/mL). (Figure 3, related to Figure S3)
Ectopically induced non-selective permeability of the envelope by recombinant porins also increases sensitivity of Mtb to a wide range of environmental stresses (Fabrino et al., 2009; Mailaender et al., 2004; Purdy et al., 2009). We therefore reasoned that TdmhMtb-dependent envelope remodeling under limiting nutrients, or in biofilms, could concomitantly sensitize bacteria to a variety of stresses. Indeed, biofilms of the mutant were significantly more tolerant than the wild-type to peroxide and lysozyme, predominant antimicrobial agents of the mammalian innate immune system (Beutler, 2004) (Fig. 3F).
Ectopic induction of TdmhMs increases nutrient influx and stress sensitivity
Despite several attempts we could not detect the physiological levels of TdmhMtb proteins in Mtb, nor could obtain its purified active form using recombinant systems. Therefore to affirm the correlation between TDM hydrolase activity and the phenotypes of ΔtdmhMtb, we investigated the effect of ectopic expression of a well-characterized heterologous Tdmh, TdmhMs, in a nutrient-rich culture of Mtb. Interestingly, despite a deleterious consequence of an exogenous exposure to TdmhMs (Yang et al., 2012b), its stable expression from a plasmid borne acetamide-inducible promoter could be achieved in Mtb without any apparent impact on the growth (Fig. 4A and 4B). The in vivo activity of the enzyme therefore is likely controlled by an unknown post-translational mechanism. Induced expression of TdmhMs in nutrient-rich culture led to significant increase in the influx of glycerol, acetate and phosphate (Fig. 4C). Moreover, Induced expression of TdmhMs also increased its sensitivity to peroxide, lysozyme and LL37, a cathelicidin-related antimicrobial peptide (Fig. 4D). Furthermore, induction of TdmhMs in Mtb(Erd) accelerated its growth in media with limiting glycerol (Fig. 4E). We therefore conclude that activity of TDM hydrolase produces structural changes in the envelope that increase its permeability to nutrients, but also expose the bacilli to environmental hazards.
Figure 4.
Ectopic induction of TDM hydrolase increases non-selective permeability of Mtb envelope. (A) and (B), Western blot and lipid analysis of acetamide induced expression of tdmhMs in mc27000:pAO10, and the empty plasmid control, mc27000:pLAM12, in Sauton’s media for time (day) indicated on each lane. Purified TDM and FMs were markers on TLC in panel B. (C) Accumulation of glycerol, phosphorus and acetate over two hours by acetamide induced expression of tdmhMs in mc27000:ΔtdmhMtb:pAO10, and the empty plasmid control, mc27000:pLAM12, in Sauton’s media. (D) Sensitivity of the two Mtb strains described in panel E to H2O2 (40mM), lysozyme (6μg/mL) and LL37 (20μM). (E) Growth of Mtb (Erd):ΔtdmhMtb:pAO10 and Mtb(Erd):ΔtdmhMtb:pLAM12 in media with limiting glycerol (0.1% v/v) and inducer (0.2% v/v acetamide).
TDM hydrolases self-restrict intracellular growth of Mtb in immunocompetent host
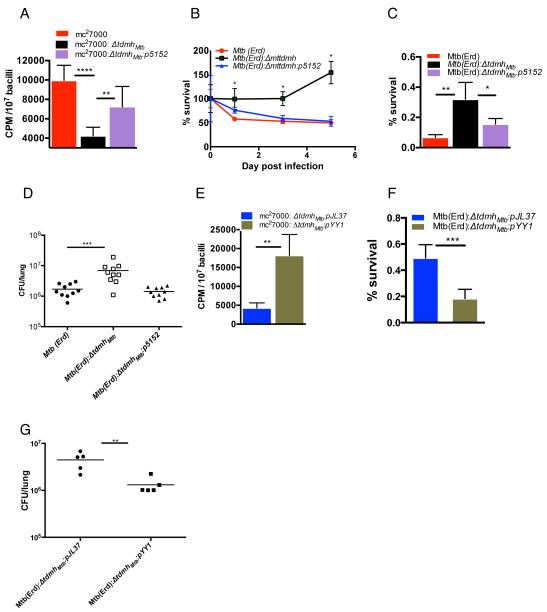
Rv3451(tdmhMtb) is among the 215 core genes induced within the first 24 hours of Mtb infection in macrophages and the upregulated expression continues during prolonged existence of the pathogen in the host cells (Rohde et al., 2012). Moreover, anti-TdmhMtb2 antibodies have been detected in the sera of TB patients (Brust et al., 2011). These suggest that Mtb utilizes the activity of TDM hydrolases in vivo. The induced expression of tdmhMtb in macrophages further suggests that the hydrolase activity could be utilized by the pathogen to increase nutrient influx in limiting intracellular condition. We tested this idea by adding 14C-acetate into media of macrophages infected with either ΔtdmhMtb, or its respective wild-type or the complemented strains, and comparing the radioactivity accumulated in the intracellular bacilli. This approach was facilitated through efficient separation of Mtb from lysates of infected macrophages with TB-Beads. TB-Beads are commercially available paramagnetic microparticles (< 5μm), which bind to Mtb bacilli with very high affinity and specificity (Wilson et al., 2010). Over 90% of the bacteria from macrophage lysates could be captured on TB-Beads (Fig. S4A). Furthermore, non-specific binding was very low, yielding a quantitative estimation of radioactivity specifically associated with intracellular Mtb (Fig. S4B). Indeed, ΔtdmhMtb accumulated 50% less radioactivity than the other strains, suggesting that the hydrolases facilitate nutrient influx in Mtb in vivo (Fig. 5A). Surprisingly, ΔtdmhMtb was more robust than wild-type, Mtb(Erd), and the complemented strains in pre-activated primary bone-marrow derived macrophages (BMM) from immunocompetent mice, C57BL/6 (Fig. 5B and S4C), suggesting that the hydrolase dependent sensitization to stresses could perhaps play a dominant role in determining the overall survival of Mtb in immunocompetent macrophages. We therefore evaluated the intracellular fitness of Mtb(Erd), ΔtdmhMtb, and the complemented strain in a stress-test, which involved direct measurement of their sensitivity to isoniazid. Figure 5C shows that intracellular ΔtdmhMtb indeed survives significantly better than the wild-type and complemented strains under isoniazid stress (Fig. 5C). Interestingly, infection of primary BMMs infected with ΔtdmhMtb produced lower levels of a proinflammatory cytokine, TNF-α, than those infected with wild-type or the complemented strains (Fig. S4D). This suggests a suboptimal activation of the mutant infected BMM, although underlying mechanisms remains unknown.
Figure 5.
The opposing effects of TdmhMtb self-restrict intracellular growth of Mtb in immunocompetent host. (A) Accumulation of radioactivity over a 48-hour period in the intracellular mc27000, mc27000: ΔtdmhMtb, and mc27000: ΔtdmhMtb:p5152 when 14C-acetate was added to the medium of infected RAW264.7 (mean ± SE, n=7). (B) Survival of Mtb (Erd), Mtb (Erd):ΔtdmhMtb and Mtb (Erd):ΔtdmhMtb:p5152 in activated BMM from C57BL/6 mice (also see Fig S4C for raw cfu). (C) Sensitivity of intracellular Mtb (Erd), Mtb (Erd):ΔtdmhMtb and Mtb (Erd):ΔtdmhMtb:p5152 to 5-day exposure of isoniazid (0.75μg/mL) added to the culture medium of infected primary BMM (C57BL/6) (mean ± SE, n=5). (D) Burden of the three Mtb strains in the lungs of C57BL/6 mice three weeks after aerosolized infection with ~50-100 bacilli/lung. Data represents two independent exposures, each with five animals/per strain (p < 0.0001, Mann-Whitney test). (E) Relative change in intracellular accumulation of radioactivity over a 48-hour period between mc27000: ΔtdmhMtb constitutively expressing tdmhMs (mc27000:ΔtdmhMtb: pYY1) and its corresponding empty vector control, mc27000: ΔtdmhMtb:pJL37, when 14C-acetate was added to the medium of infected RAW264.7 (mean ± SE, n=4). (F) Sensitivity of intracellular Mtb(Erd):ΔtdmhMtb:pYY1 and Mtb(Erd):ΔtdmhMtb:pJL37 to 5-day exposure of isoniazid (0.75μg/mL), added to the culture medium of infected primary BMM from C57BL/6 mice (mean ± SE, n=6). (G) Burden of Mtb(Erd):ΔtdmhMtb:pYY1 and Mtb(Erd):ΔtdmhMtb:pJL37 in the lung of C57BL/6 mice after three weeks of infection (p < 0.01, Mann-Whitney test). (Figure 5, related to Figure S4)
An improved survival of ΔtdmhMtb mutant in the macrophages was further supported by its better growth during early phase of infection in immunocompetent, C57BL/6, mice. During the first three weeks of infection, the mutant produced about 4-fold higher burden in lungs than the parent wild-type and complemented strains (Fig. 5D). Interestingly, severe splenomegaly and lung pathology, with greater alveolar, peribronchiolar and perivascular infiltrations, as well as conducting airway congestion were observed during chronic phase of ΔtdmhMtb infection (Fig. S4E and S4F), although the basis of severe pathology remains unclear. Consistent with the severe pathology however, mice infected with ΔtdmhMtb also had shorter lifespans than those infected with the wild-type strain (Fig. S4G). It is noteworthy that levels of phthiocerol dimycoserosate (PDIM) were same in wild-type and ΔtdmhMtb (Fig. S4H), therefore confirming that the relative increase in virulence of ΔtdmhMtb was not an artifact of a spontaneous loss of PDIM, as reported to often occur in H37Rv (Domenech and Reed, 2009).
It is noteworthy that a constitutive expression of tdmhMs from the hsp60 promoter (pYY1) in intracellular ΔtdmhMtb also led to both, a significant increase in 14C incorporation from the extracellular media as well as an increased sensitivity to extracellular isoniazid (Fig. 5E and 5F). Moreover, infection of C57BL/6 with ΔtdmhMtb:pYY1 produced 3-fold lesser burden during the first three weeks of infection, compared to the empty plasmid strain, ΔtdmhMtb:pJL37 (Fig. 5G). Taken together, complementation by tdmhMs affirms that intracellular behavior of ΔtdmhMtb is directly linked to the loss of TDM hydrolase activity in the mutant. Moreover, the enzyme clearly has an important role in self-restricting intracellular growth of Mtb in an immunocompetent host through mechanisms that include stress sensitization of bacteria.
TDM hydrolases provide growth advantage to intracellular Mtb in MyD88−/− hosts
We next argued that reduced levels of intracellular stress in an immunocompromised host could mitigate the effects of TDM-hydrolase-dependent stress sensitization, such that the hydrolase-dependent nutrient influx could then produce a net positive growth of Mtb in such host. Indeed, growth of Mtb(Erd):ΔtdmhMtb in pre-activated primary MyD88−/− BMM was significantly slower than wild-type Mtb(Erd), and complemented strain (Fig. 6A and S5A). MyD88 is a central adaptor molecule that converges multiple TLR-dependent signals to activate a strong innate antimicrobial activity against bacterial infection, including TB, in mice (Hawn et al., 2006; Scanga et al., 2004). We further observed a retarded incorporation of radiolabel in the intracellular ΔtdmhMtb when 14C-acetate was added to the medium of infected primary MyD88−/− BMM (Fig. 6B), suggesting that slower growth of the mutant in MyD88−/− BMM is likely due to slower influx of nutrients in bacteria. Importantly, constitutive expression of TdmhMs in ΔtdmhMtb produced opposing effects on its growth in pre-activated primary BMMs from C57BL/6 and MyD88−/− mice (Fig. 6C, 6D, S5B and S5C). Finally, the slower growth characteristic of ΔtdmhMtb in MyD88−/− BMM was faithfully reproduced in the lungs of the immunocompromised mice (Fig. 6E), with prolonged survival of the animals (Fig. 6F). Taken together, the overall effect of Tdmh on intracellular growth of Mtb during early phase of infection is highly responsive to the innate resistance from the host.
Figure 6.
Tdmh facilitates Mtb growth in MyD88−/− mice. (A) Growth of Mtb (Erd) or Mtb (Erd):ΔtdmhMtb and the complemented ΔtdmhMtb:p5152 strains in activated primary BMMs from MyD88−/− mice. (B) Accumulation of radioactivity over a 48-hour period in the intracellular mc27000 and mc2700:ΔtdmhMtb, when 14C-acetate was added to the medium of infected primary BMMs from MyD88−/− mice. (C) & (D) Relative difference between the survival of Mtb (Erd):ΔtdmhMtb expressing tdmhMs (Mtb(Erd): ΔtdmhMtb pYY1) and its corresponding empty vector control, Mtb (Erd):ΔtdmhMtb:pJL37, in primary BMMs from C57BL/6 (C), and MyD88−/− (D) mice. (E) Burden of Mtb(Erd), Mtb(Erd):ΔtdmhMtb and Mtb(Erd):ΔtdmhMtb:pYY1 in the lungs of MyD88−/− mice after 14 days of aerosolized infection (n= 5, p < 0.05, Mann-Whitney test). Mean burden of the three strains after 24 hours of exposure were 93, 89 and 99, respectively. (F) Survival of MyD88−/− mice infected with each of the three strains described in panel E (n = 8, p < 0.001, Mann-Whitney test). Raw cfu data for panel A, C and D are provided in figures S5A S5B and S5C, respectively. (Figure 6, related to Figure S5)
Discussion
In this study we have discovered an adaptative response in Mtb under nutrient limitation. The response involves a hydrolase-dependent increase in non-selective permeability of the envelope and subsequent enhancement in nutrient influx, through cleavage of the ester linkage of TDM. This is likely a conserved mechanism in environmental and pathogenic mycobacteria, suggested not only by the presence of tdmhMtb homologues in several mycobacterial species, but also by the functional complementarity between TdmhMs and TdmhMtb.
TdmhMtb and TdmhMs are members of a ubiquitous serine hydrolase superfamily, representing enzymes with a wide-range of function, such as carboxypeptidases, proteases, phospholipases, fungal cutinases, and phage lysins (Akoh et al., 2004; Ollis et al., 1992; Payne et al., 2009), but only a few of these have been implicated in nutrient influx during bacterial adaptation. One such serine hydrolase, a deacylase of lipid A (pagL), in Pseudomonas aeruginoasa is functionally comparable to TdmhMtb. PagL induces outer membrane remodeling under in vitro growth in limiting magnesium condition (Ernst et al., 2006). Loss of PagL activity is also associated with severe pulmonary infection (Ernst et al., 2006).
Implication of TDM hydrolase in mycobacterial adaptation to limiting nutrients is of particular significance in the context of resuscitation promoting factors (Rpf), which have been widely explored for their role in mycobacterial growth after dormancy (Mukamolova et al., 1998). Mtb genome encodes five rpf homologues, rpfA-E, of which rpfBDE deletion impairs growth revival from late stationary phase, and ΔrpfB is retarded in reactivating TB in mice (Downing et al., 2005; Tufariello et al., 2006). Moreover, RpfB has a lysozyme-like structure and interacts with a peptidoglycan hydrolase, RipA (Cohen-Gonsaud et al., 2005; Hett et al., 2010), suggesting that Rpf-dependent resuscitation of Mtb also involves a limited digestion of the envelope. However, Rpf-dependent resuscitation and TdmhMtb-dependent adaptation appear to represent distinct physiological states of Mtb. Whereas Rpf-dependent envelope remodeling is likely a specialized process exclusively associated with growth revival from dormancy, activity of TdmhMtb might occur over a dynamic range in metabolically active Mtb to facilitate its rapid adaptation and growth maintenance under conditions of limiting nutrients. While enhanced influx of small hydrophilic nutrients through induced envelope permeability is clearly a key adaptative mechanism, TdmhMtb can also be argued to facilitate recycling of trehalose and mycolic acids in Mtb. However, TdmhMtb dependent nutrient recycling is likely to be secondary to other recycling mechanisms, since trehalose recycling is critical for the survival of intracellular Mtb (Kalscheuer et al., 2010).
The regulated increase of non-selective nutrient influx through TDM hydrolysis provides a unique perspective of the structural as well as functional plasticity of the mycobacterial envelope, permeability of which is restricted by the inner plasma membrane and the outer MoM (Niederweis, 2008). Permeability across MoM appears to be primarily non-selective, facilitated by the activity of channel proteins (Niederweis, 2008). It is therefore reasonable to argue that TDM hydrolases could modulate the solute influx through changes in MoM structure, implying that TDM is likely localized in MoM. This notion is further supported by the accessibility of TDM to exogenous TdmhMs (Yang et al., 2012b). An increase in MoM permeability through hydrolase activity can be imagined to occur either through delocalized increase in fluidity of the lipid bilayer, or localized perturbation of the lipid domains around porins to induce channel openings. However, esterase-dependent stress sensitization might be a combined effect of increased influx of the small hydrophilic solutes, such as isoniazid and peroxide, as well as greater exposure of the cell envelope targets to the external hazards. The later mechanism likely provides an explanation for the Tdmh dependent sensitization to lysozyme and antimicrobial peptides.
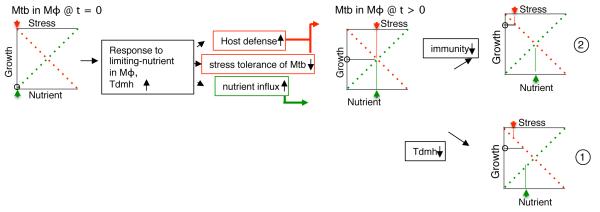
TdmhMtb dependent increase in nutrient influx during early phase of infection is not surprising given that Mtb must actively replicate in a nutrient-deprived intracellular environment in order to establish a successful infection. However, the positive effect of Tdmh on Mtb growth is clearly outweighed by the negative effects from increased sensitization of the pathogen to the intracellular stresses, as well as greater induction of inflammatory response in macrophages. Together these opposing effects of Tdmh appear to balance the overall intracellular bacterial growth in immunocompetent host (Fig. 7). The development of severe disease by ΔtdmhMtb raises a possibility that restricted bacterial growth during early phase could be crucial for controlled disease progression during chronic phase. Further immunological characterization of the host will offer crucial mechanistic insight into Tdmh-dependent disease progression. The retarded growth of ΔtdmhMtb in MyD88−/− host can be reasonably linked to the physiological deficiency of the mutant in assimilating adequate nutrients. Therefore hydrolase-dependent nutrient influx could also lead to accelerated growth of Mtb and faster reactivation of infection in other unrelated immunocompromised host conditions, including animal models described for reactivation of TB latency (Scanga et al., 1999). Analysis of ΔtdmhMtb growth and pathogenesis in such models will likely offer insight into reactivation of latent TB.
Figure 7.
Schematic representation of TdmhMtb dependent self-balanced growth of intracellular Mtb in immunocompetent host. The status of nutrient availability, stress effect and net growth of Mtb in Mφ at initial (t=0) and later (t>0) phases of infection are represented by green arrow, red arrow and open circle, respectively. Two possible scenarios under which this balance could be perturbed are depicted. Scenario 1 shows the effect of TdmhMtb inactivation on bacterial burden in immunocompetent host. Although the nutrient influx in a ΔtdmhMtb mutant is likely retarded, this deficiency is outweighed by increased tolerance of the mutant to the host-induced stresses. In scenario 2, reduced intracellular stress in an immunocompromised host can mitigate the effect of TdmhMtb on stress sensitivity, such that Tdmh-dependent nutrient assimilation can accelerate growth of wild-type Mtb in such host.
In summary, distinct consequences of TDM hydrolases on Mtb infection in immunocompetent and immunocompromised hosts not only reveal the complexities of host-pathogen dynamics, but also underscore the significance of developing distinct strategies for TB treatment depending on the status of the host immunity.
Experimental procedure
Bacterial strains and media
Except for the growth conditions described for an individual experiment in the results, strains of Mtb, M. smegmatis and E. coli were routinely maintained under standard conditions. Detailed growth conditions for these strains are provided as supplementary information.
Construction of mutants and plasmids
Construction of mc2155:ΔtdmhMs is described previously (Ojha et al., 2010). Deletion of tdmhMs in various strains of Mtb was constructed using the gene replacement technique described earlier (Bardarov et al., 2002). Detailed methods for construction of these mutants and other recombinant strains are provided in supplementary information. Lists of all plasmids, bacterial strains, and oligonucleotides used in this study are provided in supplementary tables, S1, S2 and S3.
Real-time PCR
Real-time PCR was performed on a StepOnePlus RTPCR System (Applied Biosystems) with SYBR Green mastermix following the manufacturer’s instructions. Total RNA from the desired bacterial cultures were extracted using Ribopure kit (Ambion) and processed for RT-PCR as described in the supplementary information.
In vitro activity of TDMH
14C-TDM equivalent to 100,000cpm, was homogeneously suspended in the assay buffer as described earlier. The homogenate was then mixed with either 5μg of purified TdmhMs (MSMEG_1529), 100μg lysates of mc2155: ΔtdmhMs: pJL37, mc2155: ΔtdmhMs: pRv3451 or mc2155: ΔtdmhMs: pRv3452 and incubated at 37°C for two hours. The lipids present in the reaction mixture were then extracted and analyzed as described previously (Ojha et al., 2010).
Lipid purification and analysis
1μci/ml of acetic acid, sodium salt [1-14C] (PerkinElmer Inc.) was added to either biofilms or planktonic cultures of Mtb as described in the text, and incubated for either six (for biofilms) or three (for planktonic) hours, before proceeding to the lipid extraction. Apolar and polar lipids were extracted and analyzed as described earlier (Ojha et al., 2010). Further details are described in supplementary information.
Infection of macrophages
For primary macrophages, bone marrows from femurs of 60-day old female C57BL/6 and in-house bred MyD88−/− mice (Hawn et al., 2006) were flushed, and incubated in DMEM with 0.58g/L of L-glutamine and 1mM sodium pyruvate (Corning Inc.), 10% v/v FBS (Life Technologies Inc.) and 20% v/v L929 cell-conditioned medium at 37 °C for 7 days with 5%CO2. L929 cells are cultured in DMEM with 0.58g/L of L-glutamine, 1mM sodium pyruvate, 10% v/v FBS, 2% v/v sodium bicarbonate (Fisher) and 1% v/v non-essential amino acids (Sigma-Aldrich Inc.) to get L929 cell supernatant. For RAW264.7 cells, L929 supernatant was substituted with 2% v/v sodium bicarbonate, and FBS was reduced to 10% v/v. Cells were seeded in 12-well plates (CELLSTAR) (2×105 cells/well) and incubated for 24 hours before infection. Cells were seeded in 12-well plates (Cellstar Inc.) at 200,000 cells/well, and incubated for 24 hours before infection. If necessary, bone-marrow derived primary macrophages (BMM) were activated with 30ng/mL of rINF-γ (Sino Biological Inc.) for 16 hours, followed by 1μg/mL of LPS (Sigma Aldrich Inc.) for two hours. Desired strains of Mtb were added to the cultured cells at an MOI of 10. After 6 hours, infected macrophages were washed thrice with PBS and incubated in fresh media. Cells were collected at specified time, lysed in PBS with 0.05% SDS, and intracellular bacteria were plated on 7H11OADC and incubated for 3 weeks to determine their viability. Percentage survival of bacteria at any time was calculated with respect to mean CFU obtained at 0-day of infection. For isoniazid sensitivity of intracellular Mtb, the antibiotic was added at 0.75μg/mL to the cell culture media after 4 hours of incubation of the washed infected BMM in fresh media. Bacteria from the drug exposed infected lysates were plated to determine the relative survival efficiency as compared to the parallel cultures of infected macrophages not exposed to antibiotics.
Nutrient influx assay in vitro
Influx of radiolabeled hydrophilic solutes in various bacterial strains was determined as described previously (Stephan et al., 2005). Detailed methods are provided as supplementary information.
Nutrient uptake assay for intracellular Mtb
Macrophages were seeded in 75cm2 flask (107cells/flask) and incubated for 24 hours before infection. Desired strains of Mtb (mc27000) were added to the cultured cells at an MOI of 10. After 6 hours, infected macrophages were washed thrice with PBS and incubated in fresh media with 1μci/ml of acetic acid, sodium salt [1-14C] (PerkinElmer Inc.) for 48 hours. Cells were washed thrice with PBS and lysed in 1mL of PBS with 0.05% SDS. 10μL of the lysates were diluted and plated to determine the total number of bacteria in the lysate. Then 1mL suspension of TB-Beads (Microsense Inc.) were added to the lysates and mixed for 5 minutes. After separation of TB-Beads on a magnetic plate and three washes in 1mL PBS with 0.05% Tween 80 (PBST), the beads were suspended in 1mL PBST. 10μL of the suspension was serially diluted and plated on 7H11OADC with pantothenic acids to determine the number of bacilli captured on the beads. Rest of the beads were mixed with an equal volume of 10% buffered formalin phosphate (Fisher) containing 0.1M LiCl and collected on a 0.45 μm Spin-X centrifuge tube filter (Costar). The radioactivity on the column was counted in a liquid scintillation counter (Beckman). After subtracting background counts (from the lysates of uninfected macrophages), the values across the samples were normalized to cpm/107 bacilli.
Mouse infection
All mouse procedures were performed in Animal-BSL3 laboratory using the protocol approved by Institutional Animal Care and Use Committee of the University of Pittsburgh and Seattle Biomedical Research Institute. Strains of Mtb(Erd) and its derivatives, as described, were grown to OD 0.3, washed in PBST, and resuspended to ~107 cfu/mL in PBST. 60-day old female C57BL/6 (Charles River) and male MyD88−/− mice (Osaka University) were infected with aerosolized suspension of the bacterial strains for 20 minutes using Inhalation Exposure System. After 24 hours of exposure, 4-5 C57BL/6 or two MyD88−/− mice from each infected group were euthanized, and bacterial burdens in the lungs were determined by homogenizing the tissues in PBST and plating the dilutions on 7H11OADC. Bacterial burdens in the organs were subsequently determined at time intervals as specified. For histopathological analysis, organs were fixed in phosphate-buffered formalin (10%) and paraffin-embedded, and their ultrathin sections were stained with hematoxylin and eosin (H&E).
Statistical analysis
Unless otherwise specified, significance of difference between control and experimental groups were calculated by t-test statistics (either paired or unpaired) in GraphPad Prism software. A minimum of three independent biological replicates. p < 0.05, 0.01 and 0.001 were indicated as *, ** or ***.
Supplementary Material
Highlights.
Rv3451 (TdmhMtb) and Rv3452 (TdmhMtb2) encode TDM hydrolases in Mtb.
TdmhMtb is induced in nutrient-limiting planktonic cultures and biofilms.
TdmhMtb increases nutrient influx but also sensitizes Mtb to multiple stresses.
Tdmh has opposing effects on Mtb growth in wild-type and MyD88−/− mice.
Acknowledgements
We thank David Sherman for the kind gift of PDIM-deficient mutant (H37Rv:Tn2935), Beth Juneko and Dat Mai for technical help, Joanne Flynn and the Flynn laboratory for helpful discussions in experimental design, and Graham Hatfull, Keith Derbyshire and Pallavi Ghosh for critical comments on the manuscript. The work was supported by NIH grant to AKO AI79288, UL1 RR024153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF. GDSL family of serine esterases/lipases. Prog Lipid Res. 2004;43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Bardarov S, Bardarov S, Jr., Pavelka MS, Jr., Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR., Jr. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Brust B, Lecoufle M, Tuaillon E, Dedieu L, Canaan S, Valverde V, Kremer L. Mycobacterium tuberculosis lipolytic enzymes as potential biomarkers for the diagnosis of active tuberculosis. PLoS One. 2011;6:e25078. doi: 10.1371/journal.pone.0025078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol. 2004;110:2–12. doi: 10.1016/s1521-6616(03)00210-9. [DOI] [PubMed] [Google Scholar]
- Cohen-Gonsaud M, Barthe P, Bagneris C, Henderson B, Ward J, Roumestand C, Keep NH. The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes. Nat Struct Mol Biol. 2005;12:270–273. doi: 10.1038/nsmb905. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech P, Reed MB. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: implications for virulence studies. Microbiology. 2009;155:3532–3543. doi: 10.1099/mic.0.029199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing KJ, Mischenko VV, Shleeva MO, Young DI, Young M, Kaprelyants AS, Apt AS, Mizrahi V. Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation in vitro. Infect Immun. 2005;73:3038–3043. doi: 10.1128/IAI.73.5.3038-3043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Adams KN, Moskowitz SM, Kraig GM, Kawasaki K, Stead CM, Trent MS, Miller SI. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J Bacteriol. 2006;188:191–201. doi: 10.1128/JB.188.1.191-201.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrino DL, Bleck CK, Anes E, Hasilik A, Melo RC, Niederweis M, Griffiths G, Gutierrez MG. Porins facilitate nitric oxide-mediated killing of mycobacteria. Microbes Infect. 2009;11:868–875. doi: 10.1016/j.micinf.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43:482–486. doi: 10.1038/ng.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- Hett EC, Chao MC, Rubin EJ. Interaction and modulation of two antagonistic cell wall enzymes of mycobacteria. PLoS Pathog. 2010;6:e1001020. doi: 10.1371/journal.ppat.1001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer R, Weinrick B, Veeraraghavan U, Besra GS, Jacobs WR., Jr. Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21761–21766. doi: 10.1073/pnas.1014642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology. 2004;150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- Molle V, Saint N, Campagna S, Kremer L, Lea E, Draper P, Molle G. pH-dependent pore-forming activity of OmpATb from Mycobacterium tuberculosis and characterization of the channel by peptidic dissection. Mol Microbiol. 2006;61:826–837. doi: 10.1111/j.1365-2958.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB. A bacterial cytokine. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8916–8921. doi: 10.1073/pnas.95.15.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederweis M. Nutrient acquisition by mycobacteria. Microbiology. 2008;154:679–692. doi: 10.1099/mic.0.2007/012872-0. [DOI] [PubMed] [Google Scholar]
- Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. Mycobacterial outer membranes: in search of proteins. Trends Microbiol. 2010;18:109–116. doi: 10.1016/j.tim.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR, Jr., Hatfull GF. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha AK, Trivelli X, Guerardel Y, Kremer L, Hatfull GF. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J Biol Chem. 2010;285:17380–17389. doi: 10.1074/jbc.M110.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008;33:1–3. doi: 10.1097/RLU.0b013e31815c5128. [DOI] [PubMed] [Google Scholar]
- Payne K, Sun Q, Sacchettini J, Hatfull GF. Mycobacteriophage Lysin B is a novel mycolylarabinogalactan esterase. Mol Microbiol. 2009;73:367–381. doi: 10.1111/j.1365-2958.2009.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, Gao F, Chen B, Jacobs WR, Jr., Glickman MS. Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis -induced inflammation and virulence. J Clin Invest. 2006;116:1660–1667. doi: 10.1172/JCI27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde KH, Veiga DF, Caldwell S, Balazsi G, Russell DG. Linking the transcriptional profiles and the physiological states of Mycobacterium tuberculosis during an extended intracellular infection. PLoS Pathog. 2012;8:e1002769. doi: 10.1371/journal.ppat.1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Mohan VP, Joseph H, Yu K, Chan J, Flynn JL. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroy A, Mailaender C, Harder D, Koerber S, Wolschendorf F, Danilchanka O, Wang Y, Heinz C, Niederweis M. Rv1698 of Mycobacterium tuberculosis represents a new class of channel-forming outer membrane proteins. J Biol Chem. 2008;283:17827–17837. doi: 10.1074/jbc.M800866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings CL, Glickman MS. Is Mycobacterium tuberculosis stressed out? A critical assessment of the genetic evidence. Microbes Infect. 2010;12:1091–1101. doi: 10.1016/j.micinf.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan J, Bender J, Wolschendorf F, Hoffmann C, Roth E, Mailander C, Engelhardt H, Niederweis M. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol Microbiol. 2005;58:714–730. doi: 10.1111/j.1365-2958.2005.04878.x. [DOI] [PubMed] [Google Scholar]
- Takayama K, Wang C, Besra GS. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufariello JM, Mi K, Xu J, Manabe YC, Kesavan AK, Drumm J, Tanaka K, Jacobs WR, Jr., Chan J. Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis. Infect Immun. 2006;74:2985–2995. doi: 10.1128/IAI.74.5.2985-2995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Global Tuberculosis Report. 2012. The burden of diseases caused by TB; pp. 8–28. [Google Scholar]
- Wilson S, Lane A, Rosedale R, Stanley C. Concentration of Mycobacterium tuberculosis from sputum using ligand-coated magnetic beads. Int J Tuberc Lung Dis. 2010;14:1164–1168. [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012a;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bhati A, Ke D, Gonzalez-Juarrero M, Lenaerts A, Kremer L, Guerardel Y, Zhang P, Ojha AK. Exposure to a cutinase-like serine esterase triggers rapid lysis of multiple mycobacterial species. J Biol Chem. 2012b doi: 10.1074/jbc.M112.419754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.