Abstract
Disordered gambling (DG) will soon be included along with the substance use disorders in a revised diagnostic category of the Diagnostic and Statistical Manual of Mental Disorders DSM-5 called ‘Substance Use and Addictive Disorders’. This was premised in part on the common etiologies of DG and the substance use disorders. Using data from the national community-based Australian Twin Registry, we used biometric model fitting to examine the extent to which the genetic liabilities for DG and alcohol use disorder (AUD) were shared, and whether this differed for men and women. The effect of using categorical versus dimensional DG and AUD phenotypes was explored, as was the effect of using diagnoses based on the DSM-IV and the proposed DSM-5 diagnostic criteria. The genetic correlations between DG and AUD ranged from 0.29 to 0.44. There was a significantly larger genetic correlation between DG and AUD among men than women when using dimensional phenotypes. Overall, about one-half to two-thirds of the association between DG and AUD was due to a shared genetic vulnerability. This study represents one of the few empirical demonstrations of an overlap in the genetic risk for DG and another substance-related addictive disorder. More research is needed on the genetic overlap between DG and other substance use disorders, as well as the genetic overlap between DG and other (non-substance-related) psychiatric disorders.
Keywords: disordered gambling, alcohol use disorder, genetic influences, twin study, sex differences, comorbidity
For the first time ‘behavioral addictions’ (Grant et al., 2010; Petry, 2006; Potenza, 2006) will be recognized in the official diagnostic nomenclature, with the fifth revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (Holden, 2010). A number of candidate behavioral addictions were considered, such as Internet, sex, eating, and shopping, but the only behavioral addiction to be formally codified is disordered gambling (DG), which will now be grouped together in the same category as the substance-related addictions. This decision was based in part on symptomatic and neurobiological similarities and genetic overlap between DG and the substance use disorders (Frascella et al., 2010) and the more developed research base for DG than for the other behavioral addictions. (The term ‘disordered gambling’ is used in this report to refer to the full spectrum of gambling-related problems, including pathological gambling as defined by the DSM as well as subclinical problems (Shaffer et al., 1999)).
Epidemiologic research has consistently demonstrated the frequent co-occurrence of DG and substance-related addictions. In the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC), the odds ratios of the associations between lifetime DG and alcohol, nicotine, and illicit drug dependence were 6.5, 7.2, and 4.8, respectively, with larger associations obtained among women than men (Petry et al., 2005). In particular, the odds ratios (adjusted for age, race–ethnicity, marital status, urbanicity, region, education, and income) of the associations between DG and alcohol dependence were 9.5 among women and 4.6 among men.
To our knowledge, there is only one genetically informed study that has examined the genetic overlap between DG and a substance-related addictive disorder. Results from the all-male United States Vietnam Era Twin (VET) Registry study found that the genetic correlation between DSM-III-R DG (APA, 1987) and alcohol dependence was rG = .45. Seventy-five percent of the overall association between DG and alcohol dependence was due to genetic factors (Slutske et al., 2000). Because this study was limited to men, it was not possible to examine whether the strength of the association between DG and alcohol dependence or the sources of their co-occurrence differed between men and women. In sum, the evidence base supporting an overlap in the genetic contributions to DG and substance-related addictions is quite thin, prompting experts to conclude that ‘more investigation into common and unique genetic contributions to pathological gambling and substance dependence are needed’ (Frascella et al., 2010, p. 297).
The purpose of the present investigation was to provide much-needed evidence about the genetic overlap between DG and one of the most common substance-related addictions, alcohol use disorder (AUD). The present investigation is an advance over the previous study (Slutske et al., 2000) in three important ways. First, both women and men were included. Given the sex difference in the strength of the association between DG and alcohol dependence in the NESARC study, we hypothesized that there also may be sex differences in the contribution of genetic and environmental factors to their comorbidity. Second, we incorporated diagnoses based on the proposed DSM-5 in order to see whether similar results would be obtained using the DSM-IV (APA, 1994) and the proposed DSM-5 diagnostic formulation. The DSM-5 also includes a severity-graded ordinal diagnosis of mild, moderate, and severe DG and AUD; this severity distinction is the approach that the DSM-5 plans to adopt for providing a dimensional diagnostic option (American Psychiatric Association, 2012). Third, we explored the genetic overlap between DG and AUD using continuous, dimensional phenotypes. This is compatible with the call for the DSM-5 to offer a dimensional diagnostic option (Helzer et al., 2007) and would have the benefit of yielding more statistical power for detecting sex differences in biometric models.
Method
Participants
Participants in this study were 4,764 members of the Australian Twin Registry (ATR) Cohort II (for more details about the study participants and the zygosity determination, see Slutske et al., 2009). In 2004–2007, a telephone interview containing a thorough assessment of gambling behaviors was conducted with the ATR Cohort II members (individual response rate of 80.4%). The mean age was 37.7 years (range = 32–43 years) and 57.2% of the sample was female. There were 1,875 complete twin pairs (867 monozygotic (MZ) [520 female, 347 male], 1,008 dizygotic (DZ) [367 female–female, 227 male–male, and 414 female–male]), and 1,014 individual twins from incomplete pairs (304 MZ [151 female, 153 male], 710 DZ [181 women from female–female pairs, 216 men from male–male pairs, and 207 women and 106 men from female–male pairs]).
Procedure
Twins were assessed by structured telephone interview. Interviews were administered by trained lay interviewers who were blind to the status of the cotwin. Interviewers were supervised by a clinical psychologist with over 10 years of experience. All interview protocols were reviewed either by the project coordinator or by research editors (veteran skilled interviewers from previous studies who had maintained consistently low error rates in coding). All interviews were tape-recorded and a random sample of 5% of the interview tapes was reviewed for quality control and coding inconsistencies. A small subsample of the participants (N = 166) was re-interviewed several months after their initial interview (mean interval = 3.4 months, SD = 1.4 months, range = 1.2–9.5 months) to establish the test–retest reliability of the interview measures. Individuals with a history of DG symptoms were over-sampled for the test–retest reliability study. This study was approved by the Institutional Review Boards at the University of Missouri and the Queensland Institute of Medical Research. All of the participants provided informed consent.
Measures
Two phenotyping approaches were used. The first approach was based on categorical diagnoses according to the DSM-IV and the proposed DSM-5 criteria. The second approach used dimensional phenotypes. The purpose of the latter approach was to create the most informative phenotypes for characterizing comorbidity (Helzer et al., 2007) and maximizing statistical power in biometric models (Kramer, 2007; Markon et al., 2011).
Disordered Gambling
The DG assessment was based on the National Opinion Research Center DSM-IV Screen for Gambling Problems (NODS; Gerstein et al., 1999). The DSM-IV diagnostic criteria for DG were assessed for all participants who reported that they had ever gambled at least five times within a single 12-month period; the majority of the participants, 77.5%, surpassed this gambling threshold. Five different DG phenotypes were included: (1) A dichotomous categorical DG phenotype based on a cutoff of endorsing five or more DSM-IV DG symptoms (corresponding to a DSM-IV diagnosis of pathological gambling disorder), (2) a dichotomous categorical DG phenotype based on a cutoff of endorsing four or more DSM-IV DG symptoms (corresponding to the proposed DSM-5 diagnosis of gambling disorder), (3) a DSM-5 severity-graded (mild, moderate, and severe) DG diagnosis, (4) a dichotomous categorical DG phenotype based on a cutoff of endorsing one or more DSM-IV DG symptoms, and (5) a dimensional DG phenotype. The reliabilities of these measures were excellent (see Table 1 for prevalences and reliabilities), and the validity of diagnoses of DG based on the NODS has been demonstrated in several studies (Hodgins, 2004; Slutske et al., 2011; Wickwire et al., 2008). The correlations between the categorical and dimensional DG phenotypes were all very high (r ≥ .94). The fourth and fifth phenotypes listed above were used in the biometric model-fitting analyses.
TABLE 1.
Prevalences and Reliabilities of Disordered Gambling and Alcohol Use Disorder Diagnoses
| Lifetime prevalence (%)
|
Test–retest reliability
|
|||||
|---|---|---|---|---|---|---|
| Full sample (N = 4,764) | Men (N = 2,037) | Women (N = 2,727) | Kappa | Yule’s Y | ra | |
| Disordered gambling | ||||||
| Categorical | ||||||
| DSM-IV DG | 2.2 | 3.4 | 1.2 | 0.67 | 0.79 | .92 |
| DSM-5 DG | 2.8 | 4.2 | 1.7 | 0.75 | 0.82 | .95 |
| 1+ symptom categorical DG | 12.5 | 18.2 | 8.3 | 0.79 | 0.80 | .95 |
| Dimensional | ||||||
| DSM-5 severity levelsb | 1.4/0.9/0.5 | 2.1/1.5/0.7 | 0.9/0.4/0.4 | 0.77 | — | .95 |
| Mean (standard deviation) | ||||||
|
|
||||||
| DG factor score | 0.06 (0.78) | 0.23 (0.81) | −0.07 (0.74) | — | — | — |
|
| ||||||
| Alcohol use disorder | ||||||
| Categorical | ||||||
| DSM-IV AD | 11.4 | 15.7 | 8.1 | 0.65 | 0.70 | .87 |
| DSM-5 AUD | 31.3 | 40.8 | 24.2 | 0.62 | 0.67 | .86 |
| Dimensional | ||||||
| DSM-5 severity levelsb | 19.8/6.8/4.6 | 25.0/9.6/6.6 | 16.4/4.7/3.1 | 0.58 | — | .84 |
| Mean (standard deviation) | ||||||
|
|
||||||
| DSM-IV AUD symptom count | 1.38 (1.96) | 1.82 (2.22) | 1.05 (1.66) | — | — | .78c |
Note:
Polychoric correlation;
severity levels of mild, moderate, and severe;
Pearson’s correlation.
DG = disordered gambling; AD = alcohol dependence; AUD = alcohol use disorder.
One of the phenotypes used in the biometric model-fitting analyses was a dimensional DG trait, which was developed for a genome-wide association study of DG (Lind et al., 2012). The dimensional DG trait was derived by extracting a single common factor from the 10 DSM-IV DG symptoms, 20 items from the South Oaks Gambling Screen (SOGS; Lesieur & Blume, 1987), and four additional items related to the frequency and versatility of gambling involvement (to provide coverage of the lower end of the DG distribution). The estimated factor score was weighted by the factor loadings so that better items contributed more to the factor score than did weaker items. Inspection of the relative factor loadings convincingly showed that the factor score represented an index of a continuum of DG — the five highest loading items came from the DSM-IV symptom set (three items), the SOGS item set (one item) and the gambling versatility item (a lifetime count of the number of different gambling activities ever tried, out of 11). It is noteworthy that gambling versatility was the highest loading item in the confirmatory factor analysis; previous research has also suggested that gambling versatility is a good indicator of DG (LaPlante et al., 2009; Welte et al., 2004b).
Alcohol Use Disorder
The AUD assessment came from the World Health Organization Composite International Diagnostic Interview (CIDI; Kessler et al., 1998; Robins et al., 1988). Four different AUD phenotypes were included: (1) A DSM-IV alcohol dependence (AD) diagnosis, (2) an approximate DSM-5 AUD diagnosis based on a cutoff of endorsing two or more symptoms, (3) a DSM-5 severity-graded (mild, moderate, and severe) AUD diagnosis, and (4) an 11-item dimensional DSM-IV AUD symptom count (incorporating both alcohol dependence and abuse symptoms). The DSM-5 diagnoses were approximations because one of the 11 DSM-5 symptoms, ‘craving’, was not included in the DSM-IV assessment. The reliabilities of these measures were good (see Table 1 for prevalences and reliabilities). The correlations between the categorical and dimensional AUD phenotypes were all very high (r ≥ .92). (The first, third, and fourth phenotypes listed above were used in the biometric model fitting analyses.)
Data Analysis
The DSM-IV AUD symptom count was log-transformed to reduce the skewness and kurtosis of its distribution. Biometric models were fitted directly to the raw twin data by the method of robust weighted least squares (for the analysis of categorical phenotypes) or maximum likelihood (for the analysis of continuous phenotypes) using the Mplus program (Version 6.1; Muthén & Muthén, 2007). Biometric model-fitting was conducted to partition the variation in DG and AUD, each considered in isolation, into additive genetic, shared environmental or non-additive genetic, and non-shared environmental influences, and to similarly partition the covariation between DG and AUD into genetic or environmental factors. The evidence for sex differences in the sources of comorbidity between DG and AUD was evaluated by comparing the fits of models that allowed parameter estimates for men and women to differ with models that constrained parameter estimates for men and women to be equal.
Results
Associations Between Disordered Gambling and Alcohol Use Disorder
For all of the definitions used, DG was significantly associated with AUD (see Table 2). The associations between the categorical definitions of DG and AUD did not significantly differ for men and women, but the association between dimensional DG and AUD symptoms did, with a stronger association among men than women (see Table 2). The associations between DG and AUD presented in Table 2 are based on two different indexes of association — the correlation coefficient and odds ratio. The odds ratios are presented to connect the findings of this study to the broader epidemiologic literature. The correlations are presented because this was the index used in the biometric model fitting.
TABLE 2.
Associations Between Disordered Gambling and Alcohol Use Disorder
| Alcohol use disorder phenotype | Full sample (N = 4,764) | Men (N = 2,037) | Women (N = 2,727) | Tests of sex differencesa
|
|
|---|---|---|---|---|---|
| χ2 | p-value | ||||
| Categorical DSM-IV DG (5+ symptoms) and AUD tetrachoric correlations (odds ratios) | |||||
| DSM-IV AD | 0.50 (8.17) | 0.48 (6.68) | 0.46 (8.35) | 0.27 | .60 |
| DSM-5 AUD | 0.38 (4.68) | 0.40 (4.74) | 0.27 (3.19) | 0.82 | .37 |
| Categorical DSM-5 DG (4+ symptoms) and AUD tetrachoric correlations (odds ratios) | |||||
| DSM-IV AD | 0.48 (7.04) | 0.49 (6.36) | 0.40 (6.00) | 0.03 | .89 |
| DSM-5 AUD | 0.35 (3.95) | 0.38 (4.20) | 0.23 (2.55) | 1.63 | .20 |
| Categorical DG (1+ symptoms) and AUD tetrachoric correlations (odds ratios) | |||||
| DSM-IV AD | 0.42 (4.19) | 0.44 (4.02) | 0.32 (3.28) | 0.79 | .38 |
| DSM-5 AUD | 0.35 (2.95) | 0.35 (2.78) | 0.26 (2.16) | 0.76 | .38 |
| Continuous DG and AUD Pearson’s correlations | |||||
| DSM-IV AUD symptom count | 0.34 | 0.37 | 0.26 |
t 2.91 |
p-value .004 |
Note: DG = disordered gambling; AD = alcohol dependence; AUD = alcohol use disorder.
All correlations and odds ratios are statistically significant at p < .05.
Tests of sex differences in odds ratios.
Although there was an adequate sample size to estimate the phenotypic associations between DG and AUD using the narrower DSM-IV and DSM-5 definitions of DG, there was not an adequate sample size to use these definitions in the biometric model fitting. Therefore, the DSM-IV and DSM-5 definitions of DG were not used in the remaining analyses. However, the correlations presented in Table 2 establish that, for the most part, the correlations obtained for the narrower and broader definitions of DG were similar. In a previous paper, we demonstrated that the strength of the associations between continuous personality dimensions and a categorical DG phenotype were comparable whether the cutoff used was 1, 2, 3, 4, or 5 or more symptoms of DSM-IV DG (Slutske et al., 2013), suggesting that results using the 1+ DG symptom cutoff will probably be similar to results that would have been obtained using a higher cutoff.
Twin Correlations
Inspection of Table 3 reveals the following: (1) The within-trait MZ twin correlations were larger than the DZ twin correlations for all of the definitions of DG and AUD (shaded cells), which implicates genetic influences for all of the phenotypes studied, (2) the within-trait opposite-sex DZ twin correlation was smaller than the within-trait same-sex DZ twin correlations for dimensional DG (shaded cells; 0.15 vs. 0.30 and 0.28), which implicates qualitative sex differences in the genetic contribution to variation, (3) the cross-trait twin correlations among male and female MZ pairs (cells in bold) were similar, whereas (4) the cross-trait twin correlations among male DZ pairs were larger than the cross-trait twin correlations among female and opposite-sex DZ pairs (cells in bold), which raises the possibility that the causes of the correlation between DG and AUD may differ for men and women. These observations were rigorously tested in the biometric model fitting.
TABLE 3.
Within-Trait and Cross-Trait Twin Correlations Between Disordered Gambling and Alcohol Use Disorder
| Twin 1 phenotype | Twin 2 phenotype
|
Twin 2 phenotype
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monozygotic men (347 pairs)
|
Monozygotic women (520 pairs)
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 1. DG (categorical) | 0.49 | 0.53 | ||||||||
| 2. DG (continuous) | 0.47 | 0.57 | 0.45 | 0.57 | ||||||
| 3. DSM-IV AD | 0.24 | 0.22 | 0.44 | 0.22 | 0.19 | 0.56 | ||||
| 4. DSM-5 AUD | 0.13 | 0.23 | 0.46 | 0.54 | 0.14 | 0.20 | 0.50 | 0.48 | ||
| 5. DSM-IV AUD (continuous) | 0.12 | 0.23 | 0.41 | 0.48 | 0.47 | 0.14 | 0.19 | 0.39 | 0.39 | 0.39 |
| Twin 1 phenotype | Twin 2 phenotype
|
Twin 2 phenotype
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dizygotic men (227 pairs)
|
Dizygotic women (367 pairs)
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 1. DG (categorical) | 0.24 | 0.22 | ||||||||
| 2. DG (continuous) | 0.26 | 0.30 | 0.21 | 0.28 | ||||||
| 3. DSM-IV AD | 0.19 | 0.20 | 0.11 | 0.10 | 0.04 | 0.12 | ||||
| 4. DSM-5 AUD | 0.14 | 0.21 | 0.07 | 0.20 | 0.09 | 0.03 | 0.12 | 0.19 | ||
| 5. DSM-IV AUD (continuous) | 0.08 | 0.16 | 0.12 | 0.20 | 0.19 | 0.10 | 0.07 | 0.08 | 0.15 | 0.13 |
| Female twin phenotype | Male twin phenotype
|
||||
|---|---|---|---|---|---|
| Dizygotic opposite sex (414 pairs)
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| 1. DG (categorical) | 0.23 | 0.16 | 0.08 | 0.09 | 0.07 |
| 2. DG (continuous) | 0.17 | 0.15 | 0.06 | 0.08 | 0.08 |
| 3. DSM-IV AD | 0.08 | 0.06 | 0.33 | 0.21 | 0.16 |
| 4. DSM-5 AUD | 0.09 | 0.07 | 0.21 | 0.21 | 0.10 |
| 5. DSM-IV AUD (continuous) | 0.08 | 0.09 | 0.16 | 0.19 | 0.13 |
Note: DG = disordered gambling; AD = alcohol dependence; AUD = alcohol use disorder.
Also included in the analyses were data from 1,014 single twins: 153 MZ men, 151 MZ women, 216 DZ men, 181 DZ women, and 207 women and 106 men from DZ opposite-sex pairs.
Biometric Model Fitting
Three different bivariate models were fitted to the twin data to decompose the association between: (1) dichotomous DG and dichotomous DSM-IV AD, (2) dichotomous DG and severity-graded DSM-5 AUD, and (3) dimensional DG and dimensional DSM-IV AUD symptom count. In all three instances, the best-fitting model was one that included additive genetic and non-shared environmental sources of variation — shared environmental or non-additive genetic factors did not account for significant portions of variation in liability for either AUD or DG. The three models fit very well (root mean square error of approximation ≥ 0.03).
In a previous paper (Slutske et al., 2010) we presented the results of fitting univariate twin models to the broader 1+ symptom categorical definition of DG. Similar results were obtained here — genetic factors accounted for 50% of the variation in DG risk (95% confidence interval (CI) = 0.37–0.64) and the remaining 50% was due to unique environmental factors (95% CI = 0.37–0.63). The results using the dimensional definition of DG yielded estimates of genetic and unique environmental factors of 56% (95% CI = 0.52–0.60) and 44% (95% CI = 0.40–0.48), respectively, with identical estimates obtained among men and women. There was no evidence for qualitative sex differences in the genetic contribution to categorical DG in either the previous paper (Slutske et al., 2010) or the present study. However, there was evidence for qualitative sex differences for dimensional DG in the current study. In the opposite-sex DZ twin pairs, the genetic correlation was significantly lower (rA(DZOS) = .27 [95% CI = 0.07–0.48]; Δχ2 = 4.71, df = 1, p = .03) than the correlation of 0.50 that is assumed among same-sex DZ twin pairs in biometric models (because DZ twins share on average 50% of their segregating genes). This yielded a correlation between the latent sources of genetic variation for men and women of 0.55 (95% CI = 0.13–0.96), suggesting that there may be some differences in the specific genes that contribute to variation in DG in men and women. The estimates of the contributions of genetic and unique environmental factors to the risk for DSM-IV AD, severity-graded DSM-5 AUD, and variation in dimensional DSM-IV AUD symptoms are presented in Table 4.
TABLE 4.
Results of Bivariate Model-Fitting of Disordered Gambling and Alcohol Use Disorder
| Alcohol use disorder phenotype | Proportion of variation
|
Correlations between factors explaining variation in risk for disordered gambling and alcohol use disorder
|
||
|---|---|---|---|---|
| Additive genetic | Unique environment | Genetic correlations | Environmental correlations | |
| Full sample | ||||
| DSM-IV alcohol dependencea | 0.48 (0.35, 0.61) | 0.52 (0.39, 0.65) | 0.44 (0.33, 0.49) | 0.32 (0.13, 0.42) |
| DSM-5 alcohol use disordera | 0.50 (0.42, 0.57) | 0.51 (0.43, 0.58) | 0.29 (0.16, 0.36) | 0.37 (0.28, 0.42) |
| DSM-IV AUD symptom countb | 0.43 (0.38, 0.48) | 0.57 (0.52, 0.62) | 0.35 (0.32, 0.38) | 0.22 (0.17, 0.27) |
| Men | ||||
| DSM-IV alcohol dependencea | 0.41 (0.22, 0.61) | 0.58 (0.39, 0.78) | 0.46 (0.31, 0.52) | 0.35 (0.08, 0.45) |
| DSM-5 alcohol use disordera | 0.52 (0.40, 0.65) | 0.48 (0.37, 0.59) | 0.30 (0.08, 0.39) | 0.42 (0.32, 0.46) |
| DSM-IV AUD symptom countb | 0.49c (0.41, 0.56) | 0.51 (0.44, 0.59) | 0.41d (0.38, 0.43) | 0.25 (0.17, 0.31) |
| Women | ||||
| DSM-IV alcohol dependencea | 0.54 (0.36, 0.72) | 0.46 (0.28, 0.64) | 0.39 (0.16, 0.48) | 0.25 (−0.18, 0.40) |
| DSM-5 alcohol use disordera | 0.47 (0.35, 0.58) | 0.54 (0.43, 0.65) | 0.28 (0.04, 0.37) | 0.27 (0.01, 0.37) |
| DSM-IV AUD symptom countb | 0.38c (0.31, 0.44) | 0.66 (0.56, 0.69) | 0.29d (0.24, 0.34) | 0.20 (0.11, 0.26) |
Note:
Using categorical 1+ symptom phenotype of disordered gambling;
using continuous disordered gambling phenotype, 95% confidence intervals are in parentheses.
Parameter estimate differed for men and women, Δχ2 = 8.72, df = 1, p = .003.
Parameter estimate differed for men and women, Δχ2 = 13.82, df = 1, p = .0002.
When comparing the fits of models that allowed the parameter estimates for men and women to differ to models that constrained the parameter estimates to be equal, there was no significant difference for categorical models that included DG and DSM-IV AD (Δχ2 = 4.26, df = 6, p = .64) or DG and severity-graded DSM-5 AUD (Δχ2 = 3.36, df = 6, p = .76), but there was a significant sex difference for the model that included the dimensional DG and AUD phenotypes (Δχ2 = 59.44, df = 6, p < .0001). The results for the models in which parameter estimates were constrained for men and women are presented in Table 4 under ‘Full sample’ heading. Because there were significant differences in the estimates for men and women for the dimensional DG and AUD phenotypes, the estimates provided separately for men and women are of most interest (see Table 4 and Figure 1).
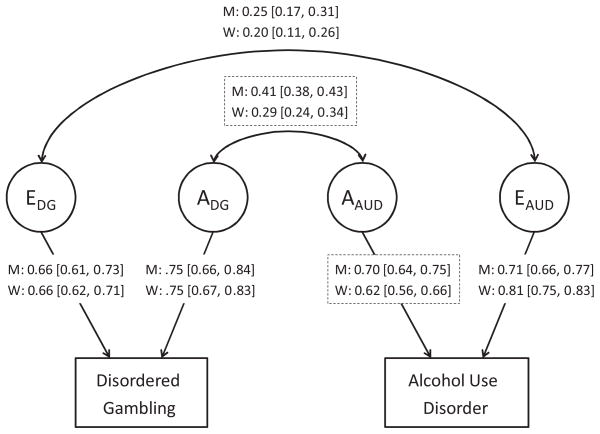
FIGURE 1.
Results of biometric twin modeling of the association between dimensional disordered gambling and alcohol use disorder illustrating the sex differences in the parameter estimates obtained. 95% confidence intervals (CI) around parameter estimates are in parentheses. Note: Parameter estimates that significantly differed between men and women are denoted with dashed boxes. Path coefficients (and upper and lower CIs) can be squared to yield the proportion of variation in disordered gambling and alcohol use disorder attributable to genetic and environmental factors. A =additive genetic influences, E =unique environmental influences, M =men, W =women, DG =disordered gambling, AUD =alcohol use disorder.
The genetic correlation between DG and DSM-IV AD (rG = .44) was substantially larger than the genetic correlation between DG and DSM-5 AUD (rG = .29) in the full sample (Table 4). The genetic correlation between dimensional DG and AUD was significantly larger among men (rG =.41) than women (rG =.29; Δχ2 = 8.72, df = 1, p = .003). The genetic correlations are an indicator of the strength of the correlation between the genetic liabilities for DG and AUD. An alternative perspective comes from estimating the proportion of the observed correlation between DG and AUD that can be explained by overlapping genetic and environmental influences (see Table 5). Again, the proportion of the overlap between DG and AUD due to genetic influences was larger for DSM-IV AD (0.59) than for DSM-5 AUD (0.44). The proportion of overlap between dimensional DG and AUD (0.68) was still larger, with nearly equally high proportions among men (0.69) and women (0.66).
TABLE 5.
Estimates of the Proportion of Covariation in Risk for Disordered Gambling and Alcohol Use Disorder Attributed to Genetic and Environmental Factors
| Alcohol use disorder phenotype | Proportion of covariation between disordered gambling and alcohol use disorder
|
|
|---|---|---|
| Additive genetic | Unique environment | |
| Full sample | ||
| DSM-IV alcohol dependencea | 0.59 (0.32, 0.87) | 0.41 (0.13, 0.69) |
| DSM-5 alcohol use disordera | 0.44 (0.18, 0.69) | 0.56 (0.31, 0.82) |
| DSM-IV AUD symptom countb | 0.68 (0.58, 0.78) | 0.32 (0.22, 0.42) |
| Men | ||
| DSM-IV alcohol dependencea | 0.58 (0.23, 0.92) | 0.43 (0.08, 0.77) |
| DSM-5 alcohol use disordera | 0.40 (0.08, 0.71) | 0.60 (0.29, 0.92) |
| DSM-IV AUD symptom countb | 0.69 (0.56, 0.82) | 0.31 (0.18, 0.44) |
| Women | ||
| DSM-IV alcohol dependencea | 0.64 (0.16, 1.0) | 0.36 (−0.12, 0.84) |
| DSM-5 alcohol use disordera | 0.52 (0.07, 0.98) | 0.48 (0.02, 0.93) |
| DSM-IV AUD symptom countb | 0.66 (0.50, 0.82) | 0.34 (0.18, 0.50) |
Note:
Using categorical 1+ symptom phenotype of disordered gambling;
using continuous disordered gambling phenotype, 95% confidence intervals are in parentheses.
Discussion
In a large community-based twin study, genetic risk factors for DG significantly correlated with the genetic risk factors for AUD. Overlapping genetic risk factors accounted for more than half of the association in the risk for DG and AUD. The results of this study are consistent with a previous study of the association between DG and alcohol dependence in the all-male VET registry (Slutske et al., 2000). The genetic correlations between DG and DSM-III-R/DSM-IV alcohol dependence in the previous study and among the men in the present study were rG = .46 and rG = .45, respectively. The results of these studies converge on the conclusion that DG and AUD have common genetic underpinnings, at least among men. Among the women in the present study, the genetic correlation between risk for DG and DSM-IV alcohol dependence was rG = .39, which did not significantly differ from the genetic correlation obtained among men for the same categorical diagnoses. This represents the first demonstration that DG and AUD also have common genetic underpinnings among women.
Individuals with a lifetime history of DSM-IV pathological gambling disorder were about eight times more likely to have a history of alcohol dependence. A sex difference in the association between DSM-IV pathological gambling and alcohol dependence (odds ratios of 8.4 among women and 6.7 among men) was similar to the sex difference observed in the NESARC study (Petry et al., 2005). Especially puzzling, however, was that the direction of the sex difference reversed when using the broader DSM-5 diagnoses (odds ratios of 2.6 among women and 4.2 among men) and the dimensional DG and AUD constructs (rs of .26 among women and .37 among men). The association between dimensional DG and AUD was significantly larger among men than women. This sex difference in the magnitude of the phenotypic correlation between dimensional DG and AUD translated into a larger genetic correlation between dimensional DG and AUD in men than women (rG of .29 among women and .41 among men).
Two different approaches to creating dimensional diagnoses were utilized — the severity specifiers proposed for the DSM-5, which resulted in three-level ordinal diagnoses for DG and AUD, and the use of continuous symptom counts. However, neither the three-level severity specifiers proposed for the DSM-5 nor the full DSM-IV symptom counts were usable as dimensional diagnoses for DG. This is because over 97% or 87% of the participants in this sample would have been assigned a score of zero using the two approaches. Thus, we had to look beyond the boundaries of the DSM to create a dimensional DG measure. This was accomplished by using the DG symptoms from the DSM, adding items from another gambling inventory, supplementing these with measures of versatility and frequency of gambling involvement, and statistically extracting a common DG factor from these 34 items (Lind et al., 2012). Although the proposed DSM-5 DG diagnosis with the severity specifiers may be useful for clinical practice, it falls short in providing a useful dimensional diagnosis for research purposes.
Although there did not appear to be sex differences in the percentage of variation in DG risk that was explained by genetic factors (about 50%), examining the contributions to variation in DG risk using dimensional measures and within a multivariate design (i.e., within the context of a co-occurring disorder) revealed small differences that were not detected in the analyses of the categorical diagnoses or the three-level diagnoses using the severity specifiers. With the dimensional diagnoses, sex differences in the proportion of variation in AUD due to genetic factors, sex differences in the magnitude of the genetic correlation between DG and AUD, and differences in the specific genes that contribute to variation in DG in men and women were observed. The value of using dimensional diagnoses has been convincingly argued by Kraemer (2007, p. S13) who stated that ‘a report of statistical non-significance may be partially or wholly due to a lack of power to detect effects due to use of categorical measures’. By harnessing the power of dimensional diagnoses and multivariate analysis, intriguing evidence for differences between men and women in the etiology of DG was uncovered. This increase in power was not at the cost of validity in that the dimensional diagnoses were very highly correlated with their categorical counterparts. Another paper based on this same sample also obtained evidence for a potential sex difference in the etiology of DG in the context of multivariate analyses of DG and dimensions of personality (Slutske et al., 2013).
A recent genome-wide association study of DG based on data from a subset of participants in the present study (the largest molecular genetic investigation of DG to date) provides some clues to the common genetic underpinnings of DG and AUD (Lind et al., 2012). Three neurobiological pathways (synaptic long-term potentiation, gonadotrophin-releasing hormone signaling, and gap junction) harboured an excess of genes of small effect that were previously implicated in alcohol addiction as well as other substance-related addictions (Li et al., 2008). Synaptic long-term potentiation is the experience-dependent strengthening of synaptic transmission that is essential for synaptic plasticity which underlies neural adaptation to substances of abuse and is considered to be important in learning and memory (Hyman, 2005). This mechanism is thought to occur in the mesolimbic dopamine pathway, a putative brain reward circuit that may be a common pathway through which many substances, such as alcohol, and behaviors, such as gambling, have their rewarding effects and potential for addiction (Grant et al., 2002; Nestler, 2005). There is also emerging evidence suggesting that the functioning of this brain circuit differs in men and women (Bolla et al., 2004; Lee et al., 2009; Martin-Soelch et al., 2011; Tranel et al., 2005).
A significant genetic correlation between DG and AUD might arise because DG and AUD have common genetic underpinnings, but it is equally plausible that genetically influenced DG constitutes part of the vulnerability to develop AUD, or that genetically influenced AUD constitutes part of the vulnerability to develop DG. Previous studies that have assessed the temporal relation between DG and AUD in individuals with a history of both disorders have generally found that AUD precedes the development of DG (Cunningham-Williams et al., 2000; Kessler et al., 2008). Also consistent with alcohol use being a potential cause of DG are the results of experimental studies demonstrating that subjects will gamble more when under the influence of a moderate dose of alcohol than when given a placebo (e.g., Kyngdon & Dickerson, 1999) and correlational surveys demonstrating that individuals who drink while gambling spend more money (Giacopassi et al., 1998) and are more likely to develop a gambling disorder (Welte et al., 2004a). In addition, experimental studies have demonstrated that the influence of alcohol use on gambling behavior is more pronounced among individuals with a history of DG (Ellery et al., 2005). Conversely, experimental studies have also shown that individuals assigned to participate in a gambling task are more likely to drink an alcoholic beverage than individuals assigned to an alternate task (Stewart et al., 2002). These findings indicate that the genetic correlation between DG and AUD is likely due to both reciprocal causal effects between drinking and gambling behaviors and common genetic underpinnings for the two disorders.
Limitations
This study has at least five limitations. First, the diagnosis used for DSM-5 AUD was an approximation because one of the symptoms (‘craving’) was not included. Examinations of the impact of the craving criterion on the DSM-5 AUD diagnosis suggest that its omission would not have made a substantial difference in the prevalence of DSM-5 AUD (Agrawal et al., 2011) or its meaning (Casey et al., 2012). Second, it was not possible to include the narrower DSM-IV and DSM-5 definitions of DG in the biometric model-fitting. However, because individual differences in DG are likely to be quantitative rather than qualitative (similar to many psychiatric disorders (Helzer et al., 2008)), results based on a broader definition will likely apply to a narrower definition. Third, the age range of the sample was relatively narrow (32–43 years). In addition to limiting the generalizability of this study to other age groups, it also potentially complicates the interpretation of sex differences. The age of onset of DG appears to be later among women than men, whereas the age of onset of AD is more comparable. In the US NESARC study, the age of onset of DG was 35 years among women and 30 years among men (Blanco et al., 2006), and the age of onset of AD was 24 years for both men and women (Falk et al., 2008). As a result, the strength of the association between DG and AUD might have been attenuated in women compared to men because more of them may not yet have progressed through the period of risk for developing a gambling disorder. Four, the majority of the participants were Caucasians of Northern European ancestry, so it is not clear the extent to which these results will apply to other racial groups. Five, it is unclear how the results of this Australian twin study will generalize to other countries.
Future Directions
This study represents a first step. We chose to focus on the genetic association between DG and AUD because AUD is one of the more common substance-related addictions, and also because we were interested in replicating the earlier study of the all-male VET registry and extending the previous findings to women. Because genetic influences on different substance-related addictions overlap (Kendler et al., 2012), it is likely that the genetic overlap observed between DG and AUD would likely extend to other substance use disorders, such as nicotine, cannabis, and cocaine dependence. This genetic overlap may also extend to other non-addictive psychiatric disorders, such as antisocial personality disorder (Slutske et al., 2001). Recent factor analyses of the NESARC diagnostic data suggested that DG loaded onto a higher order ‘externalizing’ factor, along with alcohol dependence, drug dependence, and antisocial personality disorder (Oleski et al., 2011). However, the factor loading of DG onto this externalizing factor was relatively low, and among women, DG also appeared to load onto the ‘anxious-misery’ factor (a sub-factor of a higher order ‘internalizing’ factor). Clearly, more research is needed to accurately characterize the shared and distinct risk factors for DG and other addictive and non-addictive disorders, and also how this might differ for men and women.
Acknowledgments
Supported by National Institutes of Health Grant MH66206. We thank Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for twins, and David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
References
- Agrawal A, Heath AC, Lynskey MT. DSM-IV to DSM-5: The impact of proposed revisions on diagnosis of alcohol use disorders. Addiction. 2011;106:1935–1943. doi: 10.1111/j.1360-0443.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-III-R. 3. Washington, DC: American Psychiatric Association; 1987. rev. ed. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. [Accessed on 31th October, 2012];DSM-5 development. 2012 Retrieved from www.dsm5.org.
- Blanco C, Hasin DS, Petry N, Stinson FS, Grant BF. Sex differences in subclinical and DSM-IV pathological gambling: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2006;36:943–953. doi: 10.1017/S0033291706007410. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matchik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Casey M, Adamson G, Shevlin M, McKinney A. The role of craving in AUDs: Dimensionality and differential item functioning in the DSM-5. Drug and Alcohol Dependence. 2012;125:75–80. doi: 10.1016/j.drugalcdep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Cunningham-Williams RM, Cottler LB, Compton WM, Spitznagel EL, Ben-Abdallah A. Problem gambling and comorbid psychiatric and substance use disorders among drug users recruited from drug treatment and community settings. Journal of Gambling Studies. 2000;16:347–376. doi: 10.1023/a:1009428122460. [DOI] [PubMed] [Google Scholar]
- Ellery M, Stewart SH, Loba P. Alcohol’s effects on video lottery terminal (VLT) play among probable pathological and non-pathological gamblers. Journal of Gambling Studies. 2005;21:299–324. doi: 10.1007/s10899-005-3101-0. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hilton M. Age of onset and temporal sequencing of lifetime DSM-IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug and Alcohol Dependence. 2008;94:234–245. doi: 10.1016/j.drugalcdep.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: Carving addiction at a new joint? Annals of the New York Academy of Sciences. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein D, Hoffmann JP, Larison C, Engelman L, Murphy S, Palmer A, Hill MA. Gambling impact and behavior study: Report to the National Gambling Impact Study Commission. New York: Christiansen/Cummings Associates; 1999. [Google Scholar]
- Giacopassi D, Stitt BG, Vandiver M. An analysis of the relationship of alcohol to casino gambling among college students. Journal of Gambling Studies. 1998;14:135–149. doi: 10.1023/a:1023094725055. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kushner MG, Kim SW. Pathological gambling and alcohol use disorder. Alcohol Research & Health. 2002;26:143–150. [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. American Journal of Drug and Alcohol Abuse. 2010;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Bucholz KK, Gossop M. A dimensional option for the diagnosis of substance dependence in DSM-V. International Journal of Methods in Psychiatric Research. 2007;16:S24–S33. doi: 10.1002/mpr.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Kraemer HC, Krueger RF, Wittchen H, Sirovatka PJ, Regier DA. Dimensional approaches in diagnostic classification: Refining the research agenda for DSM-V. Arlington, VA: American Psychiatric Association; 2008. [Google Scholar]
- Hodgins D. Using the NORC DSM Screen for gambling problems as an outcome measure for pathological gambling: Psychometric evaluation. Addictive Behaviors. 2004;29:1685–1690. doi: 10.1016/j.addbeh.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Holden C. Psychiatry. Behavioral addictions debut in proposed DSM-V. Science. 2010;327:935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: A disease of learning and memory. American Journal of Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15:181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, Shaffer HJ. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychological Medicine. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC. DSM categories and dimensions in clinical and research contexts. International Journal of Methods in Psychiatric Research. 2007;16:S8–S15. doi: 10.1002/mpr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyngdon A, Dickerson M. An experimental study of the effect of prior alcohol consumption on a simulated gambling activity. Addiction. 1999;94:697–707. doi: 10.1046/j.1360-0443.1999.9456977.x. [DOI] [PubMed] [Google Scholar]
- LaPlante DA, Nelson SE, LaBrie RA, Shaffer HJ. Disordered gambling, type of gambling and gambling involvement in the British Gambling Prevalence Survey 2007. European Journal of Public Health. 2009;21:532–537. doi: 10.1093/eurpub/ckp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TMC, Chan CCH, Leung AWS, Fox PT, Gao J. Sex-related differences in neural activity during risk-taking: An fMRI study. Cerebral Cortex. 2009;19:1303–1312. doi: 10.1093/cercor/bhn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLOS Computational Biology. 2008;4:e2. doi: 10.1371/journal.pcbi.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Zhu G, Montgomery GW, Madden PAF, Heath AC, Martin NG, Slutske WS. Genome-wide association study of a quantitative disordered gambling trait. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00463.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: A quantitative review. Psychological Bulletin. 2011;137:856–879. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Herscovitch P, Carson RE, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. European Journal of Neuroscience. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén B. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Oleski J, Cox BJ, Clara I, Hills A. Pathological gambling and the structure of common mental disorders. Journal of Nervous and Mental Disease. 2011;199:956–960. doi: 10.1097/NMD.0b013e3182392931. [DOI] [PubMed] [Google Scholar]
- Petry NM. Should the scope of addictive disorders be broadened to include pathological gambling? Addiction. 2006;101:152–160. doi: 10.1111/j.1360-0443.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101:142–151. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Helzer J, Cottler L, Goldring E. NIMH diagnostic interview schedule version III revised (DIS-III-R) St. Louis, MO: Department of Psychiatry, Washington University School of Medicine; 1988. [Google Scholar]
- Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: A research synthesis. American Journal of Public Health. 1999;89:1369–1376. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Eisen SA, True WR, Lyons MJ, Goldberg J, Tsuang MT. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Archives of General Psychiatry. 2000;57:666–673. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen SA, Xian H, True WR, Lyons MJ, Goldberg J, Tsuang MT. A twin study of the association between pathological gambling and antisocial personality disorder. Journal of Abnormal Psychology. 2001;110:297–308. doi: 10.1037//0021-843x.110.2.297. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian Twin Study of Gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12:63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Archives of General Psychiatry. 2010;67:624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, Martin NG. Disordered gambling as defined by the DSM-IV and the South Oaks Gambling Screen: Evidence for a common etiologic structure. Journal of Abnormal Psychology. 2011;120:743–751. doi: 10.1037/a0022879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Cho SB, Piasecki TM, Martin NG. Genetic overlap between personality and risk for disordered gambling: Evidence from a national community-based Australian twin study. Journal of Abnormal Psychology. 2013;122:250–255. doi: 10.1037/a0029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, McWilliams LA, Blackburn JR, Klein RM. A laboratory-based investigation of relations among video lottery terminal (VLT) play, negative mood, and alcohol consumption in regular VLT players. Addictive Behaviors. 2002;27:819–835. doi: 10.1016/s0306-4603(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Welte JW, Barnes GM, Wieczorek WF, Tidwell M. Simultaneous drinking and gambling: A risk factor for pathological gambling. Substance Use and Misuse. 2004a;39:1405–1422. doi: 10.1081/ja-120039397. [DOI] [PubMed] [Google Scholar]
- Welte JW, Barnes GM, Wieczorek WF, Tidwell MO, Parker JC. Risk factors for pathological gambling. Addictive Behaviors. 2004b;29:323–335. doi: 10.1016/j.addbeh.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Wickwire EM, Burke RS, Brown SA, Parker JD, Ryan KM. Psychometric evaluation of the National Opinion Research Center DSM-IV Screen for Gambling Problems (NODS) American Journal on Addictions. 2008;17:392–395. doi: 10.1080/10550490802268934. [DOI] [PubMed] [Google Scholar]