Abstract
Background
Hormonally active environmental exposures are suspected to alter onset of puberty in girls, but research on this question has been very limited.
Objective
We investigated pubertal status in relation to hormonally active environmental exposures among a multiethnic group of 192 healthy nine-year old girls residing in New York City.
Methods
Information was collected on breast and pubic hair stages, weight and height. Phytoestrogen intake was estimated from a food frequency questionnaire. Three phytoestrogens and bis-phenolA (BPA) were measured in urine. In a subset, 1,1′-dichloro-2,2′-bis(4-chlorophenyl)ethylene (DDE), polychlorinated biphenyls (PCBs) were measured in blood plasma and lead (Pb) in blood. Associations of exposures with pubertal stages (present=stage 2+ vs absent=stage 1) were examined using t-tests and Poisson multivariate regression to derive prevalence ratios (PR, 95%-confidence limits [CI]).
Results
Breast development was present in 53% of girls. DDE, Pb, and dietary intakes of phytoestrogens were not significantly associated with breast stage. Urinary phytoestrogen biomarker concentrations were lower among girls with breast development than with no development. In multivariate models, main effects were strongest for two urinary isoflavones, daidzein (PR 0.89 [0.83-0.96] per ln-μg/g creatinine) and genistein (0.94 [0.88-1.01]). Body mass index (BMI) is a hormonally relevant, strong risk factor for breast development. Therefore, BMI-modification of exposure effects was examined, and associations became stronger. Delayed breast development was observed among girls with below-median BMI and 3rd tertile (high exposure) of urinary daidzein (PR 0.46 [0.26-0.78]); a similar effect was seen with genistein, comparing to girls ≥median BMI and lowest two tertiles (combined) of these isoflavones. With urinary enterolactone a phytoestrogen effect was seen only among girls with high BMI, where breast development was delayed among those with high urinary enterolactone (PR 0.55 [0.32 - 0.96] for the upper tertile vs lower two combined). There was no main effect of PCBs on breast stage, but girls with below-median BMI and ≥median PCB levels had reduced risk for breast development (any vs none) compared with other BMI-PCB groups. No biomarkers were associated with hair development, which was present in 31% of girls.
Conclusions
Phytoestrogens and PCBs are environmental exposures that may delay breast development, especially in conjunction with BMI which governs the endogenous hormonal milieu. Further research to confirm these findings may improve our understanding of the role of early-life development in breast cancer risk and other chronic diseases related to obesity.
Keywords: puberty, environment, biomarkers, BMI, phytoestrogen, DDE, PCB, diet
Introduction
First breast development occurs at 9 years of age on average in Black and at 10 years in white girls in the U.S., while average age at menarche is 12.5-13 years (Herman-Giddens et al., 1997; Richards et al., 1992; Selevan et al., 2003; Wu et al., 2003). Racial/ethnic disparities in pubertal timing have been attributed to height and body mass index (BMI, m/kg2) (Kaplowitz et al., 2001), but variability is not entirely explained by body size characteristics and other factors such as physical activity and genetics (Richardson et al., 1983). Breast development accompanies an upsurge in steroid hormones, chiefly estrogen (Jones et al., 2007). Based on knowledge of hormonal activity of environmental contaminants, exogenous exposures have come to be of interest as potential etiologic agents for sexual maturation.
Environmental exposures are known to alter pubertal onsent in experimental models. Experimental data support a delay with lead (Pb) exposure (Ronis et al., 1998), and advanced female development after exposure to hormonally active agents, including phytoestrogens (Whitten & Naftolin, 1992), PCBs (Gellert, 1978), bisphenol A (BPA) (Honma et al., 2002), and pesticides (Walters et al., 1993). Environmental and dietary factors have been investigated little with regard to pubertal onset or even menarche among girls, with inconsistent results (Blanck et al., 2000; de Ridder et al., 1991; Gladen et al., 2000; Karmaus et al., 2002; Kato et al., 1988; Koprowski et al., 1999) except for inorganic lead which was associated with delayed breast development (Denham et al., 2005; Selevan et al., 2003; Wu et al., 2003). No data have been reported on phytoestrogen biomarkers and pubertal maturation.
Earlier puberty is associated with breast cancer, insulin resistance, bone development, and cardiovascular disease, especially among African-American women (Morrison et al., 1999). Therefore understanding its determinants may offer preventive measures for later health effects.
We examined pubertal stages in relation to hormonally active environmental factors, including exposure biomarkers as well as dietary phytoestrogen intake, among nine-year-old girls from three ethnic groups in New York City.
Materials and methods
The population, previously described in detail (Britton et al., 2004), included 192 9 year-old girls recruited at Mount Sinai Hospital in New York City and in a nearby pediatric private practice during 1996-1997. The participation rate was 89% (200/224 of those approached), and the final group for the environmental analyses was 186 girls with complete information on pubertal stages, age, race and BMI. Pediatric nurses measured the girls' heights and weights and obtained blood samples. Baseline data and dietary intake were collected by in-person interview. A food-frequency questionnaire was used (Harvard Youth/Adolescent Questionnaire YAQ (Rockett et al., 1995)) that has been validated in a multiethnic population and found to be reproducible over a year's time. We queried girls directly on each dietary item, with mothers or guardians also present. Responders were not aware of the results of the physical examination when they completed the interview. Pubertal stages were assessed by pediatricians using a form with standard drawings provided by Prof. J. Richard Udry (Morris, 1980), Carolina Population Center, Chapel Hill, NC 27516-3997 (with permission).
Methods for organochlorines in plasma, Pb, urinary phytoestrogen metabolites and creatinine (to monitor urine dilution) have been reported, including quality control measures (Berkowitz et al., 2003; Liu et al., 2005; Wolff et al., 2005). The organochlorines were measured in a randomly selected subset because of budget limitations (DDE, PCB #s 118, 153, 138, 180). As reported, limits of detection for the organochlorines were 0.07 μg/L, for Pb 0.1 μg/dL, and for for the urinary metabolites 0.79 μg/L for daidzein, 1.0 μg/L for enterolactone, 0.5 μg/L for genistein, and 0.5 μg/L for bisphenol A (defined as 3-times the blanks). There was a single non-positive non-zero value of one PCB congener which was replaced with the lowest positive value for that congener (Berkowitz et al., 2003); the four PCB congeners were added together to obtain the PCB-total variable. Because concentrations of the individual congeners were low, we did not investigate them individually.
A phytoestrogen database was compiled for 65 food items in the YAQ based on the literature and existing databases (Block et al., 1986; Horn-Ross et al., 2000; Pillow et al., 1999; usda, 2000). Dietary intake (mg/d) of phytoestrogens were grouped as isoflavones (genistein, daidzein, formononetin, biochanin-A, coumestrol); flavones (luteolin, apigenin), flavonols (quercetin, kaempherol, myricetin); phytosterols (beta-sitosterol, campesterol, stigmasterol); and lignans (enterolactone, enterodiol). In the interview, additional questions were asked about phytoestrogen foods consumed within the past 24 hr (any cruciferi, sesame, granola, bran, squash, beans and lentils, cherries, apples/apple juice, tofu, sprouts, garlic, tea, cola, onion).
In statistical analyses, biomarkers including creatinine were log-transformed. Urinary metabolites were examined both as μg/L and corrected for creatinine (μg/gC). Dietary phytoestrogens and urinary metabolites were also categorized as tertiles of intake (mg/day). We identified a minimum set of exposure covariates by fitting multiple regression models to predict the exposure variables. Covariates included those essential for the pubertal models (race, age, BMI, and height (Britton et al., 2004) as well as additional potential predictors identified in bivariate comparisons (p<0.2). The resulting minimal covariates were then used in the models predicting pubertal development from exposures. They were breast-fed for DDE models; race, breast-fed, maternal country of birth (non-US) for PCB; race, private clinic (vs hospital) for Pb; race and urinary creatinine for urinary phytoestrogens and BPA, adding maternal education for daidzein and BPA, adding BMI, maternal education for enterolactone; no covariates for dietary flavones; calories for flavonols and isoflavones; private clinic for lignans; private clinic, maternal U.S. birth for phytosterols.
Modified poisson regression with robust error variance was used to estimate adjusted prevalence ratios (relative risks) rather than odds-ratios because the outcome (pubertal development) was not rare (Zou, 2004). Prevalence ratios were computed using Proc Genmod (SAS, Inc., Cary, NC). Models were fitted for pubertal stages (any pubertal signs vs none: B2+ vs B1 for breast and H2+ vs H1 for hair) by including exposure measures (continuous variables [ln] or tertiles as indicator variables), age, race, BMI, height, and relevant predictor variables for each exposure. Backward elimination was conducted to remove covariates that did not alter the coefficient for pubertal stage by more than 10%. We assumed that hormonal exposures were unlikely to operate independent of BMI, a strong endogenous hormonal factor in pubertal development. Therefore, the BMI-exposure joint effects were also explored using four-category indicator BMI-environmental exposure variables with BMI dichotomized at the median and the environmental exposure dichotomized at the top vs bottom two tertiles of Pb, urinary phytoestrogen, BPA metabolites and dietary intake; exposure dichotomized at the median for DDE and PCBs because the numbers were small.
Results
We recruited healthy girls for this study from our hospital or a nearby affiliated private clinic. They represented 3 ethnic groups and were 9 years old (average 9.5 years), an age that we chose to provide equal proportions of breast development (B1=none; B2+=any) based on a earlier pilot study at the private clinic. Breast development was present in 53% of girls (74/192 girls were B2, 21 were B3, and 6 were B4) and pubic hair development in 31%. Socioeconomic status was broad, with half of the mothers having less than 13 years' education; proportions were similar at the hospital and private clinic (Table 1). Mothers not born in the US were mainly from Europe (n=8, 4%) and Latin America (n=40, 21%). As previously reported, pubertal stage (any vs none) was more advanced for Black ethnicity and greater weight, BMI, or height in this population (Britton et al., 2004). Average BMI (18.8 kg/m2) was close to the national 75th-percentile of 18.3 kg/m2 among 9.5 year-old girls in the year 2000; the national norm (50th-percentile) was 16.5 kg/m2 (CDC, 2000).
Table 1.
Characteristics of a Multiethnic Cohort of Girls, Mount Sinai Hospital 1997-1998
| N (%) | Mean ± SD | range | N | ||
|---|---|---|---|---|---|
| Mother was US -born | 138 (73%) | 190 | |||
| Maternal education (13+ yr) | 91 (49%) | 185 | |||
| Parity (Nonparous before this child) | 63 (48%) | 130 | |||
| Girl was breastfed | 79 (42%) | 187 | |||
| Girl's race: | Black | 54 (28%) | |||
| Hispanic | 72 (38%) | 192 | |||
| White | 66 (34%) | ||||
| Breast stage | B2+ (vs B1) | 101 (53%) | 192 | ||
| Pubic Hair stage | H2+ (vs H1) | 59 (31%) | 192 | ||
| Recruited from private clinic (vs hospital clinic) | 84 (44%) | 192 | |||
| Maternal age (yr) | 28 ± 6.9 | 14-43 | 116 | ||
| Girl's age (yr) | 9.5 ± 0.3 | 9.002-9.998 | 192 | ||
| Girl's BMI (kg/m2) | 18.8 ± 4.2 | 10.2-34.7 | 186 | ||
Plasma organochlorine (OCs) concentrations were low (Table 2), but the range was similar to that reported in other young populations during this time period, including the NHANES data for 12-19 year-olds in 1999-2000 (CDC, 2005). Median Pb was 2.4 μg/dL, typical of New York inner city children (Haley & Talbot, 2004) but higher than the CDC data for 6-11 year-olds in 1999-2000 (median 1.3 μg/dL) (CDC, 2005). The highest urinary phytoestrogen concentration was enterolactone, followed by daidzein and genistein, a pattern similar to that among children in the 1999-2000 NHANES. Median concentrations of urinary enterolactone (173 μg/L) and daidzein (70 μg/L) were somewhat lower than those found in NHANES children in 1999-2000 (353 and 101 μg/L, respectively); levels of daidzein (25 μg/L) were similar (32 μg/L for NHANES) (CDC, 2005). Our median creatinine-corrected concentrations were closer to those of NHANES children in 1999-2000 (260, 88, 28 μg/gC in our girls for enterolactone, daidzein, and genistein vs 384, 93, 28 μg/gC respectively for NHANES).
Table 2.
Biomarker Levels and Phytoestrogen Intake Means by Breast and Hair Stages in a Multiethnic Cohort of Girls, Mount Sinai Hospital 1997-1998
| Breast stage = 1 | Breast stage = 2+ | Hair stage = 1 | Hair stage = 2+ | |||||
|---|---|---|---|---|---|---|---|---|
| GM | GSD | GM | GSD | GM | GSD | GM | GSD | |
| Exposure biomarkersa | ||||||||
| DDE (ug/L plasma) | 0.43 | 2.0 | 0.39 | 2.1 | 0.42 | 2.1 | 0.39 | 1.9 |
| PCB (ug/L plasma) | 0.83 | 1.9 | 0.83 | 1.8 | 0.79 | 1.9 | 0.90 | 1.7 |
| N | 36 | 51 | 54 | 33 | ||||
| Pb (ug/dL whole blood) | 2.1 | 1.8 | 2.3 | 1.6 | 2.0 | 1.7 | 2.4 | 1.7 |
| N | 63 | 78 | 93 | 48 | ||||
| Daidzein (ug/gC) | 3.1 | 6.6 | 1.8 | 5.3 | 2.8 | 4.9 | 1.5 | 8.4 |
| Genistein (ug/gC) | 11.8 | 3.4 | 5.6 | 5.1 * | 8.4 | 3.7 | 7.1 | 6.2 |
| Enterolactone (ug/gC) | 32.3 | 2.8 | 19.6 | 3.1 * | 25.3 | 3.0 | 24.1 | 3.0 |
| BPA (ug/gC) | 0.24 | 10.3 | 0.11 | 12.9 * | 0.19 | 10.1 | 0.10 | 16.1 |
| N | 81 | 88 | 117 | 52 | ||||
| Phytoestrogen intake (mg/d) | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Flavones | 0.7 | 1.2 | 0.6 | 1.0 | 0.7 | 1.1 | 0.6 | 1.1 |
| Flavonols | 11.7 | 6.1 | 11.9 | 6.5 | 11.4 | 6.1 | 12.6 | 6.8 |
| Isoflavones | 1.7 | 2.4 | 2.0 | 3.4 | 1.7 | 2.2 | 2.2 | 4.0 |
| Lignans | 0.8 | 0.5 | 0.8 | 0.6 | 0.8 | 0.5 | 0.9 | 0.6 |
| Phytosterols | 252 | 200 | 283 | 214 | 252 | 199 | 304 | 224 |
| Calorie intake (cal/d) | 3,185 | 1197 | 2,893 | 1107 | 3060 | 1241 | 2958 | 956 |
| N | 81 | 96 | 120 | 57 | ||||
Biomarkers are presented as geometric means (GM) and standard deviations (GSD); dietary intakes are means and standard deviations. Starred rows had statistically significantly different geometric means by t-test (p<.05).
p<0.05 for t-test of means of log-transformed variables.
Phytoestrogen intake was highest for phytosterols and flavonols; quercetin comprised most of the flavonol intake (median 8.7 mg/d; data not shown), which is typical of Western diets (Hertog et al., 1993). As reported for other studies, quercetin came mainly from fruit (data not shown). Other dietary phytoestrogens, aside from the phytosterols, were derived mainly from fruit and vegetables (not shown). Phytosterols chiefly come from oils (including margarine and oil-containing foods such as bread or fried food). Urinary phytoestrogens were not correlated with phytoestrogen intakes from the YAQ; however urinary phytoestrogens were significantly correlated with having eaten fruit within the past 24 hr (apples, apple juice, or cherries; Spearman r p<0.05, not shown).
Girls with breast stage 1 (91/192) had higher mean urinary phytoestrogen levels compared with girls at B2+ (Table 2). Results were similar for creatinine-corrected concentrations (shown in Table 2) or for uncorrected values (as μg/L, not shown). Plasma OCs, Pb, and dietary phytoestrogens did not differ by breast stage, and no exposures varied by pubic hair stage.
In multivariate models, only urinary daidzein remained significantly associated with breast stage (Table 3). Genistein was weakly protective for pubic hair stage, but not significantly. Results were almost identical for urinary phytoestrogen concentrations whether they were creatinine-corrected (Table 3) or not (μg/L with or without adjusting for ln-creatinine in the model, not shown; continuous ln-variables). Flavones had a suggestive inverse association with breast stage, but the trend was not linear and not significant. For other exposures, the estimates were near 1.0 and had wide confidence intervals. Combinations of exposures within types (all blood biomarkers, all urine, all diet) were also examined; effects did not change from the single exposure models (not shown).
Table 3.
Prevalence ratios and 95%-confidence intervals for any vs no development in relation to Environmental Exposures
| PR (CI) for Breast stage 2+ vs stage 1 | PR (CI) for Pubic hair stage 2+ vs stage 1 | |||
|---|---|---|---|---|
| Blood biomarkers | ||||
| DDE (loge ug/L plasma; N=86) | 1.008 (0.72-1.40) | 1.07 (0.71-1.62) | ||
| PCB (loge ug/L plasma) | 0.92 (0.68-1.22) | 1.12 (0.73-1.73) | ||
| Pb (loge ug/dL whole blood) N=139 | 1.01 (0.79-1.30) | 1.25 (0.83-1.88) | ||
| Urinary metabolites N=164 | ||||
| Daidzein (loge ug/gC urine) | 0.89 (0.83-0.96)* | 0.96 (0.84-1.14) | ||
| Genistein (loge ug/gC urine) | 0.94 (0.88-1.01) | 0.90 (0.80-1.02) | ||
| Enterolactone (loge ug/gC urine) n=152 | 0.92 (0.81-1.14) | 1.12 (0.91-1.38) | ||
| BPA loge ug/gC urine) | 0.96 (0.92-1.01) | 0.98 (0.89-1.08) | ||
| Phytoestrogen intake, n=172 | ||||
| 1st tertile=referent | 2nd tertile | 3rd tertile | 2nd tertile | 3rd tertile |
| Flavonols | 1.36 | 1.18 | 1.67 | 1.50 |
| (0.98-1.89) | (0.81-1.72) | (0.99-2.82) | (0.89-2.50) | |
| Flavones | 0.76 | 0.75 | 1.26 | 0.91 |
| (0.56-1.02) | (0.54-1.06) | (0.77-2.06) | (0.52-1.59) | |
| Isoflavones | 1.14 | 1.14 | 1.33 | 1.18 |
| (0.83-1.54) | (0.80-1.63) | (0.80-2.22) | (0.68-2.04) | |
| Lignans | 0.95 | 1.19 | 1.04 | 1.26 |
| (0.68-1.32) | (0.86-1.64) | (0.61-1.77) | (0.79-2.03) | |
| Phytosterols | 0.86 | 1.10 | 1.04 | 1.52 |
| (0.62-1.20) | (0.79-1.53) | (0.59-1.85) | (0.91-2.56) | |
p<0.05
Models adjusted for DDE (Breast) age, BMI, height, Black race, breastfed; (Hair) BMI, height, black race, breastfed; PCB (breast) age, height, Black race, maternal country of birth (US/PR vs other); (Hair) height, Black race, maternal country of birth; Pb (breast) age, BMI, Black race; (Hair) height, Black race, private clinic.
Urinary phytoestrogen models for Breast Stage with daidzein and genistein included BMI, height, and Black race. The enterolactone model was adjusted for height, Black race, maternal education (≤ 13 yr vs 13+) and excluded urine samples with creatinine <10 mg/dL, yielding N = 152. For BPA, the model included height and Black race. For Hair Stage, models included BMI, height, and Black race, except for enterolactone which was adjusted for height, Black race, maternal education (≤ 13 yr vs 13+) and excluded urine samples with creatinine <10 mg/dL.
Phytoestrogen intake (mg/d) models for breast stage were adjusted for BMI, height, Black race, and calories; the lignan model was also adjusted for Hispanic race and private clinic enrolment; that for phytosterols added private clinic enrolment. The models for Hair stage adjusted for height and Black race; in addition the model for flavonols added age; lignans and phytosterols both added age and private clinic enrolment.
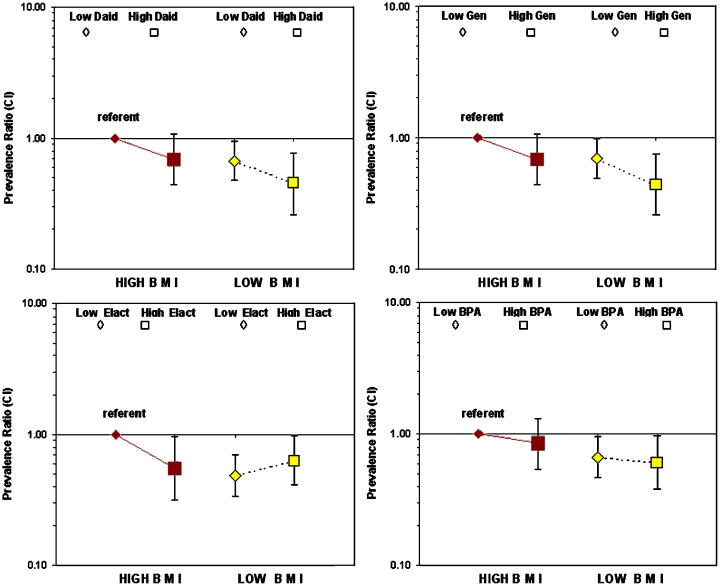
Consideration of BMI as a modifier of hormonal exposures provided further information about relationships of the biomarkers with breast development. Table 4 shows results after stratifying the effects of urinary metabolites (top vs bottom two tertiles) by BMI (< vs ≥ median, or low- vs high-BMI), and Figure 1 depicts these findings. Our hypothesis was that girls with high BMI and low phytoestrogen levels would have higher breast stage (B2+). Indeed, in this subgroup, breast stage 2 or higher was more common than other subgroups for all four urinary biomarkers; they are the reference groups in Table 4 and Figure 1. In addition, girls having low BMI and highest urinary biomarker levels had delayed breast development for 3 of the 4 biomarkers. For urinary daidzein and genistein, there was a similar effect on delayed development in both BMI groups such that high exposure lowered the prevalence ratio by ∼30% compared with the referents (high-BMI, low exposures). Still, low-BMI/high exposure girls had the most delayed development (PRs and 95% CI = 0.46 [0.26-0.78] and 0.44 [0.26-0.76] for daidzein and genistein, respectively). Without considering the joint effect of BMI, girls with higher urinary daidzein or genistein had delayed breast development (PR for both biomarkers was 0.66 CI 0.47-0.9, 3rd vs 1st and 2nd tertiles combined, adjusting for BMI in the model). Urinary enterolactone was associated with significantly delayed breast development only in high-BMI girls. However, without considering the joint effect of BMI, enterolactone had no association with breast development (PR 0.83 CI 0.62-1.19, adjusting for BMI in the model). BPA appeared to be unrelated to risk (with or without consideration of BMI) (Table 4). The trends were similar for phytoestrogen biomarkers without creatinine correction, with slight shifts in the confidence intervals (not shown). We also examined modification by BMI of DDE-, PCB-, and Pb-effects on development. DDE and Pb had no significant exposure modification. For PCBs, two subgroups had similar and null effects (low-BMI/low-PCB, high-BMI/high-PCB), but girls with low-BMI and ≥median PCB levels, were less likely to be B2+ than B1 (PR 0.60 [0.40-0.92]), all as compared with high-BMI/low-PCB (referent).
Table 4.
Prevalence ratios and 95%-confidence intervals for joint BMI and urinary biomarker exposure in relation to breast development
| PR (95%-CI) of breast stage 2+ vs stage 1 for tertiles of metabolites by: | ||||
|---|---|---|---|---|
| High BMI (≥ median) | Low BMI (< median) | |||
| Urinary metabolites, ug/gC n=164* | Tertiles 1+2 | Tertile 3 | Tertiles 1+2 | Tertile 3 |
| Daidzein | 1.0 (ref) | 0.69 (0.44 - 1.08) | 0.67 (0.48 - 0.94) * | 0.46 (0.26 - 0.78) ** |
| Genistein | 0.69 (0.44 - 1.07) | 0.70 (0.49 - 0.98)* | 0.44 (0.26 - 0.76)** | |
| Enterolactone | 0.55 (0.32 - 0.96)* | 0.49 (0.34 - 0.70)** | 0.63 (0.41 - 0.98)* | |
| BPA | 0.85 (0.54 - 1.33) | 0.67 (0.47 - 0.96)* | 0.60 (0.38 - 0.96)* | |
n (from left to right)=56, 27, 54, 27 (daidzein ug/gC); 60, 23, 50, 31 (genistein ug/gC); 57, 26, 53, 28 (enterolactone ug/gC); 58, 25, 52, 29 (BPA ug/gC).
p<0.05
p<0.01
Figure 1.
BMI, urinary metabolites and risk for breast stage 2+ vs 1. BMI is quantiled at the median; urinary metabolites are the 3rd (top) tertile (◊ high exposure) vs the 1st+2nd (□ low exposure) tertiles. Prevalence ratios and CIs are shown; models parameters are given in Table 4.
Discussion
Our main finding is that girls with high levels of urinary phytoestrogens are less likely to have experienced breast stage 2 than girls with low urinary metabolites. The protective effect of phytoestrogens may only be apparent when the joint BMI effect is considered. For instance, the effect of isoflavones (daidzein or genistein) was stronger in BMI strata, and the effect of urinary enterolactone was significant only among high-BMI girls. Of note, enterolactone is the urinary biomarker and the phytoestrogen with the highest exposure both in our population and in Western women. Girls with low BMI and high PCBs were also less likely to have breast stage 2+. We did not find significant associations of diet, plasma DDE, or Pb exposures with development. We found no effects on hair development, but our study was designed to detect effects with breast development. In addition, hormonal effects on pubertal hair development may be more responsive to adrenal androgens whereas breast maturation requires estrogen (Jones et al., 2007).
For DDE and PCBs limitations include our sample size which was small, low-level exposures, and not having lipid measurements which might alter the estimates for PCBs toward the null; low-BMI girls with higher PCB levels might also have lower lipids. Pb levels were also very low, with a median of 2 mg/dL. Analyses of NHANES girls found delayed development with high Pb in a large sample (Selevan et al., 2003; Wu et al., 2003). These reports found effects among girls with Pb >3 or >5 mg/dL, respectively. The lower bound of the upper tertile of Pb among our girls was close to 3 mg/dL, but we had only 7 girls with >5 mg/dL; therefore we may have had too few girls to detect an effect of elevated exposures.
Phytoestrogen and dietary intakes in our study were consistent with other reports (Hertog et al., 1993; Horn-Ross et al., 2000; Pillow et al., 1999) including children this age (Munoz et al., 1997; Rockett et al., 1995). Like our negative findings, other studies about dietary intake have been inconclusive (Koprowski et al., 1999b; Pedersen et al., 1991; Persky et al., 1992; Roberts et al., 1977), although two longitudinal studies have found fiber intake to have a protective effect for age at menarche (de Ridder et al., 1991; Koo et al., 2002). High fiber foods are also often high in phytoestrogens (Grace et al., 2004). Phytoestrogens have been found to perturb female reproductive function including cycle characteristics (Phipps et al., 1993) and endometriosis (Tsuchiya et al., 2007). A soy formula trial found no relationship with pubertal maturation (Strom et al., 2001). A potential limitation of our study is that pubertal onset alters dietary patterns, or that girls in puberty alter their dietary reporting. The dietary assessment may inadequately measure some phytoestrogen-rich ethnic-specific foods. On the other hand, it is likely that Hispanic girls in New York City eat a more Western than traditional Hispanic diet; this would make the YAQ an appropriate instrument. Further indication that the YAQ was not inappropriate for our population are the correlations seen between cruciferous vegetables in the 24-hr checklist and in the YAQ, though modest, are consistent with annual repeated assessments reported for this questionnaire (Rockett et al., 1995). However, questionnaire estimates like the YAQ are known to miss occult sources of phytoestrogens such as soy-fortified meat patties (Lampe et al., 1999), although our exposure categories should be relatively insensitive to underreporting because we ranked each intake as high or low. A limitation of the cross-sectional design is that phytoestrogen intake at the age of 9 years may not be relevant to pubertal stage at the same age; for example, in animal models exposures early in life are found to influence onset of puberty (Whitten et al., 1995), and diet preceding puberty was found to be associated with the growth spurt (Berkey et al., 2000). We did not have data on earlier diet. Urinary metabolites represent only recent exposure because clearance of a single dose occurs within days; however, a few studies, including one in children this age (Teitelbaum et al., 2008), have shown that phytoestrogen biomarkers are reasonably stable over a range of months to a year (Zeleniuch-Jacquotte et al., 1998), suggesting that intake of phytoestrogen-containing foods is fairly consistent. Therefore, in our study, the urinary metabolites may be more representative of the past year's phytoestrogen intake than dietary recall, as suggested for isoflavones (Atkinson et al., 2002). Additional limitations of our study include the relatively small sample size, the cross-sectional design, and possibly inadequate adjustment for socioeconomic status. There is also possible residual confounding among the urinary biomarkers, BMI, and creatinine (Barr et al., 2005). Although our findings were hypothesis-driven, we cannot eliminate the possibility of chance associations among the multiple comparisons undertaken.
Differences in the biomarker associations with development could be explained by differences in concentrations combined with varying biological potency and endogenous hormones. The proposed anti-estrogenic effects of phytoestrogens are well known (Adlercreutz, 2002). These agents may alter estrogen's effectiveness (block CYP19, reduce cell proliferation, bind to the estrogen receptor, stimulate SHBG production) (Adlercreutz, 2002). Weak estrogens may be protective by competing with endogenous hormones, and hormonal vs anti-hormonal effects of such agents may depend on the hormonal milieu (Paris et al., 2002, Sun et al., 2006). This supports our findings of stronger effects when incorporating obesity as a modifier of exposure. The PRs were around 0.5 for the group with highest concentration phytoestrogen biomarkers if considered together with their BMI.
BPA levels were much lower than phytoestrogens in our study, but BPA may may act more as hormone-agonist than antagonist. BPA has not been widely studied in humans, but it has been associated with PCOS (a hyperandrogenic condition) (Takeuchi et al., 2004). PCBs may have dioxin-like activity, and it has been reported that PCBs delay puberty in rats (Faqi et al., 1998; Lundkvist, 1990); this is consistent with our finding of delayed breast development, seen only among girls with lower BMI who had higher PCB levels compared with other BMI-PCB groups. Similar delays in maturation were reported for both boys and girls with dioxin-like exposures (Den Hond et al., 2002). In contrast, Warner reported that exposure to dioxin (the quintessential environmental anti-estrogen) in childhood was related to earlier age at menarche (Warner et al., 2004). Relatively high in utero exposures to DDE or Pb but not PCB were associated with earlier menarche (Blanck et al., 2000; Vasiliu et al., 2004), while in North Carolina no relationships were seen between perinatal DDE or PCB exposure and pubertal development (Gladen et al., 2000).
A central motivation for investigating relationships of environmental exposures with pubertal development is the idea that earlier puberty confers later risk for chronic disease, including breast cancer. Phytoestrogens have long been considered as strong candidates for ameliorating hormonally related disorders. One reason is the low breast cancer rates in Asia and the high isoflavone content of their diet (Adlercreutz, 2002). A growing body of epidemiologic research supports a protective effect of isoflavones on breast cancer risk, including adolescent soy consumption (Shu et al., 2001; Thanos et al., 2006), although the early epidemiologic studies were equivocal (Adlercreutz, 2002). Studies of dietary intake of other phytoestrogens have also found reduced breast cancer risk (Fink et al., 2007; Touillaud et al., 2005; Verheus et al., 2007), but the data are not consistent (Adebamowo et al., 2005). Measurement of phytoestrogen biomarkers has also shown some protection for breast cancer as well as heart disease (Hertog et al., 1993; Ingram et al., 1997; Liang et al., 2006; Teede et al., 2001; Zheng et al., 1999), but again there have also been many studies that found no such effects (Grace et al., 2004; Zeleniuch-Jacquotte et al., 2004). However, no studies have examined phytoestrogen biomarkers and puberty or menarche which may be the relevant window of exposure.
Conclusions
Our results support a possible effect of environmental agents on breast development, which is dependent on BMI. In particular, the delayed breast development associated with higher levels of urinary phytoestrogens is more pronounced amoung girls with high BMI. Further research is needed to confirm this in larger samples and in studies with a longitudinal design. Also, it may be important to study dietary intake earlier in life and genetic factors or other exposures that may be more relevant to puberty than current eating habits. Further investigation of environmental and lifestyle factors, including more accurate dietary assessments, may suggest approaches to prevention of early puberty.
Acknowledgments
Support by grants from EPA R825816, CDC CCU300860, AICR 97A057, US Army Medical Research and Materiel Command under Award Number DAMD 17-99-1-9303, NIEHS/EPA Children's Center grants ES09584 and R827039, ES/CA12770, and from the Rubin Shulsky Philanthropic Fund of the Jewish Communal Fund is gratefully acknowledged. Informed consent was obtained from a parent or guardian of each girl and assent from the girl; the research was overseen by the Hospital's human subjects review board. We thank Dr. Nathan Kase and Dr. Neil Leleiko for guidance in the study design and in clinical interpretations; Danielle Taylor-Thomas for research assistance; Karen Ireland for laboratory support; Geoffrey C. Kabat for advice on the dietary intake; James Godbold and Robert Lapinski for statistical support.
Abbreviations
- BMI
body mass index
- YAQ
Youth/Adolescent Questionnaire
- DDE
1,1′-dichloro-2,2′-bis(4-chlorophenyl)ethylene
- PCB
polychlorinated biphenyls
- Pb
- CI
- PR
- CDC
Centers for Disease Control
- μg/gC
micrograms-per-gram creatinine
- NHANES
National Health and Nutrition Examination Survey
- mg/d
milligrams per day
- rS
Spearman correlation coefficient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adebamowo CA, Cho E, Sampson L, Katan MB, Spiegelman D, Willett WC, Holmes MD. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Phytoestrogens and breast cancer. J Steroid Biochem Mol Biol. 2002:113–118. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wahala K, Schwartz SM, Lampe JW. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol Biomarkers Prev. 2002;11:253–260. [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Gardner JD, Frazier AL, Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol. 2000;152:446–452. doi: 10.1093/aje/152.5.446. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- Britton JA, Wolff MS, Lapinski R, Forman J, Hochman S, Kabat GC, Godbold J, Larson S, Berkowitz GS. Characteristics of pubertal development in a multi-ethnic population of nine-year-old girls. Ann Epidemiol. 2004;14:179–187. doi: 10.1016/j.annepidem.2002.08.001. [DOI] [PubMed] [Google Scholar]
- CDC. CDC Growth Charts: United States. 2000. http://www.cdc.gov/growthcharts/ [PubMed]
- CDC. National Report on Human Exposure to Environmental Chemicals. 2005. http://www.cdc.gov/exposurereport/
- de Ridder CM, Thijssen JH, Van V, van Duuren R, Bruning PF, Zonderland ML, Erich WB. Dietary habits, sexual maturation, and plasma hormones in pubertal girls: a longitudinal study [see comments] Am J Clin Nutr. 1991;54:805–813. doi: 10.1093/ajcn/54.5.805. [DOI] [PubMed] [Google Scholar]
- Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, Winneke G, Vanderschueren D, Staessen JA. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek's hypothesis revisited. Environ Health Perspect. 2002;110:771–776. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics. 2005;115:e127–e134. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- Faqi AS, Dalsenter PR, Merker HJ, Chahoud I. Effects on developmental landmarks and reproductive capability of 3,3′,4,4′-tetrachlorobiphenyl and 3,3′,4,4′,5-pentachlorobiphenyl in offspring of rats exposed during pregnancy. Hum Exp Toxicol. 1998;17:365–372. doi: 10.1177/096032719801700702. [DOI] [PubMed] [Google Scholar]
- Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol. 2007 doi: 10.1093/aje/kwk033. 2006 Dec 11. [DOI] [PubMed] [Google Scholar]
- Gellert RJ. Uterotrophic activity of polychlorinated biphenyls (PCB) and induction of precocious reproductive aging in neonatally treated female rats. Environ Res. 1978;16:123–130. doi: 10.1016/0013-9351(78)90149-4. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136:490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA, Botting NP, Dowsett M, Welch AA, Khaw KT, Wareham NJ, Day NE, Bingham SA. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev. 2004:698–708. [PubMed] [Google Scholar]
- Haley VB, Talbot TO. Geographic analysis of blood lead levels in New York State children born 1994-1997. Environ Health Perspect. 2004;112:1577–1582. doi: 10.1289/ehp.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network [see comments] Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL, Barnes S, Lee M, Coward L, Mandel JE, Koo J, John EM, Smith M. Assessing phytoestrogen exposure in epidemiologic studies: development of a database (United States) Cancer Causes Control. 2000;11:289–298. doi: 10.1023/a:1008995606699. [DOI] [PubMed] [Google Scholar]
- Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350:990–994. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- Jones ME, McInnes KJ, Boon WC, Simpson ER. Estrogen and adiposity-Utilizing models of aromatase deficiency to explore the relationship. J Steroid Biochem Mol Biol. 2007;106:3–7. doi: 10.1016/j.jsbmb.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Asakevich S, Indurkhya A, Witten J, Kruse H. Childhood growth and exposure to dichlorodiphenyl dichloroethene and polychlorinated biphenyls. J Pediatr. 2002;140:33–39. doi: 10.1067/mpd.2002.120764. [DOI] [PubMed] [Google Scholar]
- Kato I, Tominaga S, Suzuki T. Factors related to late menopause and early menarche as risk factors for breast cancer. Jpn J Cancer Res. 1988;79:165–172. doi: 10.1111/j.1349-7006.1988.tb01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo MM, Rohan TE, Jain M, McLaughlin JR, Corey PN. A cohort study of dietary fibre intake and menarche. Public Health Nutr. 2002;5:353–360. doi: 10.1079/PHN2002261. [DOI] [PubMed] [Google Scholar]
- Koprowski C, Ross RK, Mack WJ, Henderson BE, Bernstein L. Diet, body size and menarche in a multiethnic cohort. Br J Cancer. 1999;79:1907–1911. doi: 10.1038/sj.bjc.6690303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe JW, Gustafson DR, Hutchins AM, Martini MC, Li S, Wahala K, Grandits GA, Potter JD, Slavin JL. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol Biomarkers Prev. 1999;8:699–707. [PubMed] [Google Scholar]
- Liang YL, Teede H, Dalais F, McGrath BP. The effects of phytoestrogen on blood pressure and lipids in healthy volunteers. Zhonghua Xin Xue Guan Bing Za Zhi. 2006:726–729. [PubMed] [Google Scholar]
- Liu Z, Wolff MS, Moline J. Analysis of environmental biomarkers in urine using an electrochemical detector. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;819:155–159. doi: 10.1016/j.jchromb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Lundkvist U. Clinical and reproductive effects of Clophen A50 (PCB) administered during gestation on pregnant guinea pigs and their offspring. Toxicology. 1990;61:249–257. doi: 10.1016/0300-483x(90)90175-g. [DOI] [PubMed] [Google Scholar]
- Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004:513–531. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Sprecher DL, Barton BA, Waclawiw MA, Daniels SR. Overweight, fat patterning, and cardiovascular disease risk factors in black and white girls: The National Heart, Lung, and Blood Institute Growth and Health Study [see comments] J Pediatr. 1999;135:458–464. doi: 10.1016/s0022-3476(99)70168-x. [DOI] [PubMed] [Google Scholar]
- Munoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food intakes of US children and adolescents compared with recommendations. Pediatrics. 1997;100:323–329. doi: 10.1542/peds.100.3.323. published erratum appears in Pediatrics 1998 May;101(5):952-3. [DOI] [PubMed] [Google Scholar]
- Paris F, Balaguer P, Terouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193:43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Bartholomew MJ, Dolence LA, Aljadir LP, Netteburg KL, Lloyd T. Menstrual differences due to vegetarian and nonvegetarian diets. Am J Clin Nutr. 1991;53:879–885. doi: 10.1093/ajcn/53.4.879. [DOI] [PubMed] [Google Scholar]
- Persky VW, Chatterton RT, Van Horn LV, Grant MD, Langenberg P, Marvin J. Hormone levels in vegetarian and nonvegetarian teenage girls: potential implications for breast cancer risk. Cancer Res. 1992;52:578–583. [PubMed] [Google Scholar]
- Phipps WR, Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of flax seed ingestion on the menstrual cycle. J Clin Endocrinol Metab. 1993:1215–1219. doi: 10.1210/jcem.77.5.8077314. [DOI] [PubMed] [Google Scholar]
- Pillow PC, Duphorne CM, Chang S, Contois JH, Strom SS, Spitz MR, Hursting SD. Development of a database for assessing dietary phytoestrogen intake. Nutr Cancer. 1999;33:3–19. doi: 10.1080/01635589909514742. [DOI] [PubMed] [Google Scholar]
- Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol--the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992;75:624–631. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Laing PM, Rantsho JM, Swinel RW. The bearing of diverse patterns of diet on growth and menarche in four ethnic groups of South African girls. J Trop Med Hyg. 1983;86:5–12. [PubMed] [Google Scholar]
- Roberts DF, Chinn S, Girija B, Singh HD. A study of menarcheal age in India. Ann Hum Biol. 1977;4:171–177. doi: 10.1080/03014467700002061. [DOI] [PubMed] [Google Scholar]
- Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–340. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, Ringer D, Thomas PE. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J Toxicol Environ Health A. 1998;54:101–120. doi: 10.1080/009841098158944. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348:1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, Hursting SD. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33:20–25. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- Sun H, Xu LC, Chen JF, Song L, Wang XR. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem Toxicol. 2006;44:1916–1921. doi: 10.1016/j.fct.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S, Calafat AM, Britton JA, Silva MJ, Ye X, Kuklenyik Z, Reidy JA, Brenner BL, Galvez MP, Wolff MS. Temporal variability in urinary phthalate metabolites, phenols and phytoestrogens among children. Environ Res. 2008 doi: 10.1016/j.envres.2007.09.010. In press. [DOI] [PubMed] [Google Scholar]
- Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006:1253–1261. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- Touillaud MS, Pillow PC, Jakovljevic J, Bondy ML, Singletary SE, Li D, Chang S. Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr Cancer. 2005:162–169. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Miura T, Hanaoka T, Iwasaki M, Sasaki H, Tanaka T, Nakao H, Katoh T, Ikenoue T, Kabuto M, Tsugane S. Effect of soy isoflavones on endometriosis: interaction with estrogen receptor 2 gene polymorphism. Epidemiology. 2007:402–408. doi: 10.1097/01.ede.0000257571.01358.f9. [DOI] [PubMed] [Google Scholar]
- USDA. USDA; 2000. USDA nutrient database. http://www.nal.usda.gov/fnic/foodcomp/ [Google Scholar]
- Vasiliu O, Muttineni J, Karmaus W. In utero exposure to organochlorines and age at menarche. Hum Reprod. 2004;19:1506–1512. doi: 10.1093/humrep/deh292. [DOI] [PubMed] [Google Scholar]
- Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH. Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol. 2007;25:648–655. doi: 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- Walters LM, Rourke AW, Eroschenko VP. Purified methoxychlor stimulates the reproductive tract in immature female mice. Reprod Toxicol. 1993;7:599–606. doi: 10.1016/0890-6238(93)90036-7. [DOI] [PubMed] [Google Scholar]
- Warner M, Samuels S, Mocarelli P, Gerthoux PM, Needham L, Patterson DG, Jr, Eskenazi B. Serum dioxin concentrations and age at menarche. Environ Health Perspect. 2004;112:1289–1292. doi: 10.1289/ehp.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten PL, Lewis C, Russell E, Naftolin F. Phytoestrogen influences on the development of behavior and gonadotropin function. Proc Soc Exp Biol Med. 1995;208:82–86. doi: 10.3181/00379727-208-43836. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Naftolin F. Effects of a phytoestrogen diet on estrogen-dependent reproductive processes in immature female rats. Steroids. 1992;57:56–61. doi: 10.1016/0039-128x(92)90029-9. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Lioy PJ, Santella RM, Wang RY, Jones RL, Caldwell KL, Sjodin A, Turner WE, Li W, Georgopoulos P, Berkowitz GS. Exposures among pregnant women near the World Trade Center site on 11 September 2001. Environ Health Perspect. 2005;113:739–748. doi: 10.1289/ehp.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Environ Health Perspect. 2003;111:737–741. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Adlercreutz H, Akhmedkhanov A, Toniolo P. Reliability of serum measurements of lignans and isoflavonoid phytoestrogens over a two-year period. Cancer Epidemiol Biomarkers Prev. 1998;7:885–889. [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Adlercreutz H, Shore RE, Koenig KL, Kato I, Arslan AA, Toniolo P. Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br J Cancer. 2004;91:99–105. doi: 10.1038/sj.bjc.6601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Dai Q, Custer LJ, Shu XO, Wen WQ, Jin F, Franke AA. Urinary excretion of isoflavonoids and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:35–40. [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]