Abstract
Single Nucleotide Polymorphisms (SNPs) in genes involved in the DNA Base Excision Repair (BER) pathway could be associated with cancer risk in carriers of mutations in the high-penetrance susceptibility genes BRCA1 and BRCA2, given the relation of synthetic lethality that exists between one of the components of the BER pathway, PARP1 (poly ADP ribose polymerase), and both BRCA1 and BRCA2. In the present study, we have performed a comprehensive analysis of 18 genes involved in BER using a tagging SNP approach in a large series of BRCA1 and BRCA2 mutation carriers. 144 SNPs were analyzed in a two stage study involving 23,463 carriers from the CIMBA consortium (the Consortium of Investigators of Modifiers of BRCA1 and BRCA2). Eleven SNPs showed evidence of association with breast and/or ovarian cancer at p<0.05 in the combined analysis. Four of the five genes for which strongest evidence of association was observed were DNA glycosylases. The strongest evidence was for rs1466785 in the NEIL2 (endonuclease VIII-like 2) gene (HR: 1.09, 95% CI (1.03–1.16), p = 2.7×10−3) for association with breast cancer risk in BRCA2 mutation carriers, and rs2304277 in the OGG1 (8-guanine DNA glycosylase) gene, with ovarian cancer risk in BRCA1 mutation carriers (HR: 1.12 95%CI: 1.03–1.21, p = 4.8×10−3). DNA glycosylases involved in the first steps of the BER pathway may be associated with cancer risk in BRCA1/2 mutation carriers and should be more comprehensively studied.
Author Summary
Women harboring a germ-line mutation in the BRCA1 or BRCA2 genes have a high lifetime risk to develop breast and/or ovarian cancer. However, not all carriers develop cancer and high variability exists regarding age of onset of the disease and type of tumor. One of the causes of this variability lies in other genetic factors that modulate the phenotype, the so-called modifier genes. Identification of these genes might have important implications for risk assessment and decision making regarding prevention of the disease. Given that BRCA1 and BRCA2 participate in the repair of DNA double strand breaks, here we have investigated whether variations, Single Nucleotide Polymorphisms (SNPs), in genes participating in other DNA repair pathway may be associated with cancer risk in BRCA carriers. We have selected the Base Excision Repair pathway because BRCA defective cells are extremely sensitive to the inhibition of one of its components, PARP1. Thanks to a large international collaborative effort, we have been able to identify at least two SNPs that are associated with increased cancer risk in BRCA1 and BRCA2 mutation carriers respectively. These findings could have implications not only for risk assessment, but also for treatment of BRCA1/2 mutation carriers with PARP inhibitors.
Introduction
Carrying an inherited mutation in the BRCA1 or BRCA2 gene increases a woman's lifetime risk of developing breast, ovarian and other cancers. The estimated cumulative risk of developing breast cancer by the age of 70 in BRCA1 and BRCA2 mutation carriers varies between 43% to 88%; similarly, between 11% to 59% of mutation carriers will develop ovarian cancer by the age of 70 [1]–[3]. These considerable differences in disease manifestation suggest the existence of other genetic or environmental factors that modify the risk of cancer development. The Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA), was established in 2006 [4] and with more than 40,000 mutation carriers currently provides the largest sample size for reliable evaluation of even modest associations between single-nucleotide polymorphisms (SNPs) and cancer risk. CIMBA studies have so far demonstrated that more than 25 SNPs are associated with the risk of developing breast or ovarian cancer for BRCA1 or BRCA2 carriers. These were identified through genome-wide association studies (GWAS) of breast or ovarian cancer in the general population or through BRCA1- and BRCA2-specific GWAS [5]–[8]. Cells harboring mutations in BRCA1 or BRCA2 show impaired homologous recombination (HR) [9]–[11] and are thus critically dependent on other members of the DNA repair machinery such as poly ADP ribose polymerase (PARP1) involved in the Base Excision Repair (BER) pathway. The BER pathway is crucial for the replacement of aberrant bases generated by different causes [12]. A deficiency in BER can give rise to a further accumulation of double-strand DNA breaks which, in the presence of a defective BRCA1 or BRCA2 background, could persist and lead to cell cycle arrest or cell death; this makes BRCA-deficient cells extremely sensitive to PARP inhibitors, as previously demonstrated [13]. We hypothesize that SNPs in PARP1 and other members of BER may be associated with cancer risk in BRCA1 and BRCA2 mutation carriers. SNPs in XRCC1, one of the main components of BER, have been recently evaluated within the CIMBA consortium [14], however a comprehensive study has not yet been performed of either XRCC1 or the other genes participating in BER.
In the present study, we used a tagging SNP approach to evaluate whether the common genetic variation in the genes involved in the BER pathway could be associated with cancer risk in a large series of BRCA1/2 mutation carriers using a two-stage approach. The first stage involved an analysis of 144 tag SNPs in 1,787 Spanish and Italian BRCA1/2 mutation carriers. In stage II, the 36 SNPs showing the strongest evidence of association in stage I, were evaluated in a further 23,463 CIMBA mutation carriers included in the Collaborative Oncological Gene-environment Study (COGS) and genotyped using the iCOGS custom genotyping array.
Results
Breast cancer association
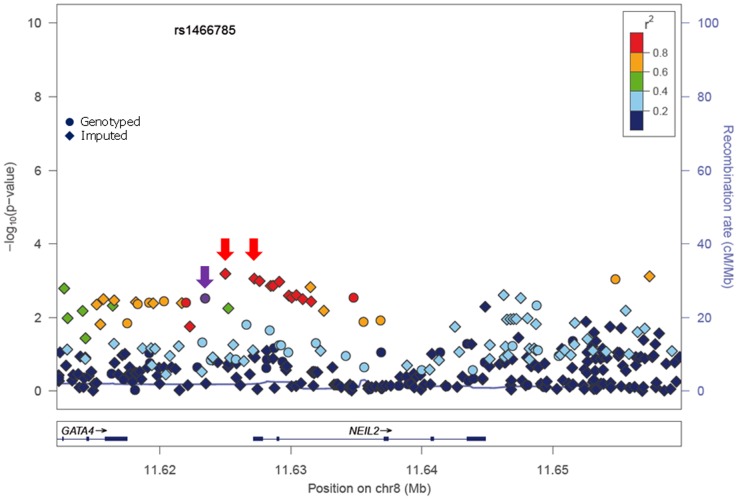
In stage I, 144 selected Tag SNPs covering the 18 selected BER genes were genotyped in 968 BRCA1 and 819 BRCA2 mutation carriers from five CIMBA centres (Spanish National Cancer ResearchCentre (CNIO), Hospital Clínico San Carlos (HCSC), Catalan Institute of Oncology (ICO), Demokritos and Milan Breast Cancer Study Group (MBCSG). Of those, 50 were excluded because of low call-rates, minor allele frequency (MAF)<0.05, evidence of deviation from Hardy Weinberg Equilibrium (p-value<10−3) or monomorphism. Associations with breast cancer risk were assessed for 94 SNPs, as summarized in Table S1. The 36 SNPs that showed evidence of association at p≤0.05 were selected for analysis in stage II. Of the 36 SNPs successfully genotyped in the whole CIMBA series comprising 15,252 BRCA1 and 8211 BRCA2 mutation carriers, consistent evidence of association with breast cancer risk (p-trend<0.05) was observed for six SNPs (Table 1). The strongest evidence of association was observed for rs1466785 in the NEIL2 gene (HR: 1.09, 95% CI (1.03–1.16), p = 2.7×10−3) for association with breast cancer risk in BRCA2 mutation carriers. We had observed a consistent association in stage I in BRCA2 mutation carriers (HR: 1.25, p = 0.06). The SNP was primarily associated with ER-negative breast cancer (HR: 1.20, 95%CI (1.06–1.37), p = 4×10−3), although the difference in HRs for ER-positive and ER-negative disease was not statistically significant. The evidence of association in Stage II was somewhat stronger when considering the genotype-specific models, with the dominant being the best fitting (HR: 1.20 95% CI: 1.09–1.37, p = 1×10−4). The associations remained significant and the estimated effect sizes remained consistent with the overall analysis when the data were reanalyzed excluding samples used in stage I of the study (data not shown). Imputation using the 1000 genomes data showed that there were several SNPs in strong linkage disequilibrium (LD) with rs1466785 showing more significant associations (p<10−3) (Figure 1).
Table 1. Associations with breast and ovarian cancer risk for SNPs observed at p-trend<0.05 in stage II of the experiment.
| BRCA1 carriers | SNP name | Gene | Unaffected (Number) | Affected (Number) | Unaffected (MAF) | Affected (MAF) | HR per allelea | HR heterozygoteb | HR homozygoteb | p-trendc | p-hetc | p-homc |
| Breast cancer | rs3847954d | UNG | 7455 | 7797 | 0.18 | 0.19 | 1.05 (1.00–1.11) | 1.09 (1.02–1.16) | 0.99 (0.84–1.16) | 0.04 | 0.011 | 0.713 |
| Ovarian cancer | rs2072668 | OGG1 | 12786 | 2461 | 0.22 | 0.23 | 1.09 (1.01–1.18) | 1.16 (1.05–1.27) | 1.03 (0.82–1.28) | 0.016 | 3×10−3 | 0.77 |
| rs2269112 | OGG1 | 12789 | 2461 | 0.17 | 0.18 | 1.11 (1.02–1.21) | 1.11 (1.01–1.23) | 1.21 (0.92–1.58) | 0.013 | 0.014 | 0.268 | |
| rs2304277 | OGG1 | 12783 | 2462 | 0.2 | 0.21 | 1.12 (1.03–1.21) | 1.19 (1.08–1.3) | 1.01 (0.79–1.30) | 4.8×10−3 | 6×10−4 | 0.69 | |
| rs10161263 | SMUG1 | 12790 | 2462 | 0.34 | 0.32 | 0.92 (0.86–0.99) | 0.88 (0.80–0.97) | 0.90 (0.78–1.04) | 0.024 | 9×10−3 | 0.49 |
Hazard Ratio per allele (1 df) estimated from the retrospective likelihood analysis.
Hazard Ratio under the genotype specific models (2df) estimated from the retrospective likelihood analysis.
p-values were based on the score test.
HR per allele of 1.69 and p-trend of 1×10−4 for BRCA2 mutation carriers in stage I of the study.
HR per allele of 1.43 and p-trend of 0.01 for BRCA1 mutation carriers in stage I of the study.
HR per allele of 1.30 and p-trend of 0.03 for BRCA1 mutation carriers in stage I of the study.
HR per allele of 0.64 and p-trend of 0.057 for BRCA2 mutation carriers in stage I of the study.
HR per allele of 1.25 and p-trend of 0.04 for BRCA1 mutation carriers in stage I of the study.
HR per allele of 1.25 and p-trend of 0.058 for BRCA2 mutation carriers in stage I of the study.
rs3093926 did not yield results under the genotype specific model due to the low minor allele frequency.
Complete description of results from stage I are included in Supplementary Table S1.
Highlighted in bold are those SNPs showing strongest associations with breast or ovarian cancer risk (p<0.01).
Figure 1. p-values of association (−log10 scale) with breast cancer risk in BRCA2 carriers for genotyped and imputed SNPs in the NEIL2 gene.
SNP rs1466785 is indicated with a purple arrow and the best causal imputed SNPs, rs804276 and rs804271 are indicated with a red arrow. Colors represent the pariwise r2. Plot generated with LocusZoom [42] (http://csg.sph.umich.edu/locuszoom/).
Ovarian cancer association
Due to lack of power we did not perform analysis of associations with ovarian cancer in stage I. However, we performed this analysis for the 36 SNPs tested in stage II. Although they had been selected based on their evidence of association with breast cancer risk, under the initial hypothesis they are also plausible modifiers of ovarian cancer risk for BRCA1 and BRCA2 mutation carriers. We found four SNPs associated with ovarian cancer risk with a p-trend<0.01 in BRCA1 or BRCA2 mutation carriers (Table 1). The strongest association was found for rs2304277 in OGG1 in BRCA1 mutation carriers (HR: 1.12, 95%CI: 1.03–1.21, p = 4.8×10−3). The association was somewhat stronger under the dominant model (HR: 1.19, 95%CI: 1.08–1.3, p = 6×10−4). Although three other SNPs were found to be associated with ovarian cancer risk in BRCA2 mutation carriers (p-trend<10−3), these results were based on a relatively small number of ovarian cancer cases. Imputed data did not show any SNPs with substantially more significant associations with ovarian cancer risk except for rs3093926 in PARP2, associated with ovarian cancer risk in BRCA2 mutation carriers for which there was a SNP, rs61995542, with a stronger association (HR: 0.67, p = 4.6×10−4) (Figure S1).
Discussion
Based on the interaction of synthetic lethality that has been described between PARP1 and both BRCA1 and BRCA2, we hypothesize that this and other genes involved in the BER pathway could potentially be associated with cancer risk in BRCA1/2 mutation carriers. Several studies have recently investigated the association of some of the BER genes with breast cancer, however, no definitive conclusions can be drawn, given that some publications suggest that SNPs in these genes can be associated with breast cancer risk with marginal p-values while others rule out a major role of these genes in the disease [15]–[21]. There is only one study from the CIMBA consortium which has evaluated the role of three of the most studied SNPs in the XRCC1 gene, c.-77C>T (rs3213245) p.Arg280His (rs25489) and p.Gln399Arg (rs25487), ruling out associations of these variants with cancer risk in BRCA1 and BRCA2 mutation carriers [14]. However, a comprehensive analysis of neither XRCC1 nor the other genes involved in the pathway in the context of BRCA mutation carriers has been performed. In the present study we have assessed the common genetic variation of 18 genes participating in BER by using a two stage strategy.
Eleven SNPs showed evidence of association with breast and/or ovarian cancer at p<0.05 in stage II of the experiment (Table 1). Of those, six showed a p-trend value<0.01 and were therefore considered the best candidates for further evaluation. Only one of those six, rs1466785 in the NEIL2 gene (endonuclease VIII-like 2) showed an association with breast cancer risk while the other five, rs2304277 in OGG1 (8-guanine DNA glycosylase), rs167715 and rs4135087 in TDG (thymine-DNA glycosylase), rs3093926 in PARP2 (Poly(ADP-ribose) polymerase 2) and rs34259 in UNG (uracil-DNA glycosylase) were associated with ovarian cancer risk.
The minor allele of NEIL2-rs1466785 was associated with increased breast cancer risk in BRCA2 mutation carriers; moreover, when considering the genotype-specific risks observed that the best fitting model was the dominant one. NEIL2 is one of the oxidized base-specific DNA glycosylases that participate in the initial steps of BER and specifically removes oxidized bases from transcribing genes [22]. By imputing using the 1000 genome data we found six correlated SNPs in strong LD with rs1466785 (r2>0.8), located closer or inside the gene and showing slightly stronger and more significant associations with the disease and therefore being better candidate causal variants. From those, we considered rs804276 and rs804271 as the best candidates given that they showed the most significant associations (p = 6×10−4 and p = 8×10−4 respectively) and there were available epidemiological or functional data supporting their putative role in cancer. SNP rs804276 has been associated with disease recurrence in patients with bladder cancer treated with Bacillus Calmette-Guérin (BCG) (HR: 2.71, 95%CI (1.75–4.20), p = 9×10−6) [23]. SNP rs804271 is located in a positive regulatory region in the promoter of the gene, between two potential cis- binding sites for reactive oxygen species responsive transcription factors in which sequence variation has been proven to alter the transcriptional response to oxidative stress [24]. Moreover, this SNP has been proposed to partly explain the inter-individual variability observed in NEIL2 expression levels in the general population and has been proposed as a potential risk modifier of disease susceptibility [25].
Several studies have been published showing associations between SNPs in NEIL2 and lung or oropharyngeal cancer risk [26], [27] but to our knowledge, no association with breast cancer risk has been reported. We hypothesize that the potential association observed in the present study could be explained by the interaction between NEIL2 and BRCA2, each of them causing a deficiency in the BER and HR DNA repair pathways, respectively. This would explain why the breast cancer risk modification due to rs1466785 would only be detected in the context of BRCA2 mutation carriers and not in the general population.
The strongest evidence of association found in BRCA1 carriers was between rs2304277 in the OGG1 gene and ovarian cancer risk. The association was more significant when considering the dominant model. OGG1 removes 8-oxodeoxyguanosine which is generated by oxidative stress and is highly mutagenic, and it has been suggested that SNPs in the gene could be associated with cancer risk [28]–[31]. This is an interesting result, given that to date only one SNP, rs4691139 in the 4q35.3 region, also identified through the iCOGS effort, has been found to modify ovarian cancer risk specifically in BRCA1 carriers [32]. SNP rs2304277 is located in the 3′UTR (untranslated region) of the gene and is probably not the causal variant, however, in this case imputations through the 1000 Genome did not show better results for a more plausible causal SNP.
We have identified four SNPs associated with ovarian cancer risk in BRCA2 mutation carriers, rs167715 and rs4135087 in the TDG gene, rs34259 in the UNG gene and rs3093926 in PARP2. However, these last results should be interpreted with caution given that the number of BRCA2 carriers affected with ovarian cancer is four-fold lower than for BRCA1 carriers and the statistical power was therefore more limited, increasing the possibility of false-positives. In the case of PARP2, imputed data showed a lower p-value of association (4×10−4) for another SNP, rs61995542, that had a slightly higher MAF than rs3093926 (0.074 vs. 0.067) (Figure S1). However, it must still be interpreted with caution due to small number of ovarian cancer cases in the BRCA2 group.
It is worth noting that, four of the five genes for which strongest evidence of association was observed, are all DNA glycosylases participating in the initiation of BER by removing damaged or mismatched bases. Apart from the already mentioned NEIL2 and OGG1, TDG initiates repair of G/T and G/U mismatches commonly associated with CpG islands, while UNG removes uracil in DNA resulting from deamination of cytosine or replicative incorporation of dUMP. We have not found strong associations with SNPs in genes involved in any other parts of the pathway, such as strand incision, trimming of ends, gap filling or ligation. It has been suggested that at least in the case of uracil repair, base removal is the major rate-limiting step of BER [33]. This is consistent with our findings, suggesting that SNPs causing impairment in the function of these specific DNA glycosylases could give rise to accumulation of single strand breaks and subsequently DNA double strand breaks that, in the HR defective context of BRCA1/2 mutation carriers would increase breast and ovarian cancer risk.
The fact that the SNPs tested are located in genes participating in the same DNA repair pathway as PARP1, make them especially interesting, not only as risk modifiers but also because they could have an impact on patients' response to treatment with PARP inhibitors. BRCA1/2 mutation carriers harboring a potential modifier SNP in DNA glycosylases could be even more sensitive to PARPi due to a constitutional slight impairment of the BER activity. This is a hypothesis that should be confirmed in further studies. The design of this study in two stages, the hypothesis-based approach adopted to select genes, and that it is based on the largest possible series of BRCA1 and BRCA2 carriers available nowadays, mean that the results obtained are quite solid However, the study still has some limitations such as the possible existence of residual confounding due to environmental risk factors for which we did not have information.
In summary, we have identified at least two SNPs, rs1466785 and rs2304277, in the DNA glycolylases NEIL2 and OGG1, potentially associated with increased breast and ovarian cancer risks in BRCA2 and BRCA1 mutation carriers, respectively. Our results suggest that glycosylases involved in the first steps of the BER pathway may be cancer risk modifiers in BRCA1/2 mutation carriers and should be more comprehensively studied. If confirmed, these findings could have implications not only for risk assessment, but also for treatment of BRCA1/2 mutation carriers with PARP inhibitors.
Materials and Methods
Subjects
Eligible subjects were female carriers of deleterious mutations in BRCA1 or BRCA2 aged 18 years or older [6]. A total of 55 collaborating CIMBA studies contributed genotypes for the study. Numbers of samples included from each are provided in Table S2. A total of 1,787 mutation carriers (968 with mutations in BRCA1 and 819 with mutations in BRCA2) from the CNIO, HCSC, ICO, Demokritos and MBCSG were genotyped in the first stage of the study. Stage II included 23,463 CIMBA samples (15,252 with mutations in BRCA1 and 8,211 with mutations in BRCA2). All carriers participated in clinical and/or research studies at the host institution under IRB-approved protocols.
Methods stage I
Selection and genotyping of SNPs
Eighteen genes (UNG, SMUG1, MBD4, TDG, OGG1, MUTYH, NTHL1, MPG, NEIL1, NEIL2, APEX1, APEX2, LIG3, XRCC1, PNKP, POLB, PARP1 and PARP2) involved in the BER pathway were selected, based on the information available at http://www.cgal.icnet.uk/DNA_Repair_Genes.html as at the 31st December, 2009. Tag SNPs for the selected genes were defined using Haploview v.4.0 (http://www.broad.mit.edu/mpg/haploview) with an r2 threshold of 0.8 and a minimum minor allele frequency of 0.05. In addition, SNPs with potentially functional effects already described in the literature were selected. A final number of 144 SNPs was included in an oligonucleotide pool assay for genotyping using the Illumina Veracode technology (Illumina Inc., San Diego, CA). Three hundred nanograms of DNA from each sample were genotyped using the GoldenGate Genotyping Assay with Veracode technology according to the published Illumina protocol. Genotype clustering and calling were carried out using the GenomeStudio software. SNPs with a call rate <0.95 were excluded from further analysis. Duplicate samples and CEPH trios (Coriell Cell Repository, Camden, NJ) were genotyped across the plates. SNPs showing Mendelian allele-transmission errors or showing discordant genotypes across duplicates were excluded.
Statistical analysis
To test for departure from Hardy-Weinberg equilibrium (HWE), a single individual was randomly selected from each family and Pearson's X2 Test (1df) was applied to genotypes from this set of individuals. The association of the SNPs with breast cancer risk was assessed by estimating hazard ratios (HR) and their corresponding 95% confidence intervals (CI) using weighted multivariable Cox proportional hazards regression with robust estimates of variance [34]. For each mutation carrier, we modeled the time to diagnosis of breast cancer from birth, censoring at the first of the following events: bilateral prophylactic mastectomy, breast cancer diagnosis, ovarian cancer diagnosis, death or date last know to be alive. Subjects were considered affected if their age at censoring corresponded to their age at diagnosis of breast cancer and unaffected otherwise. Weights were assigned separately for carriers of mutations in BRCA1 and BRCA2, by age and affection status, so that the weighted observed incidences in the sample agreed with established estimates for mutation carriers [1]; [34].
We considered log-additive and co-dominant genetic models and tested for departure from HR = 1 by applying a Wald test based on the log-HR estimate and its standard error. Additional independent variables included in all analyses were year of study, centre and country. All statistical analyses were carried out using Stata: Release 10 (StataCorp. 2007. Stata Statistical Software: Release 10.0. College Station, TX: Stata Corporation LP). Robust estimates of variance were calculated using the cluster subcommand, applied to an identifier variable unique to each family.
Methods stage II
iCOGS SNP array
Stage II of the experiment was performed as part of the iCOGS genotyping experiment. The iCOGS custom array was designed in collaboration between the Breast Cancer Association Consortium (BCAC), the Ovarian Cancer Association Consortium (OCAC), the Prostate Cancer Association Group to Investigate Cancer Associated in the Genome (PRACTICAL) and CIMBA. The final design comprised 211,155 successfully manufactured SNPs of which approximately 17.5% had been proposed by CIMBA. A total of 43 SNPs were nominated for inclusion on iCOGS based on statistical evidence of association in stage I of the present study (p≤0.05). Of these, 36 were successfully manufactured and genotyped in CIMBA mutation carriers.
iCOGS genotyping and quality control
Genotyping was performed at Mayo Clinic and the McGill University and Génome Québec Innovation Centre (Montreal, Canada). Genotypes were called using Illumina's GenCall algorithm. Sample and quality control process have been described in detail elsewhere [32], [35]. After the quality control process a total of 23,463 carriers were genotyped for the 36 selected SNPs.
Statistical analysis
Both breast and ovarian cancer associations were evaluated in stage II. Censoring for breast cancer followed the same approach as in stage I. Censoring for ovarian cancer risk occurred at risk-reducing salpingo-oophorectomy or last follow-up.
The genotype-disease associations were evaluated within a survival analysis framework, by modelling the retrospective likelihood of the observed genotypes conditional on the disease phenotypes [9], [34], [36], [37]. The associations between genotype and breast or ovarian cancer risk were assessed using the 1 d.f. score test statistic based on this retrospective likelihood. To allow for the non-independence among related individuals, we accounted for the correlation between the genotypes by estimating the kinship coefficient for each pair of individuals using the available genomic data [34], [38], [39]. These analyses were performed in R using the GenABELlibraries and custom-written functions in FORTRAN and Python.
To estimate the magnitude of the associations (HRs), the effect of each SNP was modeled either as a per-allele HR (multiplicative model) or as genotype-specific HRs, and was estimated on the log-scale by maximizing the retrospective likelihood. The retrospective likelihood was fitted using the pedigree-analysis software MENDEL. The variances of the parameter estimates were obtained by robust variance estimation based on reported family membership. All analyses were stratified by country of residence and based on calendar-year and cohort-specific breast cancer incidence rates for mutation carriers. Countries with small number of mutation carriers were combined with neighbouring countries to ensure sufficiently large numbers within each stratum. USA and Canada were further stratified by reported Ashkenazi Jewish (AJ) ancestry.
Imputation
Genotypes were imputed separately for BRCA1 and BRCA2 mutation carriers using the v3 April 2012 release (Genomes Project et al., 2012) as reference panel. To improve computation efficiency we used a two-step procedure which involved pre-phasing in the first step and imputation of the phased data in the second. Pre-phasing was carried out using the SHAPEIT software [40]. The IMPUTE version 2 software was used for the subsequent imputation [41]. SNPs were excluded from the association analysis if their imputation accuracy was r2<0.3 or MAF<0.005 in any of the data sets. For the final analysis we only took in account those SNPs with an imputation accuracy r2>0.7, MAF>0.01 and being located in the region comprised within 15 kilo bases (kb) downstream and upstream the gene where the genotyped SNP showing an association was located (Table 1). Associations between imputed genotypes and breast cancer risk were evaluated using a version of the score test as described above but with the posterior genotype probabilities replacing the genotypes.
Supporting Information
p-values of association (−log10 scale) with breast and ovarian cancer risk in BRCA1 and BRCA2 carriers for genotyped and imputed SNPs considering 15 kb upstream and downstream the genes in which SNPs described in Table 1 were located. rs numbers of SNPs from Table 1 are indicated at the top of each panel and in the graph with a purple arrow. For PARP2 gene, the imputed SNP with the strongest association, rs61995542 is indicated with a red arrow. Colors represent the pariwise r2.
(PPT)
Association with breast cancer for the 94 SNPs selected for analysis in stage I.
(XLS)
number of BRCA1 and BRCA2 carriers by study.
(XLS)
Acknowledgments
CNIO acknowledgments: Genotyping was provided by ‘Centro Nacional de Genotipado - Instituto de Salud Carlos III’ (CeGen-ISCIII; www.cegen.org).
COGS acknowledgments: this study would not have been possible without the contributions of the following: Per Hall (COGS); Douglas F. Easton (BCAC), Andrew Berchuck (OCAC), Rosalind A. Eeles, Douglas F. Easton, Ali Amin Al Olama, Zsofia Kote-Jarai (PRACTICAL), Georgia Chenevix-Trench, Antonis Antoniou, Fergus Couch and Ken Offit (CIMBA), Joe Dennis, Alison M. Dunning, Andrew Lee, and Ed Dicks (Cambridge), Javier Benitez, Anna Gonzalez-Neira and the staff of the CNIO genotyping unit, Jacques Simard and Daniel C. Tessier, Francois Bacot, Daniel Vincent, Sylvie LaBoissière and Frederic Robidoux and the staff of the McGill University and Génome Québec Innovation Centre, Stig E. Bojesen, Sune F. Nielsen, Borge G. Nordestgaard, and the staff of the Copenhagen DNA laboratory, and Julie M. Cunningham, Sharon A. Windebank, Christopher A. Hilker, Jeffret Meyer and the staff of Mayo Clinic Genotyping Core Facility.
Swedish Breast Cancer Study (SWE-BRCA): SWE-BRCA members are Ake Borg, Department of Oncology, Lund University, Lund. Anna Öfverholm, Department of Clinical Genetics, Sahlgrenska University Hospital, Gothenburg, Sweden. Anna von Wachenfeldt, Department of Oncology, Karolinska University Hospital, Stockholm, Sweden. Annelie Liljegren, Department of Oncology, Karolinska University Hospital, Stockholm, Sweden. Annika Lindblom, Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden. Beatrice Melin, Department of Radiation Sciences, Oncology, Umeå University, Umea, Sweden. Brita Arver, Department of Oncology, Karolinska University Hospital, Stockholm, Sweden. Christina Edwinsdotter Ardnor, Department of Radiation Sciences, Oncology, Umeå University, Umea, Sweden. Gisela Barbany Bustinza, Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden. Håkan Olsson, Department of Oncology, Lund University Hospital, Lund, Sweden. Hans Ehrencrona, Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, and Department of Clinical Genetics, Lund University Hospital, Lund, Sweden. Helena Jernström, Department of Oncology, Lund University, Lund, Sweden. Johanna Rantala, Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden. Karin Henriksson, Oncologic Centre, Regional Tumour Registry, Lund University Hospital, Lund, Sweden. Katja Harbst, Department of Oncology, Lund University, Lund, Sweden. Margareta Nordling, Department of Clinical Genetics, Sahlgrenska University Hospital, Gothenburg, Sweden. Maria Soller, Department of Clinical Genetics. Lund University Hospital, Lund, Sweden. Marie Stenmark-Askmalm, Division of Clinical Genetics, Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden. Maritta Hellström Pigg, Department of Immunology, Genetics and Pathology, Rudbeck Laboratory, Uppsala University, Uppsala. Monica Emanuelsson, Department of Radiation Sciences, Oncology, Umeå University, Umea, Sweden. Niklas Loman, Department of Oncology, Lund University Hospital, Lund, Sweden. Per Karlsson, Department of Oncology, Sahlgrenska University Hospital, Gothenburg, Sweden. Richard Rosenquist, Department of Immunology, Genetics and Pathology, Rudbeck Laboratory, Uppsala University, Uppsala, Sweden. Sigrun Liedgren, Division of Clinical Genetics, Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden, SWE-BRCA, Ulf Kristoffersson, Department of Clinical Genetics, Lund University Hospital, Lund, Sweden. Zakaria Einbeigi, Department of Oncology, Sahlgrenska University Hospital, Gothenburg, Sweden.
The University of Chicago Center for Clinical Cancer Genetics and Global Health (UCHICAGO) wish to thank Cecilia Zvocec, Qun Niu, physicians, genetic counselors, research nurses and staff of the Cancer Risk Clinic for their contributions to this resource, and the many families who contribute to our program.
UCSF Cancer Risk Program and Helen Diller Family Comprehensive Cancer Center: We would like to thank Ms. Salina Chan for her data management and the following genetic counselors for participant recruitment: Beth Crawford, Nicola Stewart, Julie Mak, and Kate Lamvik.
Baltic Familial Breast Ovarian Cancer Consortium (BFBOCC) study: we acknowledge Vilius Rudaitis, Laimonas Griškevičius, BFBOCC-LV acknowledge Drs. Janis Eglitis, Anna Krilova and Aivars Stengrevics.
BRCA-gene mutations and breast cancer in South African women (BMBSA) wish to thank the families who contribute to the BMBSA study.
Beckman Research Institute of the City of Hope (BRICOH) study: we wish to thank Greg Wilhoite, Linda Steele, and Marie Pinto for their work in participant enrollment and biospecimen and data management.
The City of Hope Clinical Genetics Community Research Network: we wish to thank the collaborating clinicians in the Clinical Cancer Genetics Community Research Network for patient recruitment and follow up; Kai Yang for masterful assistance with the database, and Hazel Mariveles for network coordination.
CONsorzio Studi ITaliani sui Tumori Ereditary Alla Mamella (CONSIT TEAM) acknowledgments: Monica Barile and Irene Feroce of the Istituto Europeo di Oncologia, Milan, Italy; Antonella Savarese and Aline Martayan of the Istituto Nazionale Tumori Regina Elena, Rome, Italy; Stefania Tommasi, Brunella Pilato and Rossana Lambo of the Istituto Nazionale Tumori “Giovanni Paolo II” - Bari, Italy and the personnnel of the Cogentech Cancer Genetic Test Laboratory, Milan, Italy.
Epidemiological study of BRCA1 & BRCA2 mutation carriers (EMBRACE): Douglas F. Easton is the PI of the study. EMBRACE Collaborating Centres are: Coordinating Centre, Cambridge: Susan Peock, Debra Frost, Steve D. Ellis, Elena Fineberg, Radka Platte. North of Scotland Regional Genetics Service, Aberdeen: Zosia Miedzybrodzka, Helen Gregory. Northern Ireland Regional Genetics Service, Belfast: Patrick Morrison, Lisa Jeffers. West Midlands Regional Clinical Genetics Service, Birmingham: Trevor Cole, Kai-ren Ong, Jonathan Hoffman. South West Regional Genetics Service, Bristol: Alan Donaldson, Margaret James. East Anglian Regional Genetics Service, Cambridge: Marc Tischkowitz, Joan Paterson, Amy Taylor. Medical Genetics Services for Wales, Cardiff: Alexandra Murray, Mark T. Rogers, Emma McCann. St James's Hospital, Dublin & National Centre for Medical Genetics, Dublin: M. John Kennedy, David Barton. South East of Scotland Regional Genetics Service, Edinburgh: Mary Porteous, Sarah Drummond. Peninsula Clinical Genetics Service, Exeter: Carole Brewer, Emma Kivuva, Anne Searle, Selina Goodman, Kathryn Hill. West of Scotland Regional Genetics Service, Glasgow: Rosemarie Davidson, Victoria Murday, Nicola Bradshaw, Lesley Snadden, Mark Longmuir, Catherine Watt, Sarah Gibson, Eshika Haque, Ed Tobias, Alexis Duncan. South East Thames Regional Genetics Service, Guy's Hospital London: Louise Izatt, Chris Jacobs, Caroline Langman. North West Thames Regional Genetics Service, Harrow: Huw Dorkins. Leicestershire Clinical Genetics Service, Leicester: Julian Barwell. Yorkshire Regional Genetics Service, Leeds: Julian Adlard, Gemma Serra-Feliu. Cheshire & Merseyside Clinical Genetics Service, Liverpool: Ian Ellis, Catherine Houghton. Manchester Regional Genetics Service, Manchester: D Gareth Evans, Fiona Lalloo, Jane Taylor. North East Thames Regional Genetics Service, NE Thames, London: Lucy Side, Alison Male, Cheryl Berlin. Nottingham Centre for Medical Genetics, Nottingham: Jacqueline Eason, Rebecca Collier. Northern Clinical Genetics Service, Newcastle: Fiona Douglas, Oonagh Claber, Irene Jobson. Oxford Regional Genetics Service, Oxford: Lisa Walker, Diane McLeod, Dorothy Halliday, Sarah Durell, Barbara Stayner. The Institute of Cancer Research and Royal Marsden NHS Foundation Trust: Ros Eeles, Susan Shanley, Nazneen Rahman, Richard Houlston, Elizabeth Bancroft, Elizabeth Page, Audrey Ardern-Jones, Kelly Kohut, Jennifer Wiggins, Elena Castro, Emma Killick, Sue Martin, Gillian Rea, Anjana Kulkarni. North Trent Clinical Genetics Service, Sheffield: Jackie Cook, Oliver Quarrell, Cathryn Bardsley. South West Thames Regional Genetics Service, London: Shirley Hodgson, Sheila Goff, Glen Brice, Lizzie Winchester, Charlotte Eddy, Vishakha Tripathi, Virginia Attard, Anna Lehmann. Wessex Clinical Genetics Service, Princess Anne Hospital, Southampton: Diana Eccles, Anneke Lucassen, Gillian Crawford, Donna McBride, Sarah Smalley. Medical Genetics Services for Wales, Cardiff thanks Alexandra Murray, Mark T. Rogers, Emma McCann. North West Thames Regional Genetics Service, Harrow thanks Huw Dorkins. Leicestershire Clinical Genetics Service, Leicester thanks Julian Barwell. Nottingham Centre for Medical Genetics, Nottingham thanks Jacqueline Eason, Rebecca Collier. North of Scotland Regional Genetics Service, Aberdeen thanks Zosia Miedzybrodzka, Helen Gregory.
Fox Chase Cancer Center (FCCC study): we thank Ms. JoEllen Weaver and Dr. Betsy Bove for their technical support.
The German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC): we are very thankful to all family members who participated in this study, Wolfram Heinritz, Center Leipzig, and Dieter Schäfer, Center Frankfurt, for providing DNA samples and Juliane Köhler for excellent technical assistance.
Genetic Modifiers of Cancer Risk in BRCA1/2 Mutation Carriers (GEMO) study: National Cancer Genetics Network «UNICANCER Genetic Group», France. We wish to thank all the GEMO collaborating groups for their contribution to this study. GEMO Collaborating Centers are: Coordinating Centres, Unité Mixte de Génétique Constitutionnelle des Cancers Fréquents, Hospices Civils de Lyon - Centre Léon Bérard, & Equipe «Génétique du cancer du sein», Centre de Recherche en Cancérologie de Lyon: Olga Sinilnikova, Sylvie Mazoyer, Francesca Damiola, Laure Barjhoux, Carole Verny-Pierre, Alain Calender, Sophie Giraud, Mélanie Léone; and Service de Génétique Oncologique, Institut Curie, Paris: Dominique Stoppa-Lyonnet, Marion Gauthier-Villars, Bruno Buecher, Claude Houdayer, Virginie Moncoutier, Muriel Belotti, Carole Tirapo, Antoine de Pauw. Institut Gustave Roussy, Villejuif: Brigitte Bressac-de-Paillerets, Olivier Caron. Centre Jean Perrin, Clermont–Ferrand: Yves-Jean Bignon, Nancy Uhrhammer. Centre Léon Bérard, Lyon: Christine Lasset, Valérie Bonadona, Sandrine Handallou. Centre François Baclesse, Caen: Agnès Hardouin, Pascaline Berthet. Institut Paoli Calmettes, Marseille: Hagay Sobol, Violaine Bourdon, Tetsuro Noguchi, Audrey Remenieras, François Eisinger. CHU Arnaud-de-Villeneuve, Montpellier: Isabelle Coupier, Pascal Pujol. Centre Oscar Lambret, Lille: Jean-Philippe Peyrat, Joëlle Fournier, Françoise Révillion, Philippe Vennin, Claude Adenis. Hôpital René Huguenin/Institut Curie, St Cloud: Etienne Rouleau, Rosette Lidereau, Liliane Demange, Catherine Nogues. Centre Paul Strauss, Strasbourg: Danièle Muller, Jean-Pierre Fricker. Institut Bergonié, Bordeaux: Emmanuelle Barouk-Simonet, Françoise Bonnet, Virginie Bubien, Nicolas Sevenet, Michel Longy. Institut Claudius Regaud, Toulouse: Christine Toulas, Rosine Guimbaud, Laurence Gladieff, Viviane Feillel. CHU Grenoble: Dominique Leroux, Hélène Dreyfus, Christine Rebischung, Magalie Peysselon. CHU Dijon: Fanny Coron, Laurence Faivre. CHU St-Etienne: Fabienne Prieur, Marine Lebrun, Caroline Kientz. Hôtel Dieu Centre Hospitalier, Chambéry: Sandra Fert Ferrer. Centre Antoine Lacassagne, Nice: Marc Frénay. CHU Limoges: Laurence Vénat-Bouvet. CHU Nantes: Capucine Delnatte. CHU Bretonneau, Tours: Isabelle Mortemousque. Groupe Hospitalier Pitié-Salpétrière, Paris: Florence Coulet, Chrystelle Colas, Florent Soubrier. CHU Vandoeuvre-les-Nancy: Johanna Sokolowska, Myriam Bronner. CHU Besançon: Marie-Agnès Collonge-Rame, Alexandre Damette. Creighton University, Omaha, USA: Henry T. Lynch, Carrie L. Snyder.
G-FAST: we acknowledge the contribution of Kim De Leeneer and Anne De Paepe. We wish to thank the technical support of Ilse Coene en Brecht Crombez.
Hospital Clínico San Carlos (HCSC) study: we acknowledge Alicia Tosar for her technical assistance.
Helsinki Breast Cancer Study (HEBCS) would like to thank Drs. Kristiina Aittomäki, Carl Blomqvist and Kirsimari Aaltonen and Taru A. Muranen and RN Irja Erkkilä for their help with the HEBCS data and samples.
The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) consists of the following Collaborating Centers: Coordinating center: Netherlands Cancer Institute, Amsterdam, NL: M. A. Rookus, F. B. L. Hogervorst, F. E. van Leeuwen, S. Verhoef, M. K. Schmidt, J. L. de Lange; Erasmus Medical Center, Rotterdam, NL: J. M. Collée, A.M.W. van den Ouweland, M. J. Hooning, C. Seynaeve, C. H. M. van Deurzen, I. M. Obdeijn; Leiden University Medical Center, NL: C. J. van Asperen, J. T. Wijnen, R. A. E. M. Tollenaar, P. Devilee, T. C. T. E. F. van Cronenburg; Radboud University Nijmegen Medical Center, NL: C. M. Kets, A. R. Mensenkamp; University Medical Center Utrecht, NL: M. G. E. M. Ausems, R. B. van der Luijt; Amsterdam Medical Center, NL: C. M. Aalfs, T. A. M. van Os; VU University Medical Center, Amsterdam, NL: J. J. P. Gille, Q. Waisfisz, H. E. J. Meijers-Heijboer; University Hospital Maastricht, NL: E. B. Gómez-Garcia, M. J. Blok; University Medical Center Groningen, NL: J. C. Oosterwijk, A. H. van der Hout, M. J. Mourits, G. H. de Bock. The Netherlands Foundation for the detection of hereditary tumours, Leiden, NL: H. F. Vasen. Hungarian Breast and Ovarian Cancer Study: we wish to thank to Hungarian Breast and Ovarian Cancer Study Group members (Janos Papp, Tibor Vaszko, Aniko Bozsik, Judit Franko, Maria Balogh, Gabriella Domokos, Judit Ferenczi, Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary) and the clinicians and patients for their contributions to this study.
Interdisciplinary Health Research Internal Team Breast Cancer susceptibility (INHERIT): we would like to thank Dr Martine Dumont, Martine Tranchant for sample management and skillful technical assistance. J.S. is Chairholder of the Canada Research Chair in Oncogenetics.
Kathleen Cuningham Consortium for Research into Familial Breast Cancer (kConFab): we wish to thank Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow Up Study for their contributions to this resource, and the many families who contribute to kConFab.
National Israeli Cancer Control Center (NICC): we wish to thank the NICCC National Familial Cancer Consultation Service team led by Sara Dishon, the lab team led by Dr. Flavio Lejbkowicz, and the research field operations team led by Dr. Mila Pinchev.
The Ohio State University Comprehensive Cancer Center (OSUCCG) acknowledges Leigha Senter, Kevin Sweet, Caroline Craven and Michelle O'Conor were instrumental in accrual of study participants, ascertainment of medical records and database management. Samples were processed by the OSU Human Genetics Sample Bank.SMC team wishes to acknowledge the assistance of the Meirav Comprehensice breast cancer center team at the Sheba Medical Center for assistance in this study.
Sheba Medical Centre (SMC): SMC team wishes to acknowledge the assistance of the Meirav Comprehensice breast cancer center team at the Sheba Medical Center for assistance in this study.
Funding Statement
The CNIO study was supported by Mutua Madrileña Foundation (FMMA), Spanish Association against Cancer (AECC08), RTICC 06/0020/1060 and FISPI12/00070. Funding for the iCOGS infrastructure came from: the European Community's Seventh Framework Programme under grant agreement n° 223175 (HEALTH-F2-2009-223175) (COGS), Cancer Research UK (C1287/A10118, C1287/A 10710, C12292/A11174, C5047/A8384, C5047/A15007, C5047/A10692), the National Institutes of Health (CA128978) and Post-Cancer GWAS initiative (No. 1 U19 CA 148537 - the GAME-ON initiative), the Department of Defence (W81XWH-10-1-0341), the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer, Komen Foundation for the Cure, the Breast Cancer Research Foundation, and the Ovarian Cancer Research Fund. SWE-BRCA collaborators are supported by the Swedish Cancer Society. BRCA-gene mutations and breast cancer in South African women (BMBSA) was supported by grants from the Cancer Association of South Africa (CANSA) to EJvR. UCHICAGO is supported by NCI Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA125183), R01 CA142996, 1U01CA161032 and by the Ralph and Marion Falk Medical Research Trust, the Entertainment Industry Fund National Women's Cancer Research Alliance and the Breast Cancer research Foundation. OIO is an ACS Clinical Research Professor. UPENN study is supported by Basser Research Center (SMD, KN, TRR), Breast Cancer Research Foundation (KN), Komen Foundation for the Cure (SMD). The Women's Cancer Program (WCP) at the Samuel Oschin Comprehensive Cancer Institute is funded by the American Cancer Society Early Detection Professorship (SIOP-06-258-01-COUN). BCFR study: This work was supported by grant UM1 CA164920 from the National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the BCFR. BFBOCC is supported by: Lithuania (BFBOCC-LT): Research Council of Lithuania grant LIG-07/2012 and Hereditary Cancer Association (Paveldimo vėžio asociacija); Latvia (BFBOCC-LV) is partly supported by LSC grant 10.0010.08 and in part by a grant from the ESF Nr.2009/0220/1DP/1.1.1.2.0/09/APIA/VIAA/016 and Liepaja's municipal council. BRICOH study: NIH R01CA74415 and P30 CA033752. SLN was partially supported by the Morris and Horowitz Families Professorship. CBCS work was supported by the NEYE Foundation. The City of Hope Clinical Cancer Genetics Community Research Network is supported by Award Number RC4A153828 (PI: JNW) from the National Cancer Institute and the Office of the Director, National Institutes of Health. The members of CONSIT TEAM were funded by grants from the Italian Association for Cancer Research (AIRC) to PP, LO and PR; FiorGen Foundation for Pharmacogenomics to LP; Associazione CAOS Varese to MGT and by funds from Italian citizens who allocated the 5×1000 share of their tax payment in support of the Fondazione IRCCS Istituto Nazionale Tumori, according to Italian laws (INT-Institutional strategic projects ‘5×1000’) to SMan and PR. Demokritos: This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program of the General Secretariat for Research & Technology: ARISTEIA. Investing in knowledge society through the European Social Fund. We wish to thank Hellenic Cooperative Oncology Group (HeCOG) and the Hellenic Foundation for Cancer Research (HFCR). EMBRACE is supported by Cancer Research UK Grants C1287/A10118 and C1287/A11990. GE and FLa are supported by an NIHR grant to the Biomedical Research Centre, Manchester. The Investigators at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust are supported by an NIHR grant to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. RE and Elizabeth Bancroft are supported by Cancer Research UK Grant C5047/A8385. FCCC study: The authors acknowledge support from The University of Kansas Cancer Center (P30 CA168524) and the Kansas Bioscience Authority Eminent Scholar Program. AKG was funded by 5U01CA113916, R01CA140323, and by the Chancellors Distinguished Chair in Biomedical Sciences Professorship. The German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) is supported by the German Cancer Aid (grant no 109076, RKS) and by the Center for Molecular Medicine Cologne (CMMC). This study was kindly supported by the German Cancer Aid to RKS (grant no 109076) and by the Center for Molecular Medicine Cologne (CMMC). GEMO study: The study was supported by the Ligue National Contre le Cancer; the Association “Le cancer du sein, parlons-en!” Award; and the Canadian Institutes of Health Research for the “CIHR Team in Familial Risks of Breast Cancer” program. GOG study: This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office and Tissue Bank (CA 27469), the GOG Statistical and Data Center (CA 37517), and GOG's Cancer Prevention and Control Committee (CA 101165). MHG, PLM and Sharon A. Savage were supported by funding from the Intramural Research Program, NCI. This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) CA 27469, CA 37517, and CA 101165. HCSC study was supported by a grant RD06/0020/0021 from RTICC (ISCIII), Spanish Ministry of Economy and Competitivity. The HEBCS was financially supported by the Helsinki University Central Hospital Research Fund, Academy of Finland (132473), the Finnish Cancer Society and the Sigrid Juselius Foundation. The HEBON study is supported by the Dutch Cancer Society grants NKI1998-1854, NKI2004-3088, NKI2007-3756, the Netherlands Organization of Scientific Research grant NWO 91109024, the Pink Ribbon grant 110005 and the BBMRI grant NWO 184.021.007/CP46. Hungarian Breast and Ovarian Cancer Study was supported by Hungarian Research Grant KTIA-OTKA CK-80745. IHCC study: KJB is a fellow of International PhD program, Postgraduate School of Molecular Medicine, Warsaw Medical University, supported by the Polish Foundation of Science. The ILUH group was supported by the Nordic Cancer Union, Icelandic Association “Walking for Breast Cancer Research” and by the Landspitali University Hospital Research Fund. INHERIT: This work was supported by the Canadian Institutes of Health Research for the “CIHR Team in Familial Risks of Breast Cancer” program, the Canadian Breast Cancer Research Alliance-grant #019511 and the Ministry of Economic Development, Innovation and Export Trade – grant # PSR-SIIRI-701. IOVHBOCS study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca and Ministero della Salute. kConFab is supported by grants from the National Breast Cancer Foundation, the National Health and Medical Research Council (NHMRC) and by the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. GCT and Amanda B. Spurdle are NHMRC Senior Research Fellows. MAYO is supported by NIH grant CA128978, an NCI Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA116201), a U.S. Department of Defence Ovarian Cancer Idea award (W81XWH-10-1-0341) and grants from the Breast Cancer Research Foundation and the Komen Foundation for the Cure. MSKCC was supported by the Sharon Levine Corzine Fund, Breast Cancer Research Foundation, Niehaus Clinical Cancer Genetics Initiative, Andrew Sabin Family Foundation and Lymphoma Foundation. NCI study: The research of MHG, PLM and Sharon A. Savage was supported by the Intramural Research Program of the US National Cancer Institute, NIH, and by support services contracts NO2-CP-11019-50 and N02-CP-65504 with Westat, Inc, Rockville, MD. NICCC is supported by Clalit Health Services in Israel. Some of its activities are supported by the Israel Cancer Association and the Breast Cancer Research Foundation (BCRF), NY. OSUCCG is supported by the Ohio State University Comprehensive Cancer Center. SMC study: This project was partially funded through a grant by the Israel cancer association and the funding for the Israeli Inherited breast cancer consortium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, et al. (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72: 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, et al. (2006) Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol 24: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milne RL, Osorio A, Cajal TR, Vega A, Llort G, et al. (2008) The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res 14: 2861–2869. [DOI] [PubMed] [Google Scholar]
- 4.Chenevix-Trench G, Milne RL, Antoniou AC, Couch FJ, Easton DF, et al. (2007) An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res 9: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniou AC, Kuchenbaecker KB, Soucy P, Beesley J, Chen X, et al. (2012) Common variants at 12p11, 12q24, 9p21, 9q31.2 and in ZNF365 are associated with breast cancer risk for BRCA1 and/or BRCA2 mutation carriers. Breast Cancer Res 14: R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniou AC, Spurdle AB, Sinilnikova OM, Healey S, Pooley KA, et al. (2008) Common breast cancer-predisposition alleles are associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Am J Hum Genet 82: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoniou AC, Sinilnikova OM, McGuffog L, Healey S, Nevanlinna H, et al. (2009) Common variants in LSP1, 2q35 and 8q24 and breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet 18: 4442–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou AC, Wang X, Fredericksen ZS, McGuffog L, Tarrell R, et al. (2010) A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet 42: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou AC, Sinilnikova OM, Simard J, Leone M, Dumont M, et al. (2007) RAD51 135G→C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. Am J Hum Genet 81: 1186–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moynahan ME, Chiu JW, Koller BH, Jasin M (1999) Brca1 controls homology-directed DNA repair. Mol Cell 4: 511–518. [DOI] [PubMed] [Google Scholar]
- 11.Patel KJ, Yu VP, Lee H, Corcoran A, Thistlethwaite FC, et al. (1998) Involvement of Brca2 in DNA repair. Mol Cell 1: 347–357. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Herzig M, Rotrekl V, Walter CA (2008) Base excision repair, aging and health span. Mech Ageing Dev 129: 366–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, et al. (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434: 917–921. [DOI] [PubMed] [Google Scholar]
- 14.Osorio A, Milne RL, Alonso R, Pita G, Peterlongo P, et al. (2011) Evaluation of the XRCC1 gene as a phenotypic modifier in BRCA1/2 mutation carriers. Results from the consortium of investigators of modifiers of BRCA1/BRCA2. Br J Cancer 104: 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Newcomb PA, Egan KM, Titus-Ernstoff L, Chanock S, et al. (2006) Genetic polymorphisms in base-excision repair pathway genes and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 15: 353–358. [DOI] [PubMed] [Google Scholar]
- 16.Zipprich A, Kuss O, Rogowski S, Kleber G, Lotterer E, et al. (2010) Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut 59: 963–968. [DOI] [PubMed] [Google Scholar]
- 17.Popanda O, Seibold P, Nikolov I, Oakes CC, Burwinkel B, et al. (2013) Germline variants of base excision repair genes and breast cancer: A polymorphism in DNA polymerase gamma modifies gene expression and breast cancer risk. Int J Cancer 132: 55–62. [DOI] [PubMed] [Google Scholar]
- 18.Roberts MR, Shields PG, Ambrosone CB, Nie J, Marian C, et al. (2011) Single-nucleotide polymorphisms in DNA repair genes and association with breast cancer risk in the web study. Carcinogenesis 32: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangrajrang S, Schmezer P, Burkholder I, Waas P, Boffetta P, et al. (2008) Polymorphisms in three base excision repair genes and breast cancer risk in Thai women. Breast Cancer Res Treat 111: 279–288. [DOI] [PubMed] [Google Scholar]
- 20.Ming-Shiean H, Yu JC, Wang HW, Chen ST, Hsiung CN, et al. (2010) Synergistic effects of polymorphisms in DNA repair genes and endogenous estrogen exposure on female breast cancer risk. Ann Surg Oncol 17: 760–771. [DOI] [PubMed] [Google Scholar]
- 21.Zipprich J, Terry MB, Brandt-Rauf P, Freyer GA, Liao Y, et al. (2010) XRCC1 polymorphisms and breast cancer risk from the New York Site of the Breast Cancer Family Registry: A family-based case-control study. J Carcinog 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee D, Mandal SM, Das A, Hegde ML, Das S, et al. (2011) Preferential repair of oxidized base damage in the transcribed genes of mammalian cells. J Biol Chem 286: 6006–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei H, Kamat A, Chen M, Ke HL, Chang DW, et al. (2012) Association of polymorphisms in oxidative stress genes with clinical outcomes for bladder cancer treated with Bacillus Calmette-Guerin. PLoS One 7: e38533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinslow CJ, El-Zein RA, Rondelli CM, Hill CE, Wickliffe JK, et al. (2010) Regulatory regions responsive to oxidative stress in the promoter of the human DNA glycosylase gene NEIL2. Mutagenesis 25: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinslow CJ, El-Zein RA, Hill CE, Wickliffe JK, Abdel-Rahman SZ (2008) Single nucleotide polymorphisms 5′ upstream the coding region of the NEIL2 gene influence gene transcription levels and alter levels of genetic damage. Genes Chromosomes Cancer 47: 923–932. [DOI] [PubMed] [Google Scholar]
- 26.Dey S, Maiti AK, Hegde ML, Hegde PM, Boldogh I, et al. (2012) Increased risk of lung cancer associated with a functionally impaired polymorphic variant of the human DNA glycosylase NEIL2. DNA Repair (Amst) 11: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai X, Zhao H, Liu Z, Wang LE, El-Naggar AK, et al. (2008) Functional variants of the NEIL1 and NEIL2 genes and risk and progression of squamous cell carcinoma of the oral cavity and oropharynx. Clin Cancer Res 14: 4345–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arcand SL, Provencher D, Mes-Masson AM, Tonin PN (2005) OGG1 Cys326 variant, allelic imbalance of chromosome band 3p25.3 and TP53 mutations in ovarian cancer. Int J Oncol 27: 1315–1320. [PubMed] [Google Scholar]
- 29.Rossner P Jr, Terry MB, Gammon MD, Zhang FF, Teitelbaum SL, et al. (2006) OGG1 polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 15: 811–815. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, He XF, Qin JB, Su J, Li SX, et al. (2012) Association between the OGG1 Ser326Cys and APEX1 Asp148Glu polymorphisms and lung cancer risk: a meta-analysis. Mol Biol Rep 39: 11249–11262. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Yang H, Miller JH, Shih DM, Hicks GG, et al. (2008) Cells deficient in oxidative DNA damage repair genes Myh and Ogg1 are sensitive to oxidants with increased G2/M arrest and multinucleation. Carcinogenesis 29: 722–728. [DOI] [PubMed] [Google Scholar]
- 32.Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, et al. (2013) Genome-Wide Association Study in BRCA1 Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk. PLoS Genet 9: e1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visnes T, Akbari M, Hagen L, Slupphaug G, Krokan HE (2008) The rate of base excision repair of uracil is controlled by the initiating glycosylase. DNA Repair (Amst) 7: 1869–1881. [DOI] [PubMed] [Google Scholar]
- 34.Antoniou AC, Goldgar DE, Andrieu N, Chang-Claude J, Brohet R, et al. (2005) A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol 29: 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Gaudet MM, Kuchenbaecker KB, Vijai J, Klein RJ, Kirchhoff T, et al. (2013) Identification of a BRCA2-Specific Modifier Locus at 6p24 Related to Breast Cancer Risk. PLoS Genet 9: e1003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes DR, Lee A, Easton DF, Antoniou AC (2012) Evaluation of association methods for analysing modifiers of disease risk in carriers of high-risk mutations. Genet Epidemiol 36: 274–291. [DOI] [PubMed] [Google Scholar]
- 37.Barnes DR, Antoniou AC (2012) Unravelling modifiers of breast and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: update on genetic modifiers. J Intern Med 271: 331–343. [DOI] [PubMed] [Google Scholar]
- 38.Amin N, van Duijn CM, Aulchenko YS (2007) A genomic background based method for association analysis in related individuals. PLoS One 2: e1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leutenegger AL, Prum B, Genin E, Verny C, Lemainque A, et al. (2003) Estimation of the inbreeding coefficient through use of genomic data. Am J Hum Genet 73: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaneau O, Zagury JF (2012) Haplotype inference. Methods Mol Biol 888: 177–196. [DOI] [PubMed] [Google Scholar]
- 41.Howie BN, Donnelly P, Marchini J (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26: 2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
p-values of association (−log10 scale) with breast and ovarian cancer risk in BRCA1 and BRCA2 carriers for genotyped and imputed SNPs considering 15 kb upstream and downstream the genes in which SNPs described in Table 1 were located. rs numbers of SNPs from Table 1 are indicated at the top of each panel and in the graph with a purple arrow. For PARP2 gene, the imputed SNP with the strongest association, rs61995542 is indicated with a red arrow. Colors represent the pariwise r2.
(PPT)
Association with breast cancer for the 94 SNPs selected for analysis in stage I.
(XLS)
number of BRCA1 and BRCA2 carriers by study.
(XLS)