Abstract
Background
The single-dose benzimidazoles used against Trichuris trichiura infections in humans are not satisfactory. Likewise, the benzimidazole, fenbendazole, has varied efficacy against Trichuris suis whereas Oesophagostomum dentatum is highly sensitive to the drug. The reasons for low treatment efficacy of Trichuris spp. infections are not known.
Methodology
We studied the effect of fenbendazole, albendazole and levamisole on the motility of T. suis and O. dentatum and measured concentrations of the parent drug compounds and metabolites of the benzimidazoles within worms in vitro. The motility and concentrations of drug compounds within worms were compared between species and the maximum specific binding capacity (Bmax) of T. suis and O. dentatum towards the benzimidazoles was estimated. Comparisons of drug uptake in living and killed worms were made for both species.
Principal findings
The motility of T. suis was generally less decreased than the motility of O. dentatum when incubated in benzimidazoles, but was more decreased when incubated in levamisole. The Bmax were significantly lower for T. suis (106.6, and 612.7 pmol/mg dry worm tissue) than O. dentatum (395.2, 958.1 pmol/mg dry worm tissue) when incubated for 72 hours in fenbendazole and albendazole respectively. The total drug concentrations (pmol/mg dry worm tissue) were significantly lower within T. suis than O. dentatum whether killed or alive when incubated in all tested drugs (except in living worms exposed to fenbendazole). Relatively high proportions of the anthelmintic inactive metabolite fenbendazole sulphone was measured within T. suis (6–17.2%) as compared to O. dentatum (0.8–0.9%).
Conclusion/Significance
The general lower sensitivity of T. suis towards BZs in vitro seems to be related to a lower drug uptake. Furthermore, the relatively high occurrence of fenbendazole sulphone suggests a higher detoxifying capacity of T. suis as compared to O. dentatum.
Author Summary
The human whipworm Trichuris trichiura is together with the roundworm Ascaris lumbricoides and the hookworms Ancylostoma duodenale and Necator Americanus the most common intestinal worms worldwide. Together they place more than 5 billion people at risk of infection. The current global control strategy against these worms is regular administration of anthelmintic drugs, mostly albendazole and mebendazole, both belonging to the drug-class benzimidazoles. Both drugs have a low effect against T. trichiura infections, but the reasons for this are not known. We evaluated the in vitro effect of two benzimidazoles; i.e., albendazole, fenbendazole, and another type of anthelmintic, levamisole, on the whipworm (T. suis) and the nodular worm (Oesophagostomum dentatum) of the pig. Oesophagostomum dentatum is highly sensitive towards benzimidazoles in comparison to T. suis. We measured and compared the drug uptake in both species in both living and killed worms. Our results suggest that the reason for the difference in sensitivity is due to a lower drug uptake into T. suis as compared to O. dentatum. Furthermore, T. suis was able to metabolise fenbendazole into an inactive metabolite to a much larger extent than O. dentatum, suggesting a higher detoxifying capacity of T. suis as compared to O. dentatum.
Introduction
The whipworm Trichuris trichiura has been estimated to infect 600 million people worldwide resulting in an estimated 1.6–6.4 million disability adjusted life-years lost globally [1]. The current control strategy against T. trichiura and other soil-transmitted helminths (STHs) is administration of single-dose anthelmintic drugs [1], [2]. The benzimidazoles (BZs) i.e. albendazole (ALB) and mebendazole (MBD) are widely used in large-scale control programs where they are administered regularly, at a dosage of 400 mg (ALB) or 500 mg (MBD) [2]. However, the efficacy of single-dose BZ against T. trichiura is not satisfactory. A meta-analysis of 20 randomized, placebo-controlled trials reported an average cure rate (CR) of 28% for ALB (400 mg) and 36% for MBD (500 mg) [3]. Other randomized controlled trials have reported similar low CR and egg reduction rates (ERR) ranging from 31.5–40.3% (CR) and 9.8–54.0% (ERR) for ALB and 22.9–66.7% (CR) and 18.8–81.0% (ERR) for MBD [4]–[7].
The use of the T. muris-mouse model for estimating drug efficacy on T. trichiura is well established [8]–[11]. Trichuris suis is regarded a different but closely related species to T. trichiura [12], [13], hence, T. suis can be considered a valid model for T. trichiura. Another BZ, fenbendazole (FBZ) has shown poor efficacy against T. suis infection in pigs when administered as a single-dose [14], therefore the T. suis-pig model and FBZ may be considered an interesting alternative for studying low treatment efficacy of Trichuris spp. In one controlled trial an oral dose as high as 15 mg/kg, three times the recommended dose of 5 mg/kg for other pig nematodes, was required to obtain a worm count reduction (WCR) of 96.7% [14]. In another controlled study the same oral dose resulted in only a 65.1% reduction in worm burden and a dose of 30 mg/kg resulted in an efficacy of 96.6% [15]. Multiple doses of FBZ (3 mg/kg per day for 3 consecutive days) have shown varied efficacy against T. suis in controlled tests ranging from 66% [16] to 99.8% [14], [17] in WCR. The current recommendation for treatment of T. suis infections in pigs with FBZ is either a single dose of 25 mg/kg, or a long-term treatment where the recommended therapeutic dose is distributed over 7 days [18], [19].
Another nematode of the pig is the nodular worm, Oesophagostomum dentatum which in the adult stage, opposed to T. suis, is highly sensitive to FBZ. An oral dose level as low as 0.25 mg/kg has shown an efficacy of 99.9% and doses of 1, 2.5 and 3.5 mg/kg FBZ have resulted in efficacies of 100% in controlled tests based on worm counts [20], [21]. Trichuris suis and O. dentatum both inhabit the lower part of the intestine namely the caecum and the colon [22]–[25], but in their adult stage, their microhabitat varies significantly. The thin anterior part of T. suis is embedded in the mucosa creating a tunnel-like construction of epithelial cells whereas the thicker posterior part of the body is protruding freely into the lumen [26]. In contrast to T. suis, the adult stage of O. dentatum is not attached to the mucosa but roams freely in the intestinal lumen [27], [28].
Levamisole (LEV), belonging to another class of anthelmintics, the imidazothiazoles, was introduced in 1968 [29] and has like BZs been used against parasitic infections in both animals and humans. In order for BZs and imidothiazoles to exert their pharmacological effect, they need to reach their specific receptors within the target parasites i.e. BZs bind to beta-tubulin [30] and the imidazothiazoles to acetylcholine-gated channels [29], [31]. Passive diffusion through the external surface has been proposed as the main pathway of BZs (i.e. FBZ, oxfendazole (OXF) and triclabendazole sulphoxide (TCBZSO)) in the three main classes of helminth parasites represented by: Moniezia benedeni (cestode), Fasciola hepatica (trematode) and Ascaris suum (nematode) [32]. The uptake of LEV has likewise been demonstrated to occur via a transcuticular mechanism in A. suum, but was observed to take place in four distinct stages, thus suggesting a non-passive up-take mechanism [33]. Once inside an organism, drugs are generally being metabolised. However, our knowledge of the metabolism of anthelmintics in helminths is very limited, although drug metabolising enzymes are well described in mammals and serve as an efficient defense mechanism against potential harmful substances. In brief drugs are (if not excreted unchanged) biotransformed by unique enzymes into more polar compounds that are easier to excrete by the organism in metabolic reactions named phase I-III. In mammals the major phase I reaction is oxidation catalysed by cytochrome P450 superfamily (CYPs) [34]. For many years attempts to detect CYPs in parasitic nematodes were unsuccessful [35] but with the discovery of 75 predicted CYP genes in the free-living nematode Caenorhabditis elegans as well as genomic and transcriptomic-based predictions of proteins produced by helminths, the knowledge has improved [36]. The ability of parasitic helminths to metabolise anthelmintics may serve as an advantageous defence mechanism. Previously, the first step of phase I oxidation of ALB into albendazole sulphoxide (ALBSO) (sulphoxidation) has been reported for F. hepatica, M. expansa, A. suum [37], Dicrocoelium dendriticum [38] and Haemonchus contortus [39]. This metabolite has a lower pharmacological activity than the parent compound [40] and lower effect on nematode motility [41]. The second step of ALB oxidation (sulphonation) into albendazole sulphone (ALBSO2) was reported for D. dendriticum [38]. A similar sulphonation process has been reported for F. hepatica exposed to triclabendazole sulphoxide (TCBZSO) in vitro [42]. To the best of our knowledge no studies has been conducted on the metabolism of FBZ within parasitic nematodes. Comparative in vitro studies of the oxidative metabolism of FBZ by hepatic microsomal fractions from a variety of vertebrate species showed that all species readily produced the sulphoxide metabolite ( = oxfendazole, OXF) and the sulphone metabolite fenbendazole sulphone (FBZSO2) [43]. Oxfendazole is a widely used anthelmintic whereas FBZSO2, similar to ALBSO2, are considered pharmacological inactive [40], [44].
We find the different sensitivity of T. suis and O. dentatum to FBZ in vivo highly interesting because these two species are located in the same compartment of the intestine and thus theoretically exposed to similar concentrations of drugs. We speculate that the difference in sensitivity may be related to differences in uptake and/or metabolism of the drug inside the worms. We hypothesized that the reason for a low or variable treatment efficacy of T. suis infections may be due to a lower drug uptake and/or a higher drug metabolism of T. suis in comparison to O. dentatum. The aim of this study was therefore to examine the motility of T. suis and O. dentatum adult worms in vitro when exposed to FBZ, ALB and LEV and to assess whether these drugs accumulate in the same concentrations within the two species.
Materials and Methods
2.1 Drugs
Fenbendazole, ALB and LEV were purchased from Sigma-Aldrich (Schnelldorf, Germany), and stock solutions of the drugs (100.000 µM) were prepared in 100% dimethylsulfoxid (DMSO) (Sigma-Aldrich, Schnelldorf, Germany) and stored at 5°C until use within 1 week.
2.2 Experimental animals and parasite infections
Fourteen pigs were purchased and acclimatized for 1 week prior to experimental infection. The animals had free access to water and were fed restrictively, according to national feeding requirements. For the FBZ in vitro assay, six pigs were orally infected by stomach tube with 2,000 embryonated T. suis eggs (kindly provided by Parasite Technologies A/S, Hørsholm, DK) and two pigs with 5,000 L3 O. dentatum larvae (CEP-strain). The CEP-strain was originally isolated from a farm with no prior use of anthelmintics according to the owner [45], and was later characterized as FBZ susceptible [21]. The T. suis isolate has been used in an in vivo study where experimentally infected pigs were exposed to repeated administration of FBZ (i.e. 5 mg/kg given orally on three consecutive days). Worm count reductions of 51.5 and 98.5% were obtained 24 hours after single and triple dose treatments, respectively; therefore, this isolate was considered FBZ susceptible. For the ALB and LEV in vitro assay, 3 pigs were infected with 5,000 embryonated T. suis eggs and 3 pigs with 4,000 L3 O. dentatum larvae (same strains as above). Due to practicalities the experimental infections for ALB and LEV were performed after the FBZ assay. Patency of infections was confirmed by faecal egg count (EPG) using the modified McMaster technique [46].
2.3 Ethic statement
The current study was approved by the Experimental Animal Unit, University of Copenhagen, (Denmark) based on national regulations from the Danish Animal Experiments Inspectorate (permission no. 2010/561-1914, C5).
2.4 Recovery of nematodes
For the FBZ in vitro assay, the O. dentatum infected pigs were euthanized at day 40 post infection (p.i.) and the T. suis infected pigs at day 63 p.i. For the ALB and LEV in vitro assay the O. dentatum and the T. suis infected pigs were euthanized at day 28 and 49 days p.i., respectively. Adult O. dentatum were isolated from the intestinal content according to Slotved et al. [47] and adult T. suis were collected from the intestine by manual plucking. Both parasite species were washed following a common washing procedure which consisted of 4 consecutive washing steps (each 15 min. in 39°C Hanks Balanced Salt Solution (HBSS)) followed by 4 consecutive washing steps (each 60 min. in 39°C RPMI-1640 medium). Both the HBSS and RPMI-1640 media were supplemented with 1% (v/v) amphotericin B-penicillin-streptomycin solution (10,000 U/ml penicillin, 10,000 µg/ml streptomycin, 25 µg/ml amphotericin B) and 0.5% (v/v) gentamicin (10 mg/ml) (All media, antibiotics and anti-mycotic were purchased from Life Technologies, Naerum, DK).
2.5 In vitro motility assay
Since FBZ concentrations above 30 µM precipitated during incubation, we tested the following concentrations of FBZ and ALB: 0.01, 0.1, 1, 10 and 30 µM.
Final concentrations of LEV included 0.01, 0.1, 1, 10 and 200 µM. All dilutions contained DMSO (2% v/v) and were made in RPMI-1640 medium supplemented with antibiotics and fungicide as described for the washing procedure. Thirty worms of each species selected at random were placed in a large petri dish (Th. Geyer, Roskilde, DK) containing 40 ml of each of the dilutions described above. Each concentration was tested in triplicate, thus for each drug and each concentration a total of 90 worms were used. Worms incubated in RPMI-1640 with DMSO 2% (v/v) without anthelmintics served as controls. All worms were incubated at 39°C (5% CO2, 21% O2, 90% relative humidity) for 24 or 72 hours. In the motility assay, 21 worms (i.e. 7 worms from each petri dish) of both species were scored by stereomicroscope at 6.3× magnification according to motility grades specific for each species. The motility of T. suis was graded as follows: 3: normal motility (movement of the whole body), 2: low motility (slower movement of the whole body), 1: very low motility (movement of the anterior part only), 0: no movements. The motility of O. dentatum was graded as follows: 3: normal motility (swimming), 2: low motility (slow swimming or jerking movements), 1: very low motility (only movement of the anterior tip of the body), 0: no movements. All motility measurements were blinded except for worms incubated in FBZ, due to lack of resources.
2.6 Comparison of in vitro drug uptake in living and killed nematodes
In order to compare the accumulation of drugs in living and killed worms, a number of worms obtained after the common washing procedure was killed by freezing (liquid nitrogen for 1 min.) and thawed at 5°C. Thirty living and 30 killed worms of each species were then incubated for 24 hours in FBZ, ALB or LEV at a final concentration of 10 µM in RPMI-1640 medium with DMSO (2% v/v) using the same conditions as described above. All incubations were performed in triplicates.
2.7 Preparation of nematodes and HPLC analysis
After motility measurements and the 24 hour incubation period of living and killed T. suis and O. dentatum, all worms were carefully rinsed in 50 ml HBSS for a maximum of 30 sec. The in vitro assay with FBZ was conducted first, and since the drug concentration within worms was unknown, all worms from each incubation concentration were pooled into one sample to ensure a detectable drug level. Subsequently, triplicates were made for worms incubated in each of the five concentrations of ALB and LEV. After rinsing, worms were transferred to pre-weighed Eppendorf vials, frozen in liquid nitrogen and kept at −20°C until HPLC-analysis.
Vials with worms were thawed and dried under phosphorous pentoxide until constant weight. Each vial with dried worm (10–50 mg) was mixed with 200 µl 0,05M phosphate buffer (pH 7.4) with internal standard (see below). After gentle homogenization with a plastic pestle another 200 µl buffer was added and the homogenization repeated before addition of 400 µl 6M guanidine HCl. The sample was vortexed for 1 minute and left at 20°C for 15 minutes before centrifugation at 8000× g for 10 minutes. The supernatant was transferred to a clean tube and an additional 400 µl of 6M guanidine HCl was added to the sample residue. The procedure was repeated and the two supernatants were pooled and loaded on an activated cartridge (Oasis HLB, 60 mg, 3 mL). The cartridge was activated with 2 mL methanol (100%) followed by 2 mL of water. The loaded cartridge was washed with 2 mL 5% methanol and dried under vacuum for 1 minute, before eluting the analyte with 2 mL methanol. The eluate was evaporated under air at 37°C and the residuum was dissolved in 100 µL 50% methanol and centrifuged at 8000× g before 50 µL were injected into the HPLC-system. Standards in phosphate buffer and guanidine HCl were run in parallel. Concentration of analyte in worms was expressed as µg per g dry worm.
The HPLC system was equipped with an autosampler, 2 HPLC pumps, and a UV detector. HPLC conditions for FBZ, ABZ and LEV are described below:
Fenbendazole
No internal standard was used in the FBZ analysis. The UV detector was set to 294 nm. Separation of analytes was accomplished at 30°C on a Novapak C18 (5 µ, 15 cm). The mobile phase consisted of a gradient mixed from acetonitrile and 0.025M ammonium acetate (pH 7.2) at a flow rate of 1 ml/min. The proportion of acetonitrile was 30% acetonitrile for the first 3 minutes, progressing linearly to 40% at 3.5 minutes, held constant at 40% until 11 minutes and finally reduced to 30% at 11.5 min for the remaining run time of 17 minutes. Retention times for FBZ, OXF and FBZSO2 were 13 min, 2.5 min and 4.5 min, respectively. Standards of FBZ, OXF and FBZSO2 were prepared from stock solutions in DMSO. Peak area of each analyte was used to calculate concentration. The limit of quantification for FBZ, OXF and FBZSO2 was 2 ng/mg dry worm.
Albendazole
FBZSO2 was used as internal standard in a concentration of 1 µg/ml. The UV detector was set to 290 nm. Separation of analytes was accomplished at 30°C on a Novapak C18 (5 µ, 15 cm).The mobile phase consisted of a gradient mixed from acetonitrile and 0.025M ammonium acetate (pH 7.2) at a flow rate of 1 ml/min. The proportion of acetonitrile was 25% acetonitrile for the first 2 minutes, progressing linearly to 50% at 2.5 minutes, held constant at 50% until 9 minutes and finally reduced to 25% at 9.5 min for the remaining run time of 17 minutes. Retention times for ALB, ALBSO, ALBSO2 and FBZSO2 (IS) were 9 min, 2 min, 3.5 min and 7 min, respectively. Standards of ALB, ALBSO, ALBSO2 were prepared from stock solutions in DMSO. Peak high of analyte to internal standard was used to calculate the concentration of analyte. The limit of quantification for ALB, ALBSO and ALBSO2 was 2, 0.1, 5 ng/g dry worm, respectively.
Levamisole:
Lidocaine was used as internal standard in a concentration of 5 µg/ml. The UV detector was set to 214 nm. Separation of analyte was accomplished at 30 C on a X-bridge C18 (5 µ, 15 cm). The mobile phase consisted of 25% acetonitrile and 75% phosphoric acid (0.1%) containing 0.1% octansulphone acid at a flow rate of 1 ml/min. Retention times for levamisole and lidocaine (IS) were 6.5 min and 10 min, respectively. Standards of LEV and lidocaine (IS) were prepared from stock solutions in water. Peak high of analyte to internal standard was used to calculate the concentration of analyte. The limit of quantification for LEV was 2 ng/g dry worm.
2.8 Statistical analysis
All motility scores were normalized into percentages relative to controls within species. For each drug the effect of all factors (species, time and log_concentration) and biological meaningful interactions between the factors were tested for statistical significance (P<0.05) using Analysis of Covariance (ANCOVA) with variance heterogeneity using SAS version 9.3 and JMP version 8 (SAS Institute, Cary, North Carolina). Due to significant effects of time, the effect of drug concentrations in the media on the relative motility of the two species was then calculated for 24 and 72 hours separately. Variance heterogeneity was used since the variances between the species were different. Total drug concentrations (parent compound and its metabolites) in living and killed worms of each species were compared using Student's t-test with variance heterogeneity (JMP version 8). Drug concentrations in worms exposed to 5 concentrations of FBZ and ALB were compared using the model ‘One site fit total and nonspecific binding’ (GraphPad Prism 5, GraphPad Software, San Diego, California) which calculates the parameter estimates Kd and Bmax by the following equation: Y = Bmax*X/(Kd+X)+NS*X+background. X and Y are drug concentrations in media and worms, respectively. Kd is the concentration of a ligand which is needed in order to achieve half-maximum binding at equilibrium. Bmax is the maximum specific binding, thus giving the maximum binding capacity of an object or organism. NS is the slope of non-specific binding. Background and NS was constrained to 0 since no binding was observed when measuring the negative controls. The difference of Kd and Bmax between the species was evaluated on a significance level of α = 0.05. Drug concentrations in worms exposed to LEV were compared using Student's t-test (JMP version 8) because only the two highest concentrations yielded detectable levels within the worms. Thus, concentration difference between and within species was evaluated when worms were exposed to 10 and 200 µM LEV respectively. For each drug, all data sets were tested for normality.
Results
3.1 Motility
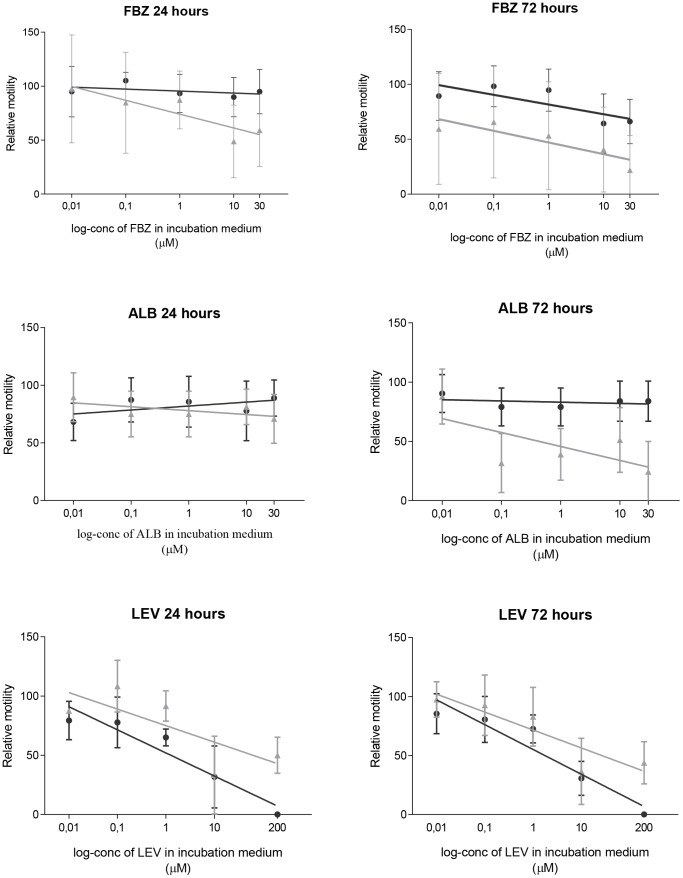
The relative motility of T. suis and O. dentatum after exposure to FBZ, ALB and LEV for 24 and 72 hours are presented in Fig. 1. No significant difference in motility between species was observed with increasing concentration over time for FBZ, ALB or LEV (species*time*log_concentration). The motility of T. suis was found to be less affected by time (24 vs. 72 h) than O. dentatum when exposed to FBZ (P = 0.015) and ALB (P<0.0001), but not LEV (species*time). The motility of T. suis was significantly less affected than that of O. dentatum after 24 hours incubation in FBZ (P = 0.003) but not 72 hours (P = 0.73) (species*log_concentration). Although the interaction was not significant after 72 hours, the motility of T. suis was still significantly less affected than the motility of O. dentatum (P<0.0001) (species) and the increasing concentration of FBZ resulted in a significant motility decrease for both species (P = 0.012) (log_concentration). When exposed to increasing concentrations of ALB, the motility of T. suis was less affected than O. dentatum after both 24 hours (P = 0.003) and 72 hours (P<0.0001) (species*log_conc). The opposite was observed for increasing concentrations of LEV where the motility of T. suis was reduced more than O. dentatum after 24 (P<0.007) and 72 hours (P<0.007) (species*log_conc).
Figure 1. Mean relative motility (± SD, n = 21) and a tendency line for Trichuris suis (dark gray circle) and Oesophagostomum dentatum (light gray triangle) after exposure to FBZ, ALB, and LEV for 24 and 72 hours.
3.2 Drugs concentrations within living and killed worms
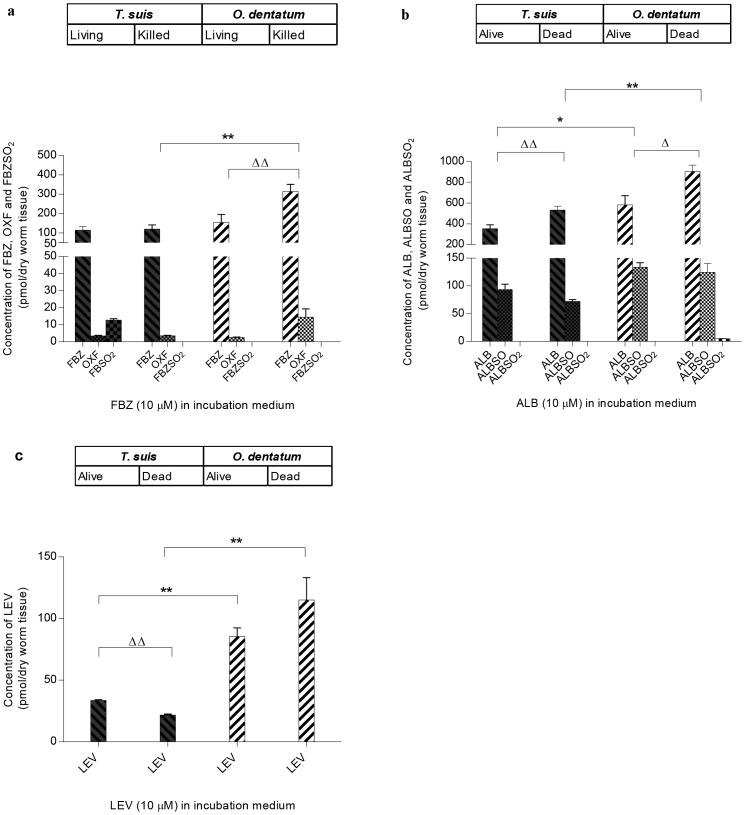
The mean concentrations of the parent compounds FBZ, ALB and LEV and the metabolites of FBZ (OXF, FBZSO2) and ALB (ALBSO, ALBSO2) in living and killed worms after incubation in 10 µM of the drug for 24 hours are shown in Fig. 2. In general, the total drug concentrations within both living and killed worm species varied according to type of drug (Fig. 2a, 2b, 2c), with ALB and its metabolite ALBSO occurring at the highest concentration level followed by FBZ and its metabolites and LEV. When incubated in ALB and LEV, the total drug concentrations were found to be significantly lower in T. suis than O. dentatum and this was observed for both living (ALB: P = 0.02, LEV: P = 0.02) and killed (ALB: P = 0.002, LEV: P = 0.008) worms. In both living and dead worms, the total concentration of FBZ and its metabolites was found to be lower in T. suis than O. dentatum. For the dead worms, the difference was significant (P = 0.004) but did not reach significance for living worms (131.1±17.1 pmol/mg dry worm tissue vs. 155.8±33.3 pmol/mg dry worm tissue for T. suis and O. dentatum, respectively).
Figure 2. Mean concentration (± SD, n = 3, each replicate consist of 30 worms) of a) FBZ, OXF and FBZSO2, b) ALB, ALBSO and ALBSO2 and c) LEV measured in living and killed Trichuris suis and Oesophagostomum dentatum after incubation for 24 hours in 10 µM of each of the parent compound.
Significant difference in total concentration (parent compound+metabolites) between species is indicated with *, significant difference within the species (living and killed) is indicated with ▵. P-values were obtained using Student's t-test with variance heterogeneity. *P<0.05 and **P<0.01 and ▵ P<0.05 and ▵▵ P<0.01.
For O. dentatum the concentration of drug was higher in killed worms as compared to living worms for all three anthelmintics, and the difference was found to be significant when incubated in FBZ (P = 0.006) and ALB (P = 0.011). For T. suis no difference between the living and the killed was observed when incubated in FBZ, whereas the anthelmintic concentration was significantly higher within killed worms when incubated in ALB (P = 0.009) and significantly lower when incubated in LEV (P<0.001). The mean concentrations of OXF in living and killed worms, respectively, were found to be 3.4 and 3.5 pmol/mg dry worm tissue for T. suis and 2.6 and 14.4 pmol/mg dry worm tissue for O. dentatum. The pharmacological inactive metabolite FBZSO2 (mean: 12.7 pmol/mg dry worm tissue) was only observed in living T. suis and amounted 9.7% of the total anthelmintic concentration measured within the worms. The mean concentrations of ALBSO in living and killed worms were 93.8 and 71.9 pmol/mg dry worm tissue, respectively, for T. suis and 133.8 and 124.4 pmol/mg dry worm tissue for O. dentatum. Only trace amount of ALBSO2 (4.71 pmol/mg dry worm tissue) were measured in killed O. dentatum.
3.3 Concentrations of total drug within living worms exposed to different drug levels
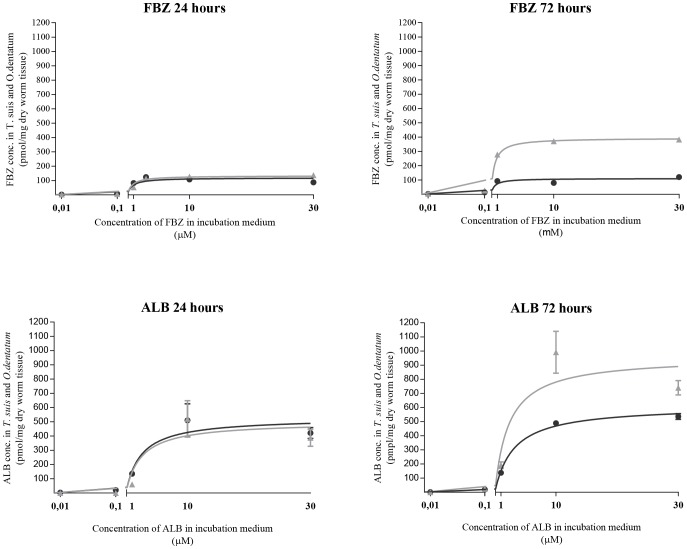
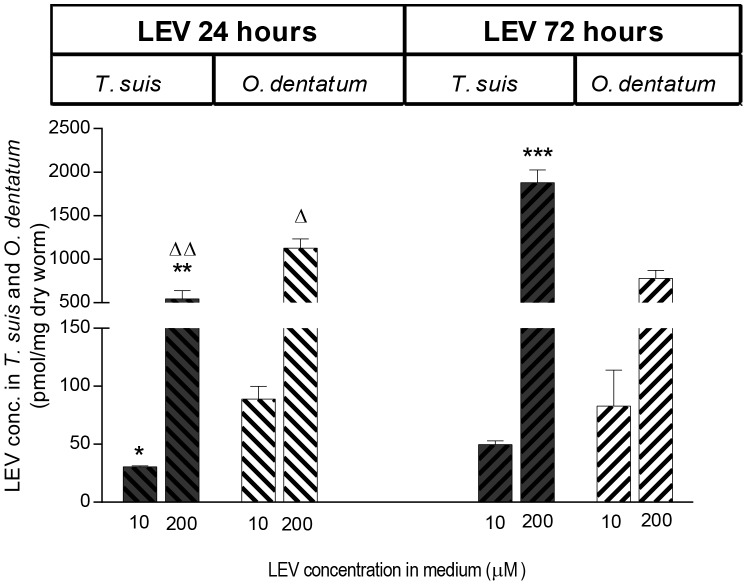
The concentration of FBZ and ALB inside living T. suis and O. dentatum after incubation in 0.01, 0.1, 1, 10 and 30 µM of FBZ and ALB for 24 and 72 hours is shown in Fig. 3. The Kd and Bmax values for each species at 24 and 72 hours are given in Table 1. For both anthelmintic drugs no significant difference in the Kd – values were observed between the species neither after 24 or 72 hours of incubation. The Bmax – values were similar for the two species after 24 hours exposure to both BZs, but after 72 hours incubation, these were significantly lower for T. suis than O. dentatum when exposed to FBZ (P<0.0001) and ALB (P = 0.033). The concentrations of LEV found within the worms after exposure to 0.01, 0.1, 1, 10 and 200 µM LEV for 24 and 72 hours were only above the detection limit when exposed to the two highest concentrations (Fig. 4). The concentrations of LEV found within the worms were significantly lower in T. suis than O. dentatum when incubated in 10 and 200 µM for 24 hours (P = 0.01, P = 0.0009). When incubated in 200 µM for 72 hours the concentration of LEV was higher in T. suis (452.5 ng/mg dried worm tissue) than in O. dentatum (187.9 ng/mg dried worm tissue) (P<0.0001). The concentration of LEV within T. suis thus increased significantly with incubation time (P<0.0001) when incubated in 200 µM LEV, whereas the concentration was lower after 72 hours than 24 hours incubation within O. dentatum (P = 0.02).
Figure 3. Non- specific binding of FBZ and ALB accumulated inside living Trichuris suis (dark gray circle) and Oesophagostomum dentatum (light gray triangle) after incubation in 0.01, 0.1, 1,10 or 30 µM FBZ or ALB for 24 and 72 hours.
Mean concentrations (pmol/mg dry worm tissue) of ALB (± SD, n = 3) are shown, while the values of FBZ represents one sample only for each concentration at 24 and 72 hours, respectively.
Table 1. Comparison of the binding constant at equilibrium (Kd) and the maximum specific binding capacity (Bmax) of Trichuris suis and Oesophagostomum dentatum incubated in FBZ and ALB for 24 and 72 hours.
| T. suis | O. O. dentatum | |||
| 24 hours | 72 hours | 24 hours | 72 hours | |
| FBZ | ||||
| Kd | 0.37 | 0.33 | 1.36 | 0.54 |
| Bmax | 110.9 | 106.6a | 147.0 | 395.2a *** |
| ALB | ||||
| Kd | 2.03 | 3.57 | 2.87 | 2.28 |
| Bmax | 514.1 | 612.7b | 513.9 | 958.1b * |
Kd is given in µM and Bmax in pmol/mg dry worm tissue. Comparisons are made between nematode species for Kd – and Bmax -values for each time point and P-values indicated for values with same superscript:
* P<0.05,
*** P<0.0001,
: comparison of Bmax-values after exposure to FBZ and ALB respectively, for 72 hours.
Figure 4. Mean concentration of LEV (± SD, n = 3) measured in living Trichuris suis and Oesophagostomum dentatum after incubation in 10 or 200 µM LEV for 24 and 72 hours.
Statistically different concentration between species when exposed to either 10 or 200 µM LEV for 24 and 72 hours, respectively are indicated with: *P<0.05, **P<0.01 and ***P<0.001. Statistically different concentration values within the species between 24 and 72 hours are indicated with: ▵ P<0.05 and ▵▵ P<0.01.
3.4 Concentrations of drug metabolites in worms exposed to different levels of anthelmintics
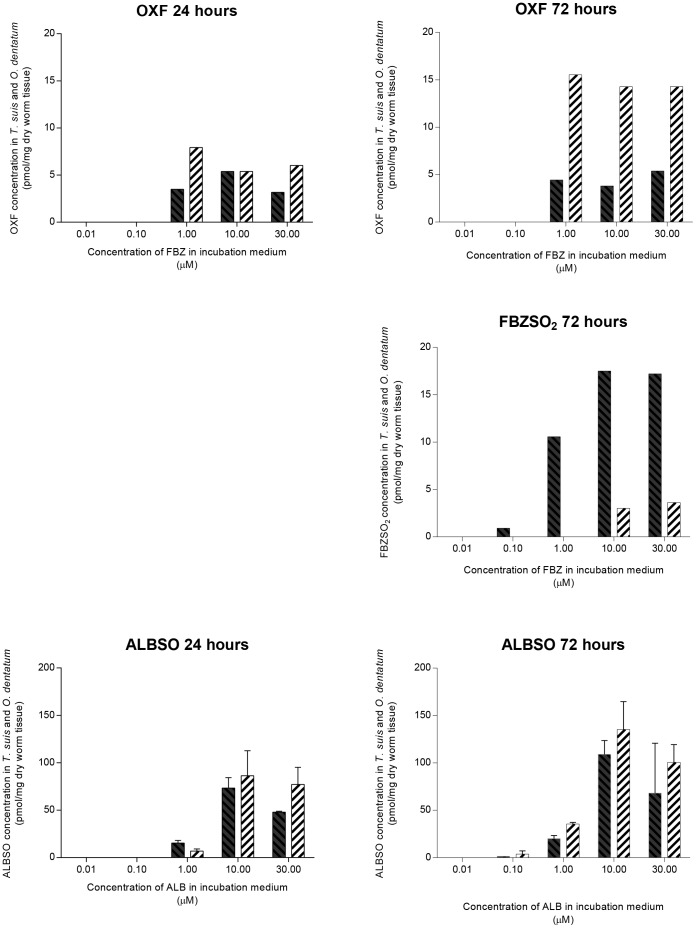
The concentrations of the metabolites OXF, FBZSO2 and ALBSO measured within living T. suis and O. dentatum are given in Fig. 5. The concentrations of OXF and FBZSO2 within the two worm species were much lower than ALBSO (Fig. 5). Incubation concentrations below 0.1 µM of FBZ and ALB did not result in detectable levels of metabolites. The concentration of OXF within T. suis did not show a concentration or time dependent increase (3.2–5.4 pmol/mg dry worm tissue and 3.8–5.4 pmol/mg dry worm tissue after incubation periods of 24 and 72 hours, respectively) whereas a clear time dependent increase was observed for O. dentatum (5.4–7.9 pmol/mg dry worm tissue and 14.2–15.6 pmol/mg dry worm tissue after 24 and 72 hours, respectively). After 24 hours incubation the inactive metabolite FBZSO2 was only detected in T. suis. Results were inconsistent and are thus not given. After 72 hours incubation, FBZSO2 was detected within T. suis at an incubation concentration as low as 0.1 µM FBZ whereas FBZSO2 only appeared in O. dentatum when incubated in 10 and 30 µM. After 72 hours a concentration dependent formation of FBZSO2 (0.9–17.5 pmol/mg dry worm tissue) was measured within T. suis where it represented between 6–17.2% of the total drug concentration whereas in O. dentatum it only constituted 0.8–0.9%. For both species, the formation of FBZSO2 appeared to be both time- and concentration-dependent as consistent results only were obtained after 72 hours incubation. The ALBSO metabolite showed a clear tendency to reach a higher concentration within O. dentatum than T. suis when incubated for both 24 and 72 hours. The formation of ALBSO within the worms appeared to be both time- and concentration-dependent at incubation concentrations ranging from 0.1 µM to 30 µM. Incubation in 30 µM ALB resulted in ALBSO concentrations equal to or below the concentrations formed when incubated in 10 µM. The metabolite ALBSO2 was not detected within any of the two species. The metabolites OXF and ALBSO showed a clear tendency to reach a higher concentration level within O. dentatum than T. suis when incubated for both 24 and 72 hours, but in relation to the total drug concentration, the average proportion of the metabolites were approximately the same (OXF: T. suis; 4% at 24 hours and 3.6% at 72 hours; O. dentatum: 5.6% and 4%, ALBSO: T. suis; 11.1% and 13.8%, O. dentatum; 15% and 12.2%).
Figure 5. Concentrations of OXF, FBZSO2 and mean concentrations of ALBSO (± SD, n = 3) measured in living Trichuris suis (black columns) and Oesophagostomum dentatum (hatched columns) after incubation in 0.01, 0.1, 1, 10 or 30 µM FBZ (upper three graphs) or ALB (lower two graphs) for 24 and 72 hours.
Discussion
In the present work, we have combined worm motility with concentration measurements of drug-uptake and drug metabolism in two nematode species that inhabit the same part of the large intestine, but differ significantly in their intestinal microhabitat. Our results show that the motility of T. suis was less affected than the motility of O. dentatum when exposed to FBZ for 24 hours and ALB for 72 hours, thus indicating a lower sensitivity of T. suis as compared to O. dentatum towards these compounds. The maximum binding capacity of FBZ and ALB was significantly lower for T. suis than O. dentatum after 72 hours incubation and the total drug concentrations were significantly lower in living and killed T. suis as compared to O. dentatum when incubated in ALB. When living and killed worms were incubated in FBZ, only killed T. suis contained a significantly lower drug concentration than O. dentatum. However, collectively these results suggest T. suis to have a lower uptake of FBZ and ALB than O. dentatum. Furthermore, a relatively higher concentration of FBZSO2 was measured in T. suis than O. dentatum, thus suggesting a higher metabolism of FBZ (or OXF) into FBZSO2 in T. suis. Fenbendazole sulphone is considered anthelmintic inactive due to weak ovicidal activity and lack of inhibition of mammalian tubulin polymerization [44]. The equivalent sulphone metabolite of ALB, ALBSO2, has not only shown complete loss of activity in both egg hatch inhibition assays and inhibition of mammalian tubulin polymerization but also decreased binding affinity to nematode tubulin [40]. Whether the latter also applies for FBZSO2 is not known but due to lack of polymerization inhibition, low ovicidal activity and assumed decreased binding affinity to nematode tubulin, FBZSO2 will in the following be considered “inactive”. However, caution must be taken. Due to uncertainty of detection levels within worms in the first trial, triplicates were not made for T. suis and O. dentatum incubated at different drug levels of FBZ (i.e. 0.01–30 µM). Although triplicates were not obtained, concentration agreement was found within the living worms incubated in 10 µM FBZ in the assay of living and killed worms. Furthermore, the formation of FBZSO2 showed a dose dependent formation.
We found that the motility of T. suis as compared to O. dentatum was less affected by increasing concentrations of FBZ and ALB. A low sensitivity to high concentrations of ALB has also been described for T. muris where doses up to 200 µg/ml (equivalent to 754 µM) of ALB were tested against adult and L3 stages of T. muris in vitro [9]. This dose level, which is approximately 25 times higher than the highest concentration used in our study (30 µM) did not reduce the motility of T. muris by 50% (IC50) after an incubation period of 72 hours. In contrast to T. suis, O. dentatum was found to be more sensitive to increasing concentrations of FBZ and ALB when incubated for 24 and 72 hours respectively. The high sensitivity towards increasing concentrations of ALB and FBZ has also been reported by Petersen et al. [41] who found that a concentration of 0.1 µM was able to inhibit migration of O. dentatum through a mesh by 61% for ALB and 69% for FBZ. An increase in concentration to only 3.16 µM increased the inhibition of migration to 75.3% for ALB and 76.2% for FBZ. The high sensitivity towards increasing concentrations of ALB and FBZ reported by Petersen et al. [41], is in agreement with our results in vitro, but more importantly, it is also in concordance with the high efficacy of FBZ against O. dentatum reported in vivo [20], [21]. Likewise, low sensitivity of T. muris towards ALB in vitro has also been shown to correlate with low treatment efficacy in vivo [9]. Trichuris suis was more sensitive towards increasing concentrations of LEV than O. dentatum. At the highest dose (200 µM) no movement of T. suis was observed neither after 24 or 72 hours incubation. A high sensitivity towards LEV has also been observed for T. muris in vitro (IC50 = 33.1 µg/ml equivalent to 68.5 µM) and in vivo where the worm burden was reduced by 95.9% with a single oral dose of LEV (200 mg/kg) in mice [9]. In pigs, the efficacy of a single oral dose of LEV (7.5–8 mg/kg) has shown varying efficacy on T. suis ranging from 26% [16] to 100% [48], [49].
In the in vitro assay with living and killed worms we found that the total concentrations of anthelmintic drugs were lower in T. suis than O. dentatum (Fig. 2). This applied to all three anthelmintics tested, although the difference was not found to be significant when living parasites were incubated in FBZ (Fig. 2). Incubation in increasing concentrations of FBZ and ALB, ranging from 0.01 to 30 µM for 72 hours revealed similar Kd values for T. suis and O. dentatum which suggests that approximately the same concentrations of FBZ and ALB are needed for both species in order to achieve binding of half of the binding sites at equilibrium. The Bmax values were significantly lower for T. suis than O. dentatum suggesting that T. suis has a significantly lower binding capacity of FBZ and ALB than O. dentatum (Fig. 3, Table 1) which is in accordance with lower effect of these two anthelmintics on motility. The Bmax values measured in O. dentatum were higher after 72 hours than 24 hours incubation. The accumulation of FBZ and ALB may be due to a lower secretion capacity of O. dentatum, in comparison to T. suis, which is supported by the formation of FBZSO2 in T. suis. The concentration of LEV within living worms were below the detection level of the HPLC analysis when incubated in 0.01, 0.1, and 1 µM, but interestingly the concentration of LEV within T. suis was more than two times higher than in O. dentatum when incubated in 200 µM LEV for 72 hours, which was translated into an absence of motor activity in the motility assay.
In the in vitro assay of living and killed worms we found that only living T. suis were able to metabolize FBZ, or possibly OXF, to the inactive metabolite FBZSO2 (Fig. 2), amounting 9.7% of the total anthelmintic concentration measured within the worms. When incubating the worms in increasing concentrations of FBZ for 24 hours we obtained inconsistent results for FBZSO2 (i.e. FBZSO2 was only detected in T. suis, and only when incubated in 1 µM FBZ) (data not shown). After 72 hours a concentration dependent formation of FBZSO2 was measured within T. suis where it represented between 6–17.2% of the total drug concentration whereas in O. dentatum it only constituted 0.8–0.9%. In relation to the maximum binding of FBZ, we measured a significantly lower value for T. suis than O. dentatum (Fig. 3 and Table 1). We therefore suggest that the poor effect of FBZ on T. suis may be related to a lower drug uptake and/or a higher detoxifying capacity of this species, however, some care should be taken with the latter. Albendazole and FBZ are able to undergo spontaneous oxidation to their corresponding derivatives ALBSO and OXF when mixed with DMSO [50]. The average proportions of the metabolites OXF and ALBSO were approximately the same within T. suis and O. dentatum when incubated in increasing concentrations of ALB and FBZ. Furthermore, these metabolites occurred in killed worms of both species and even trace amounts of ALBZSO2 were detected in killed O. dentatum. Therefore these findings indicate that OXF and ALBSO were formed by spontaneous oxidation, and that the formation of FBZSO2 observed in T. suis may be related to the presence and further transformation of OXF. As FBZSO2 were not detected in any of the killed worms or in living O. dentatum when incubated in 10 µM FBZ for 24 hours, it is most likely that the relative high concentrations of FBZSO2 measured in T. suis were not formed by spontaneous oxidation, but by T. suis itself. A trace amount of ALBSO2 (4.71 pmol/mg dry worm tissue) was measured in killed O. dentatum when incubated for 24 hours in 10 µM ALB but was not detected in any of the two species when incubated in increasing concentrations of ALB or in dead T. suis. Therefore it is most likely that occurrence of this compound is a detection uncertainty, which needs to be confirmed in future studies.
The above mentioned findings raise the following questions: a) why is the total drug concentrations of BZs generally lower in T. suis than O. dentatum? b) Why is the difference between concentration of anthelmintic within living and killed worms more pronounced for O. dentatum than T. suis? Considering the first question, possible entry routes of anthelmintic drugs into parasitic nematodes are oral ingestion or passive or active transport across the cuticle. In a study performed by Ho et al. [51], transport across the cuticle was demonstrated to be the main route of entry of lipophilic compounds (hydrocortisone and p-nitrophenol) into the nematode A. suum [51]. This route was confirmed by Mottier et al. [32] who also suggested that as a general rule helminths uptake BZs by passive diffusion [32]. Since previous work indicated that passive diffusion across the cuticle is the main route of uptake of lipophilic anthelmintics, and a transcuticular route also has been shown for the water soluble anthelmintic LEV [33], we therefore assumed that this also was the case for T. suis and O. dentatum. Oral ingestion of anthelmintic was controlled in the present study by killing the worms, but the concentration of all three anthelmintics was lower in T. suis than O. dentatum whether killed or alive, with the exception of living worms exposed to FBZ (Fig. 2). Furthermore, the binding capacity of T. suis was significantly lower than the binding capacity of O. dentatum when exposed to both FBZ (P<0.0001) and ALB (P = 0.033). The average proportions of the metabolites OXF and ALBSO were approximately the same for both species, whereas concentration levels above 5 pmol/mg dry tissue of FBZSO2 were only detected in T. suis. We therefore speculate that the lower total drug concentration of BZs measured both in living (i.e. Bmax values after 72 hours incubation in ALB and FBZ) and killed T. suis may be due to structural differences in the cuticle or different lipid contents. Considering the second question regarding the different concentration of anthelmintic within living and killed worms, Mottier et al. [32] found that the concentration of FBZ was lower within living A. suum as compared to killed worms. These findings correspond to our observation for O. dentatum exposed to all three anthelmintics, although the difference was not significant when the worms were incubated in LEV (P = 0.09). For T. suis, a significantly lower concentration within living worms in relation to the killed, was only observed when exposed to ALB. The rate of drug diffusion across the cuticle of A. suum and other nematodes is restricted by the lipid barrier in the hypodermis, the pK a of the drug, the pH of the aqueous environment within the cuticula and the negatively charged aqueous filled pores within the collagen matrix [52]. Mottier et al. [32] suggested that the lower concentration within living worms is related to the acidic environment at the nematode surface that is created by excretion of acidic organic metabolites from the worms [53]. Benzimidazoles are weak bases [54] and may therefore largely exist in their ionized form in the acidic environment at the nematode surface. The ionized form is not readily diffusible through the lipid layer of the cuticle therefore a smaller amount of BZs may enter the living parasites compared to the killed. This mechanism may be the reason why we observed a lower concentration of anthelmintic in living O. dentatum, and to a lesser extent in living T. suis, compared to the killed specimens.Nevertheless, damage of the cuticle due to freezing and a subsequent increase in permeability or possibly higher drug concentrations trapped in the cuticle of killed worms cannot be ruled out. Furthermore, inactivation of possible ATP-dependent efflux pumps i.e. the ATP-binding cassette (ABC) transporter P-glycoprotein (Pgp) [34], [55] may also contribute to the increased drug concentration observed within the killed worms. Interestingly, we did not observe the same for T. suis when exposed to FBZ and LEV which further supports our hypothesis that the lower drug concentration measured within this species is also related to a lower drug uptake.
An answer to the intriguing question for low to varied treatment efficacy of T. trichiura infections in humans has been sought from a variety of angles. The majority of these has taken an empiric approach by evaluating the effect of different treatment strategies in clinical trials such as: a) comparing the efficacy of single-dose BZs treatment (i.e. ALB (400 mg) and MBD (500 mg)) with the efficacy of combination therapy (i.e. BZs in combination with LEV (40 or 80 mg), ivermectin (200 µg/kg) or diethylcarbamazine (150 mg) [4], [5], b) comparing the efficacy of single-doses with triple-doses of ALB and MBD [6] or c) comparing the efficacy of single and double doses of ALB and MBD given alone or in combination [56]. In the above-mentioned clinical trials the highest CR (70.7%) was obtained using 3×500 mg MBD given over 3 consecutive days [6]. Empiric approaches have also been performed using T. muris as a model where the effect of single-drugs (i.e. monepantel, ALB, LEV, pyrantel pamoate and oxantel pamoate) and drug combinations between ALB, LEV, MBD, pyrantel pamoate, oxantel pamoate and ivermectin (IVM) have been assessed in both in vitro assays and in vivo studies [9], [57], [58]. Albendazole, given as a single-drug, showed poor effect in vivo (600 mg/kg) and low efficacy in vitro (50–200 µg/ml) [9], whereas the combinations of ALB-MBD, MBD-IVM, MBD-LEV and oxantel pamoate-MBD revealed a strong synergistic effect suggesting combination therapy as a future possibility [57]. Yet other approaches have been used in order to find explanations for low to mediocre treatment efficacy of BZs against Trichuris spp. infections. Specific variants of the beta-tubulin gene (i.e. single nucleotide polymorphisms (SNPs) in codon 167, 198 and 200) have been reported to convey BZ-resistance in parasitic nematodes of veterinary importance [59]–[63] and SNPs in codon 200 have been identified in T. trichiura obtained from a human population expected to be unexposed to BZs [64]. Furthermore, there is evidence demonstrating a higher frequency of the resistant genotype in codon 200 (TAC/TAC) in eggs of T. trichiura isolated from human populations in Haiti and Kenya after treatment with ALB [65], indicating that anthelmintic resistance may be involved in the low to mediocre treatment efficacy of BZs reported for this genus. However, such SNPs were not found in other Trichuris spp. [66], and not systematically in human populations [67].
The present work represents yet another approach to address the intriguing question for low to varied treatment efficacy of T. trichiura infections in humans. Based on worm motility, concentration of anthelmintic drugs and their metabolites within the worms and the difference in binding capacity of FBZ and ALB, we suggest that the lower sensitivity of T. suis towards these drugs in vitro is, in comparison to O. dentatum, due to a lower drug uptake. Furthermore, our data indicate that T. suis is able to transform FBZ or OXF into the inactive metabolite FBZSO2. Whether the drug uptake of T. suis in vitro mirrors the drug uptake in vivo is still unresolved. In the host, Trichuris spp. are attached to the mucosa with the anterior part which may give the worms a mechanical advantage in relation to anthelmintic treatment (they do not easily get detached even when temporarily deprived for energy or paralysed). Furthermore, such attachment mayserve as a protective barrier of the anterior part against active drugs in the intestinal lumen and instead render the worms more exposed to less potent anthelmintic metabolites in the blood. However, the posterior part is largely exposed to drugs in the lumen. We do not know whether the majority of the drug acting on Trichuris spp. comes from the intestinal lumen or whether it arrives via the blood supplying the intestine or both, but by using T. suis as a model we have shown that the varied and low drug efficacy against Trichuris spp. in animals and humans may be related to low drug-uptake in the worms.
Acknowledgments
The authors gratefully acknowledge Allan Roepstorff and Christian Kapel from Parasite Technologies A/S, Hørsholm, DK for providing T. suis eggs. Furthermore, we acknowledge Helena Mejer, Lise-Lotte Christiansen, Gonçalo Pacheco, Gerda Larsen, Anna Sofie Eckhoff and Rikke Jess for technical assistance.
Funding Statement
This work was funded by the University of Copenhagen, Denmark. The funder had no role in study design, data collection and analysis or preparation of the manuscript.
References
- 1.WHO. (2011) Helminths control in school-age children: a guide for managers of control programmes. 2nd ed. Geneva: World Health Organisation. [Google Scholar]
- 2.WHO. (2013) Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. Geneva: World Health Organisation. [Google Scholar]
- 3. Keiser J, Utzinger J (2008) Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA 299: 1937–1948. [DOI] [PubMed] [Google Scholar]
- 4. Belizario VY, Amarillo ME, Leon WU, Reyes AE, Bugayong MG, et al. (2003) A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bull World Health Organ 81: 35–42. [PMC free article] [PubMed] [Google Scholar]
- 5. Knopp S, Mohammed KA, Speich B, Hattendorf J, Khamis IS, et al. (2010) Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: A randomized controlled trial. Clin Infect Dis 51: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 6. Steinmann P, Utzinger J, Du Z, Jiang J, Chen J, et al. (2011) Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: A randomized controlled trial. PLoS ONE 6: e25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, et al. (2003) Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ 81: 343–352. [PMC free article] [PubMed] [Google Scholar]
- 8. Silbereisen A, Tritten L, Keiser J (2011) Exploration of novel in vitro assays to study drugs against Trichuris spp. J Microbiol Methods 87: 169–175. [DOI] [PubMed] [Google Scholar]
- 9. Tritten L, Silbereisen A, Keiser J (2011) In vitro and In vivo efficacy of monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl Trop Dis 5: e1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wimmersberger D, Tritten L, Keiser J (2013) Development of an in vitro drug sensitivity assay for Trichuris muris first-stage larvae. Parasit Vectors 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keiser J, Tritten L, Adelfio R, Vargas M (2012) Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo . Parasit Vectors 5: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cutillas C, Callejon R, Rojas Md, Tewes B, Ubeda JM, et al. (2009) Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop 111: 299–307. [DOI] [PubMed] [Google Scholar]
- 13. Nissen S, Al-Jubury A, Hansen TVA, Olsen A, Christensen H, et al. (2012) Genetic analysis of Trichuris suis and Trichuris trichiura recovered from humans and pigs in a sympatric setting in Uganda. Vet Parasitol 13: 68–77. [DOI] [PubMed] [Google Scholar]
- 14. Batte EG (1978) Evaluation of fenbendazole as a swine anthelmintic. Vet Med Small Anim Clin 73: 1183–1186. [PubMed] [Google Scholar]
- 15. Enigk K, Dey-Hazra A, Batke J (1974) The efficacy of fenbendazol against gastro-intestinal nematodes in swine. Dtsch Tierarztl Wochenschr 81: 177–182. [PubMed] [Google Scholar]
- 16. Marti OG, Stewart TB, Hale OM (1978) Comparative efficacy of fenbendazole, dichlorvos, and levamisole HCl against gastrointestinal nematodes of pigs. J Parasitol 64: 1028–1031. [PubMed] [Google Scholar]
- 17. Marchiondo AA, Szanto J (1987) Efficacy of dichlorvos, fenbendazole, and ivermectin in swine with induced intestinal nematode infections. Am J Vet Res 48: 1233–1235. [PubMed] [Google Scholar]
- 18.Anon. (2013) Veterrinærmedicinsk produktkatalog. Copenhagen, Denmark: Veterinærmedicinsk Industriforening. 1329 p. [Google Scholar]
- 19.Taylor DJ. (1999) Pig diseases. Glasgow, Scotland: St Edmundsbury Press. 412 p. [Google Scholar]
- 20. Kirsch R, Duwel D (1975) Laboratory investigations on pigs with the new anthelmintic fenbendazole. Res Vet Sci 19: 327–329. [PubMed] [Google Scholar]
- 21. Praslicka J, Bjorn H, Varady M, Nansen P, Hennessy DR, et al. (1997) An in vivo dose-response study of fenbendazole against Oesophagostomum dentatum and Oesophagostomum quadrispinulatum in pigs. Int J for Parasitol 27: 403–409. [DOI] [PubMed] [Google Scholar]
- 22. Kringel H, Roepstorff A (2006) Trichuris suis population dynamics following a primary experimental infection. Vet Parasitol 139: 132–139. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen S, Saeed I (2000) Experimental infection of pigs with three dose levels of Trichuris suis . Parasite 7: 275–281. [DOI] [PubMed] [Google Scholar]
- 24. Christensen CM, Barnes EH, Nansen P, Roepstorff A, Slotved HC (1995) Experimental Oesophagostomum dentatum infection in the pig: Worm populations resulting from single infections with three doses of larvae. Int J Parasitol 25: 1491–1498. [DOI] [PubMed] [Google Scholar]
- 25. Roepstorff A, Bjorn H, Nansen P, Barnes EH, Christensen CM (1996) Experimental Oesophagostomum dentatum infections in the pig: Worm populations resulting from trickle infections with three dose levels of larvae. Int J Parasitol 26: 399–408. [DOI] [PubMed] [Google Scholar]
- 26. Batte EG, McLamb RD, Muse KE, Tally SD, Vestal TJ (1977) Pathophysiology of swine trichuriasis. Am J Vet Res 38: 1075–1079. [PubMed] [Google Scholar]
- 27. Kotlan A (1948) Studies on the life-history and pathological significance of Oesophagostomum spp. of the domestic pig. Acta Vet Hung 1: 14–30. [Google Scholar]
- 28. Mccracken RM, Ross JG (1970) The histopathology of Oesophagostomum dentatum infections in pigs. J Comb Pathol 80: 619–623. [DOI] [PubMed] [Google Scholar]
- 29. McKellar QA, Jackson F (2004) Veterinary anthelmintics: Old and new. Trends Parasitol 20: 456–461. [DOI] [PubMed] [Google Scholar]
- 30. Lacey E (1988) The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol 18: 885–936. [DOI] [PubMed] [Google Scholar]
- 31. Coles GC, East JM, Jenkins SN (1975) The mechanism of action of the anthelmintic levamisole. Gen Pharmacol 6: 309–313. [Google Scholar]
- 32. Mottier L, Alvarez L, Ceballos L, Lanusse C (2006) Drug transport mechanisms in helminth parasites: Passive diffusion of benzimidazole anthelmintics. Exp Parasitol 113: 49–57. [DOI] [PubMed] [Google Scholar]
- 33.Verhoeven HLE, Willemsens G, Van den Bossche H. (1976) Uptake and distribution of levamisole of Ascaris suum. Biochemistry of parasites and host-parasite relationships: 573–579.
- 34. Cvilink V, Lamka J, Skalova L (2009) Xenobiotic metabolizing enzymes and metabolism of anthelminthics in helminths. Drug Metab Rev 41: 8–26. [DOI] [PubMed] [Google Scholar]
- 35. Barrett J (1998) Cytochrome P450 in parasitic protozoa and helminths. Comp Biochem Physiol C Pharmaol Toxicol Endocrinol 121: 181–183. [DOI] [PubMed] [Google Scholar]
- 36. Brophy PM, Mackintosh N, Morphew RM (2012) Anthelmintic metabolism in parasitic helminths: Proteomic insights. Parasitology 139: 1205–1217. [DOI] [PubMed] [Google Scholar]
- 37. Solana HD, Rodriguez JA, Lanusse CE (2001) Comparative metabolism of albendazole and albendazole sulphoxide by different helminth parasites. Parasitol Res 87: 275–280. [DOI] [PubMed] [Google Scholar]
- 38. Cvilink V, Szotáková B, Křížová V, Lamka J, Skálová L (2009) Phase I biotransformation of albendazole in lancet fluke (Dicrocoelium Dendriticum). Res Vet Sci 86: 49–55. [DOI] [PubMed] [Google Scholar]
- 39. Cvilink V, Skalova L, Szotakova B, Lamka J, Kostiainen R, et al. (2008) LC-MS-MS identification of albendazole and flubendazole metabolites formed ex vivo by Haemonchus contortus . Anal Bioanal Chem 391: 337–343. [DOI] [PubMed] [Google Scholar]
- 40. Lubega GW, Prichard RK (1991) Interaction of benzimidazole anthelmintics with Haemonchus contortus tubulin: Binding affinity and anthelmintic efficacy. Exp Parasitol 73: 203–213. [DOI] [PubMed] [Google Scholar]
- 41. Petersen MB, Friis C, Bjørn H (1997) A new in vitro assay of benzimidazole activity against adult Oesophagostomum dentatum . Int J Parasitol 27: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 42. Robinson MW, Lawson J, Trudgett A, Hoey EM, Fairweather I (2004) The comparative metabolism of triclabendazole sulphoxide by triclabendazole-susceptible and triclabendazole-resistant Fasciola hepatica . Parasitol Res 92: 205–210. [DOI] [PubMed] [Google Scholar]
- 43. Short CR, Flory W, Hsieh LC, Barker SA (1988) The oxidative metabolism of fenbendazole: A comparative study. J of Vet Pharmacol and Ther 11: 50–55. [DOI] [PubMed] [Google Scholar]
- 44. Lacey E, Brady RL, Prichard RK, Watson TR (1987) Comparison of inhibition of polymerisation of mammalian tubulin and helminth ovicidal activity by benzimidazole carbamates. Vet Parasitol 23: 105–119. [DOI] [PubMed] [Google Scholar]
- 45. Roepstorff A, Bjorn H, Nansen P (1987) Resistance of Oesophagostomum spp. in pigs to pyrantel citrate. Vet Parasitol 24: 229–239. [DOI] [PubMed] [Google Scholar]
- 46.Roepstorff A, Nansen P, Food and Agriculture Organization of the United Nations (1998) Epidemiology, diagnosis and control of helminth parasites of swine. Rome: Food and Agriculture Organization of the United Nations. 161 p. [Google Scholar]
- 47. Slotved HC, Barnes EH, Bjørn H, Christensen CM, Eriksen L, et al. (1996) Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet Parasitol 63: 237–245. [DOI] [PubMed] [Google Scholar]
- 48. Ferguson DL, White RG (1975) Anthelmintic activity of levamisole against Ascaris, Trichuris and Metastrongylus infection in swine. J Anim Sci 40: 838–843. [DOI] [PubMed] [Google Scholar]
- 49. Jacobs DE, Lean IJ, Oakley GA (1977) Levamisole: Efficacy against Trichuris suis . Vet Rec 100: 49. [DOI] [PubMed] [Google Scholar]
- 50. Lacey E, Snowdon KL (1988) A routine diagnostic assay for the detection of benzimidazole resistance in parasitic nematodes using tritiated benzimidazole carbamates. Vet Parasitol 27: 309–324. [DOI] [PubMed] [Google Scholar]
- 51. Ho NFH, Geary TG, Barsuhn CL, Sims SM, Thompson DP (1992) Mechanistic studies in the transcuticular delivery of antiparasitic drugs II: Ex vivo/in vitro correlation of solute transport by Ascaris suum . Mol Biochem Parasitol 52: 1–13. [DOI] [PubMed] [Google Scholar]
- 52. Thompson DP, Ho NFH, Sims SM, Geary TG (1993) Mechanistic approaches to quantitate anthelmintic absorption by gastrointestinal nematodes. Parasitol Today 9: 31–35. [DOI] [PubMed] [Google Scholar]
- 53. Sims SM, Magas LT, Barsuhn CL, Ho NFH, Geary TG, et al. (1992) Mechanisms of microenvironmental pH regulation in the cuticle of Ascaris suum . Mol Biochem Parasitol 53: 135–148. [DOI] [PubMed] [Google Scholar]
- 54.Riviere JE, Papich MG, Adams HR. (2009) Veterinary pharmacology and therapeutics. Ames, Iowa: Wiley-Blackwell. 1524 p [Google Scholar]
- 55. Lespine A, Ménez C, Bourguinat C, Prichard RK (2012) P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. Int J Parasitol Drugs Drug Resist 2: 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Namwanje H, Kabatereine NB, Olsen A (2011) Efficacy of single and double doses of albendazole and mebendazole alone and in combination in the treatment of Trichuris trichiura in school-age children in Uganda. Trans R Soc Trop Med Hyg 105: 586–590. [DOI] [PubMed] [Google Scholar]
- 57. Keiser J, Tritten L, Adelfio R, Vargas M (2012) Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo . Parasit Vectors 5: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Keiser J, Tritten L, Silbereisen A, Speich B, Adelfio R, et al. (2013) Activity of oxantel pamoate monotherapy and combination chemotherapy against Trichuris muris and hookworms: Revival of an old drug. PLoS Negl Trop Dis 7: e2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samson-Himmelstjerna Gv, Blackhall WJ, McCarthy JS, Skuce PJ (2007) Single nucleotide polymorphism (SNP) markers for benzimidazole resistance in veterinary nematodes. Parasitology 134: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 60. Ghisi M, Kaminsky R, Maser P (2007) Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet Parasitol 144: 313–320. [DOI] [PubMed] [Google Scholar]
- 61. Kwa MSG, Veenstra JG, Roos MH (1994) Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta -tubulin isotype 1. Mol Biochem Parasitol 63: 299–303. [DOI] [PubMed] [Google Scholar]
- 62. Hodgkinson JE, Clark HJ, Kaplan RM, Lake SL, Matthews JB (2008) The role of polymorphisms at beta tubulin isotype 1 codons 167 and 200 in benzimidazole resistance in cyathostomins. Int J Parasitol 38: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 63. Silvestre A, Humbert JF (2002) Diversity of benzimidazole-resistance alleles in populations of small ruminant parasites. Int J Parasitol 32: 921–928. [DOI] [PubMed] [Google Scholar]
- 64. Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DAP, et al. (2009) Assays to detect beta -tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides . PLoS Negl Trop Dis 3: e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, et al. (2013) Association between response to albendazole treatment and beta -tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl Trop Dis 7: e2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hansen TVA, Nejsum P, Olsen A, Thamsborg SM (2013) Genetic variation in codons 167, 198 and 200 of the beta-tubulin gene in whipworms (Trichuris spp.) from a range of domestic animals and wildlife. Vet Parasitol 193: 141–149. [DOI] [PubMed] [Google Scholar]
- 67. Hansen TVA, Thamsborg SM, Olsen A, Prichard R, Nejsum P (2013) Genetic variations in the beta-tubulin gene and the internal transcribed spacer 2 region of Trichuris species from man and baboons. Parasit Vectors 6: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]