Abstract
Background
Salivary proteins of Triatoma infestans elicit humoral immune responses in their vertebrate hosts. These immune responses indicate exposure to triatomines and thus can be a useful epidemiological tool to estimate triatomine infestation. In the present study, we analyzed antibody responses of guinea pigs to salivary antigens of different developmental stages of four T. infestans strains originating from domestic and/or peridomestic habitats in Argentina, Bolivia, Chile and Peru. We aimed to identify developmental stage- and strain-specific salivary antigens as potential markers of T. infestans exposure.
Methodology and Principal Findings
In SDS-PAGE analysis of salivary proteins of T. infestans the banding pattern differed between developmental stages and strains of triatomines. Phenograms constructed from the salivary profiles separated nymphal instars, especially the 5th instar, from adults. To analyze the influence of stage- and strain-specific differences in T. infestans saliva on the antibody response of guinea pigs, twenty-one guinea pigs were exposed to 5th instar nymphs and/or adults of different T. infestans strains. Western blot analyses using sera of exposed guinea pigs revealed stage- and strain-specific variations in the humoral response of animals. In total, 27 and 17 different salivary proteins reacted with guinea pig sera using IgG and IgM antibodies, respectively. Despite all variations of recognized salivary antigens, an antigen of 35 kDa reacted with sera of almost all challenged guinea pigs.
Conclusion
Salivary antigens are increasingly considered as an epidemiological tool to measure exposure to hematophagous arthropods, but developmental stage- and strain-specific variations in the saliva composition and the respective differences of immunogenicity are often neglected. Thus, the development of a triatomine exposure marker for surveillance studies after triatomine control campaigns requires detailed investigations. Our study resulted in the identification of a potential antigen as useful marker of T. infestans exposure.
Author Summary
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi, and currently affects approximately 8 million people in Latin American countries. Although vector control campaigns against the most effective Chagas disease vector, Triatoma infestans, have been highly successful, T. infestans is re-establishing in once-endemic regions. To monitor re-establishing triatomines, new epidemiological tools are needed. Antibody responses of hosts to triatomine salivary proteins represent a promising tool to detect biting bugs, and highly immunogenic salivary antigens may be used as markers of triatomine exposure. Therefore, we analyzed the antibody response of guinea pigs, common peridomestic hosts of T. infestans, to salivary proteins of nymphs and adults of four different T. infestans strains from Argentina, Bolivia, Chile and Peru. Developmental stage- and strain-specific proteins in the saliva of T. infestans influenced the antibody response of guinea pigs, and different salivary antigens were recognized by guinea pig sera. Despite the variations of immunogenic salivary antigens, a 35 kDa antigen was recognized by almost all guinea pig sera and this antigen may be a useful marker of T. infestans exposure.
Introduction
Arthropod-borne diseases, such as malaria, leishmaniasis, Lyme disease and Chagas disease, greatly impact human and animal health worldwide [1]–[4]. For the improvement of vector control measures, much effort is being devoted to develop novel, simple, rapid and sensitive tools to monitor populations of hematophagous arthropods [5]–[8]. These tools may identify human beings and animals at risk of exposure to vector bites and parasite infection. A promising, immunological approach is based on the immunogenicity of salivary proteins from hematophagous arthropods. Salivary proteins of these arthropods are injected into their hosts while blood-feeding to counteract the vertebrate's hemostasis, inflammation, and immunity [9]–[11]. In vertebrates salivary proteins induce a humoral immune response, amongst others, and these antibody responses have been used to identify highly immunogenic salivary proteins that can serve as an immunological tool such as markers of exposure to arthropod bites [12].
Schwartz et al. [13] studied, as one of the first researchers, the relationship between arthropod exposure and antibody level. They discovered that outdoor workers who had been exposed to tick bites of Ixodes dammini had higher anti-saliva IgG antibody levels compared to workers that had not been exposed to ticks. Following these findings, several other studies characterized antibody responses of different animals to the saliva of hematophagous arthropods such as sand flies [e.g. 14–16], mosquitoes [e.g. 17,18], ticks [e.g. 19–21] and black flies [22], [23]. Furthermore, antibody responses of humans and/or animals to Anopheles gambiae, Triatoma infestans and Phlebotomus argentipes saliva were also analyzed to test the efficacy of insecticide-treated nets to protect humans and animals against vector bites [24]–[26]. These studies provided a proof of concept for the application of anti-saliva antibodies as immunological tool for vector control interventions.
The major difficulties in developing an immunological test to detect vector exposure include a) problems in rearing sufficient numbers of the respective arthropod, b) the collection of arthropod saliva, such as from sand flies or mosquitoes and c) technical difficulties in dissecting salivary glands if saliva cannot be obtained [17], [27]. To surmount these difficulties, recombinant immunogenic salivary proteins were developed for immunoassays to replace saliva. These recombinant proteins improved the sensitivity, specificity and reproducibility of the tests. An example is the well-studied 10 kDa salivary protein of Anopheles gambiae, a highly conserved protein in the Anopheles genus [28]. This protein was expressed recombinantly (gSG6), and characterized for its immunogenicity and especially its Anopheles-specificity using sera of children exposed to A. gambiae [29], [30]. This protein is an ideal candidate exposure marker for the main African tropical malaria vectors because levels of anti-gSG6 antibodies dropped quickly after the end of the mosquito season [31], [32]. Other examples of successfully characterized recombinant exposure markers are a recombinant calreticulin protein that was evaluated as a promising marker of tick exposure using human sera [33], [34] and two heterologous synthesized salivary proteins of Lutzomyia longipalpis that proved to be more effective and sensitive in immunoassays compared to crude sand fly saliva [35], [36].
A recombinant salivary exposure marker, rTiSP14.6, was developed recently to detect exposure to Triatoma infestans, the most effective vector of Chagas disease [37]–[39]. RTiSP14.6 was very effective in detecting differences in infestation levels of T. infestans in Bolivian households by analyzing IgG levels against the corresponding salivary protein using chicken sera. IgM antibodies of chicken sera also reacted with rTiSP14.6, but compared to IgG immune responses of chickens no differences were detectable in the overall antibody reactions to either crude saliva or rTiSP14.6 comparing chicken sera from lowly or highly T. infestans infested households [38]. Guinea pig sera were also tested with rTiSP14.6, but the recombinant antigen only reacted very weakly with guinea pig antibodies in immunoassays, and no differences between T. infestans infestation levels were evident using laboratory or field sera [37]. Compared to chickens, mammalian hosts of T. infestans, such as guinea pigs and dogs, are of high importance in the epidemiology of Chagas disease, because they are reservoir hosts of T. cruzi [40], [41] and thus represent an important risk factor of peridomestic and domestic T. cruzi transmission to animals and humans [42], [43]. Thus, the development of an immunological marker that would react with guinea pig sera as a detector of triatomine exposure and a risk marker of T. cruzi infection, respectively, would be beneficial for surveillance in T. infestans vector control.
Saliva of hematophagous species can differ in its composition between populations of the same species such as analyzed for sand flies [44] and triatomines [37], [45], [46]. Additionally, salivary proteins of arthropods vary not only between populations but also between developmental stages [47], [48]. Such variations in the saliva composition between arthropod populations and between developmental stages may induce different humoral responses in the vertebrate's hosts. In order to develop an appropriate T. infestans exposure marker, in particular a salivary antigen that will be recognized by sera of guinea pigs exposed to any developmental stage or strain of T. infestans, we characterized the antibody response of guinea pigs exposed to a low number of nymphal or adult T. infestans. Experimental animals were exposed to different T. infestans strains, originating from Argentina, Bolivia, Chile and Peru.
Materials and Methods
Ethics statement
All experimental exposures of animals to triatomines carried out in the Czech Republic were in accordance with the Animal Protection Law of the Czech Republic (§17, Act No. 246/1992 Sb) and with the approval of the Academy of Science of the Czech Republic (protocol approval no. 172/2010) which complies with the regulations of the European Directive 2010/63/EU on the protection of animals used for scientific purposes in Europe. All animal experiments performed in Peru were approved by the Institutional Animal Care and Use Committee (IACUC) of the Universidad Peruana Cayetano Heredia (protocol approval no. 60068). This committee acts in accordance with the US federal laws and thus all animal experiments in Peru followed the guidelines of the US Animal Welfare Act.
Triatoma infestans and saliva collection
The four different strains of T. infestans originated from Argentina, Bolivia, Chile and Peru (for further details, see Table S1). Triatomine colonies were reared at an air temperature of 28±1°C, a relative humidity of 60–70% and with a 12 h/12 h light/dark cycle. For colony maintenance the bugs were regularly fed on guinea pigs or rabbits. All experiments were performed with saliva of T. infestans, starved for one week after the last blood meal. This period of time after a blood meal is necessary to re-synthesize saliva components injected into the host during the previous feeding [49]. The saliva (0.5–1 µl per bug) was collected typically from either 100 nymphs (5th instar) or 50 adults (females and males) using glass Pasteur pipettes as previously described [37]. The protein concentration was determined using the BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer's instructions and the saliva was and stored at −80°C until use. For the analysis of the developmental stage-specific salivary proteins of T. infestans saliva was collected from 20–50 bugs of each instar including males and females separately.
Exposure experiments
Guinea pigs (four months old) used for exposure experiments in the Czech Republic were obtained from our in-house breeding and animal facility and guinea pigs (four months old) in Peru were purchased from an animal distributor in Lima, Peru and had never been in contact with triatomines. In the laboratories of the Czech Republic and in Peru guinea pigs were exposed weekly to T. infestans over a period of 10 weeks to induce a strong anti-triatomine saliva antibody response. Two different experimental procedures were carried out in the Czech Republic and in Peru as described in the following. In the Czech Republic a group of 18 guinea pigs was divided into three subgroups of six animals, and each subgroup was either exposed to a triatomine strain from Argentina or Bolivia or Chile. Within each subgroup, three guinea pigs were either challenged with five 5th instars or five adult T. infestans. The triatomines were allowed to probe 50 times for 1 min on the animals during each exposure experiment. In Peru a group of 3 guinea pigs was exposed to a mixture of 5th instars and adults of a Peruvian T. infestans strain (10 nymphs and 10 adults) for 1 h. A control group of three guinea pigs was not exposed to triatomines in both laboratories and all guinea pigs in the Czech Republic and Peru were bled from the hind leg from the vena saphena. Pre-exposure sera were taken from all animals as negative controls for immunoassays. Starting at five days after the first exposure to triatomines, blood was sampled in weekly intervals.
ELISA
IgG and IgM antibodies in guinea pig sera were analyzed by ELISA as previously described using either 0.5 µg protein of crude nymphal (5th instar) or adult saliva/well [37]. Briefly, IgG antibodies were detected using horseradish peroxidase (HRP) conjugated rabbit anti-guinea pig IgG secondary antibodies (Sigma-Aldrich) diluted 1∶20,000 in PBS-Tween20 (PBST) with 2% dried skimmed milk. For the detection of IgM antibodies, HRP goat anti-guinea pig IgM secondary antibodies (Immunology Consultants Laboratory) were used and diluted 1∶10,000 in PBST with 2% dried skimmed milk. The ninety-six well plates (Medisorp, Nunc) were developed using orthophenylenediamine (Sigma-Aldrich) with hydrogen peroxide. The reaction was stopped with 2 M H2SO4, and optical densities (OD) were measured at 490 nm with a Multiskan MCC/340 spectrophotometer (Labsystems Oy). All samples were analyzed in duplicates and tested in two independent ELISA assays. A sample O.D.490 nm was determined by calculating the mean O.D.490 nm of the duplicated sample and by subtracting the O.D.490 nm of the pre-exposure sample.
1D-gel electrophoresis
For the analysis of 1) the salivary protein profiles of all developmental stages of the Bolivian T. infestans strain and 2) of 5th instars and adults of the different T. infestans strains, crude saliva (1 µg protein/lane) was separated by SDS-PAGE on 15% acrylamide gels under reducing conditions as previously described [37]. Briefly, denatured salivary proteins were separated electrophoretically at 150 V for 50 min following 300 V for 1.5 h. Afterwards acrylamide gels were stained using the SilverQuest staining kit (Invitrogen). Molecular weights were calculated with reference to the mobility of the standard proteins from the SeeBluePlus2 Prestained Standard (Invitrogen) using the software TotalLab TL120 (TotalLab). The level of similarity between the salivary profiles of each developmental stage or between the strains of T. infestans was analyzed using the software FreeTree [50]. Based on the presence or absence of salivary bands a distance matrix was created from which the Nei-Li/Dice's coefficient of similarity was calculated and used to construct an UPGMA (Unweighted Pair Group Method with Arithmetic Mean) phenogram. The robustness of trees was assessed by bootstrap analysis (1000 bootstrap replicates). The dendrogram was displayed using TreeView [51].
Immunoblot
Nymphal (5th instar) or adult salivary proteins (50 µg protein in a 2D well) were separated by SDS-PAGE as described above and transferred onto a nitrocellulose membrane at 25 V for 30 min using the Pierce Fast Semi-dry Blotter (Thermo Scientific). After the transfer, the membrane was blocked with 5% skimmed milk in PBS overnight at 4°C, washed three times in PBST and incubated with guinea pig sera diluted 1∶100 in PBST with 5% skimmed milk for 1 h at room temperature using the Mini-Protean II Multiscreen apparatus (Bio-Rad). Following repeated washing steps, the Western blot membrane was incubated for 1 h at room temperature with HRP conjugated rabbit anti-guinea pig IgG secondary antibodies (Sigma-Aldrich) diluted 1∶10,000 in PBST with 5% dried skimmed milk or HRP goat anti-guinea pig IgM secondary antibodies (Immunology Consultants Laboratory), diluted 1∶5,000 in PBST with 5% dried skimmed milk. The proteins were visualized using the Pierce ECL Western Blotting Substrate (Thermo Scientific) and digitized using the LAS-3000 machine (Fujifilm).
2D-gel electrophoresis
Polyacrylamide gel strips with a non-linear pH gradient 3–11 (13 cm, Immobiline DryStrip, GE Healthcare) were incubated in a rehydration buffer (2 M thiourea, 7 M urea, 2% chaps, 15 mM DTT) containing 0.5% carrier ampholytes, pH 3–11 (GE Healthcare) for 12 h at room temperature. Afterwards, desalted crude saliva of 5th instars or adult T. infestans (150 µg protein) was applied by cup loading and separated by isoelectric focusing (IEF) using an IEF 100 unit (Hoefer). The saliva was diluted in rehydration buffer and focused at 20°C with a maximum current setting of 200 µA/strip using the following voltage gradient: 0–250 V for 30 min; 250–500 V for 1 h; 500–1000 V for 1 h; 1000–8000 V for 2.5 h; constant 8000 V for 1 h. For the second dimension, the IPG strips were equilibrated in equilibration buffer (6 M urea, 75 mM Tris-HCl, 29.3% (v/v) glycerol, 2% SDS, 65 mM DTT, 0.002% (w/v) bromphenol blue) for 15 min followed by a 15 min incubation with 135 mM iodoacetamide. The strips were placed onto 15% SDS-PAGE gels and the proteins were separated at 300 V and 25 mA for 6 h and stained with silver as described above.
2D-western blots
For the Western blot analyses polyacrylamide gel strips with a non-linear pH gradient 3–5.6 (13 cm, Immobiline DryStrip, GE Healthcare) were rehydrated as describe above. Afterwards, 40 µg protein of desalted, nymphal (5th instar) or adult saliva was focused at 20°C with a maximum current setting of 200 µA/strip using cup loading and the following voltage gradient: 0–250 V for 30 min; 250–500 V for 1 h; 500–1000 V for 1 h; 1000–8000 V for 2.5 h; constant 8000 V for 1 h, followed by second dimension as described above but using a transfer time of 1 h. For the Western blot analyses replica gels of the 2D-gel electrophoresis were probed with early sera (5th week) from guinea pigs exposed to nymphal (5th instar) or adult T. infestans. Afterwards, Western blot images were compared with the silver stained 2D-gels using program Progenesis SameSpot (Nonlinear dynamics).
Statistical analysis
The mean O.D.490 nm of each subgroup of guinea pigs (3 guinea pigs/subgroup) was calculated for each independent ELISA assay. Therefore, the mean O.D.490 nm of the calculated mean sample O.Ds.490 nm (mean O.Ds.490 nm of duplicated samples and subtraction of the O.D.490 nm of the pre-exposure sample) of the three guinea pigs from each subgroup was calculated. This calculation was performed for the O.D. values (subgroups) of the two independent ELISA assays separately. Afterwards, the final mean O.Ds.490 nm for all guinea pig subgroups from the two independent ELISA assays was calculated.
ELISA data (final mean O.D.490 nm of two ELISA assays) from the long-term exposure study with guinea pigs were statistically analyzed using SPSS, version17.0 (IBM). To compare antibody levels of experimentally exposed guinea pigs, the average from the final mean O.D.490 nm (as described above from two independent ELISA assays) of three measured guinea pig sera per each animal subgroup was calculated and the averages of each subgroup were statistically compared. Because the ELISA data did not follow a Gaussian distribution, the non-parametric Wilcoxon signed-rank test was used to compare the antibody levels of guinea pigs tested with only nymphal saliva with the levels of animals analyzed with adult triatomine saliva. The Friedman test was used to uncover differences in the antibody responses between guinea pigs exposed to different T. infestans strains. Differences in all tests were considered as statistically significant at a level of p<0.05.
Correlations between the sum of feeding triatomines (number of triatomines used at each feeding event plus the number of triatomines already fed in previous events) per feeding event of each T. infestans strain and the corresponding guinea pig IgG antibody levels (mean O.D.490 nm) were analyzed using the non-parametric Spearman rank correlation test. Because a set of nymphal and adult Peruvian T. infestans and a higher number of Peruvian triatomines were used in guinea pig experiments compared to guinea pigs challenged with T. infestans from Bolivia, Chile and Argentina, datasets from the Peruvian T. infestans exposure experiments were analyzed separately. In all cases, antibody levels either measured against nymphal or adult saliva in ELISA assays were used in separate correlation tests in order to differentiate between developmental specific antibody responses. We further fit parametric models to describe the relationship between the cumulative number of insect bites and the O.D.490 nm of each sample taken at each time point using R [52]. We compared simple linear relationships to logistic curves; in all cases the logistic models had lower residual errors than the linear models.
Results
Salivary proteins of the developmental stages and strains of T. infestans
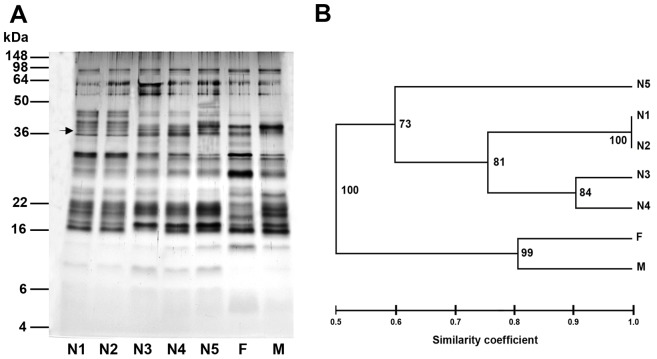
Analysis of the saliva content of all developmental stages of T. infestans (pooled saliva of 20–50 bugs per developmental stage) revealed a complex pattern of salivary proteins ranging from 5 kDa to 92 kDa as demonstrated for the Bolivian T. infestans strain (Figure 1A). Most differences in the salivary profiles were detected between nymphs and adults, e.g. a protein band of 37 kDa was present in the saliva of all nymphal stages but not in adult saliva (Figure 1A, marked with an arrow). An unrooted phenogram constructed from the electrophoretic saliva profiles of the different developmental stages disclosed two main groups that separate nymphal salivary proteins from adult proteins with the Dice's similarity coefficient of 0.5 (Figure 1B). The first two nymphal instars (N1 and N2) shared the same protein bands (similarity coefficient = 1.0), and between the following nymphal instars the degree of similarity decreased. Thus, the salivary profile of the 5th instar was the most different compared to all other nymphal profiles (similarity coefficient = 0.60). The salivary protein pattern of female and male T. infestans were quite similar (similarity coefficient = 0.80).
Figure 1. Salivary profiles of the developmental stages of T. infestans.
(A) Proteins in crude saliva of all nymphal (N1–N5) and adult (female, F and male, M) stages of a Bolivian T. infestans strain were separated by SDS-PAGE. A nymphal-specific salivary protein of 37 kDa is marked with an arrow. (B) An unrooted phenogram of the salivary profiles was constructed using the UPGMA method of the FreeTree software [50]. Bootstrap values are displayed at the nodes of the tree and the scale bar represents the Dice's similarity coefficient.
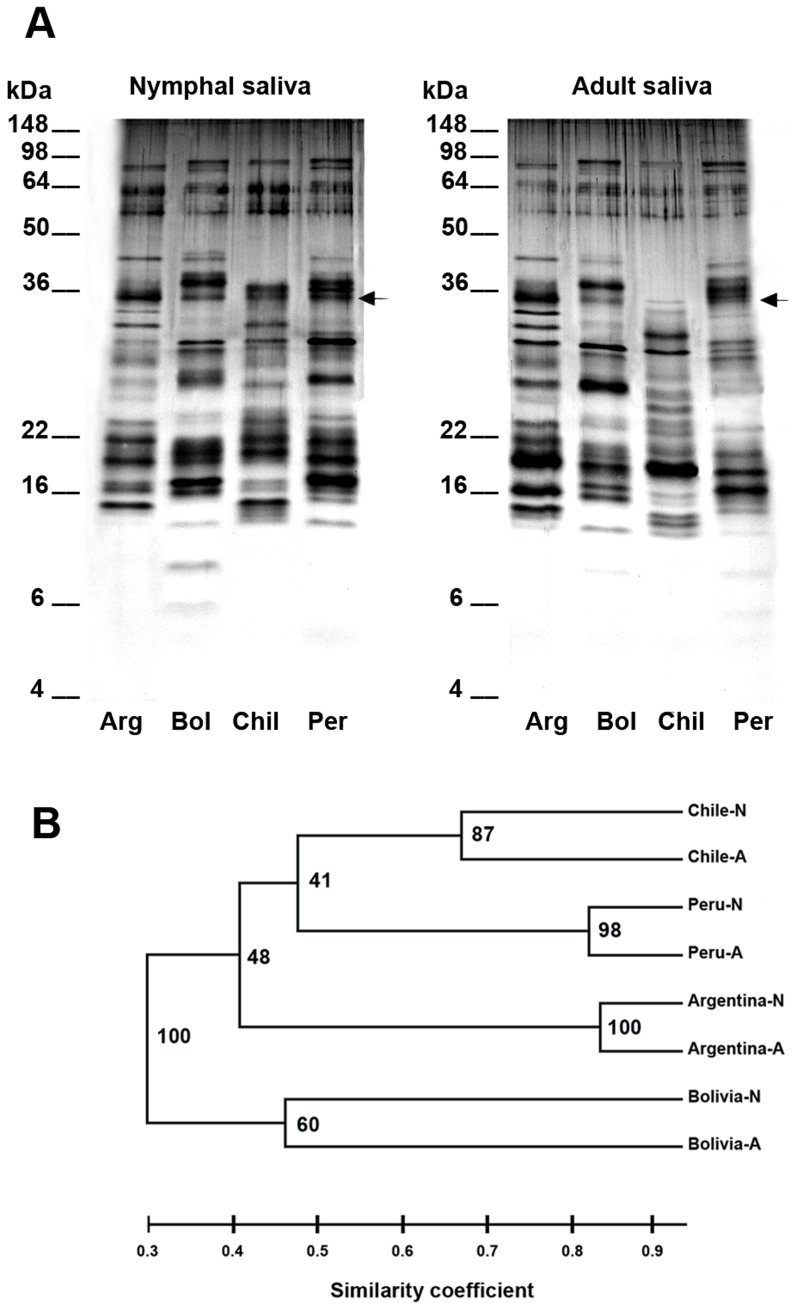
Due to the phenogram's analysis saliva of the 5th instar and adults of each T. infestans strain were separated by gel electrophoresis in order to compare the saliva composition of different T. infestans strains (Figure 2A). Salivary protein profiles of the different T. infestans strains showed less similarity (similarity coefficient = 0.3) compared to profiles of nymphs and adults of each strain (similarity coefficient = 0.46–0.84, Figure 2B). Comparing all salivary protein profiles, nymphal and adult profiles of the Bolivian T. infestans strain were most different to the profiles of all other strains (similarity coefficient = 0.3, Figure 2B). Despite these variations in the saliva composition, several protein bands were shared between the different T. infestans strains such as of the 25 kDa, 30 kDa, 33 kDa, 35 kDa (Figure 2A, marked with arrows), 55 kDa and 62 kDa proteins.
Figure 2. Nymphal and adult salivary profiles of four different T. infestans strains.
(A) Saliva of starved nymphs (fifth instar) and adults (pooled saliva of females and males) of four different T. infestans strains from Argentina (Arg), Bolivia (Bol), Chile (Chil) and Peru (Per) were analyzed by SDS PAGE. Arrows mark the protein band of 35 kDa that is common for all T. infestans strains. (B) An unrooted phenogram was constructed from the electrophoretic salivary profiles of nymphs (N) and adults (A) of the different T. infestans strains using the UPGMA method of the FreeTree software [50]. Bootstrap values are displayed at the tree nodes and the scale bar represents the Dice's similarity coefficient.
Antibody responses of guinea pigs to salivary antigens of T. infestans
Because most differences in the salivary profiles were detected between the 5th nymphal T. infestans stage and adult triatomines, both stages were used to induce antibody responses in guinea pigs. To characterize the development of IgG and IgM antibody responses guinea pigs were either bitten a) by bugs of the 5th instar or adults from the Argentinean, Bolivian or Chilean T. infestans strain or b) by a set of nymphs and adults of the Peruvian strain. All sera of guinea pigs (including sera from guinea pigs exposed to Peruvian triatomines) were either analyzed with saliva of the 5th instar or adults of the different T. infestans strains.
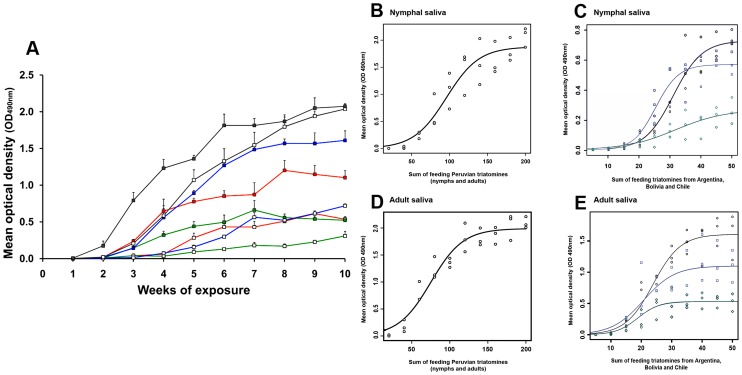
The level of IgG antibodies increased in guinea pigs bitten by nymphal and/or adult triatomines from Argentina, Bolivia, Chile and Peru with serial exposures to T. infestans (Figure 3A). Anti-saliva IgG antibodies of guinea pigs exposed to the Peruvian T. infestans strain were detectable after the first exposure, although at a very low level (mean O.D. 490 nm = 0.003, Figure 3A). All other IgG antibody levels were detectable after the second exposure, at which some antibody levels were also very low (mean O.D. 490 nm range = 0.003–0.016). The highest antibody levels were reached in guinea pigs exposed to the Peruvian strain (mean max. O.D. 490 nm = 2.073).
Figure 3. IgG antibody response of guinea pigs to saliva of nymphal and adult T. infestans of four different strains.
(A) Eighteen guinea pigs were either exposed to the 5th instar (n = 5, white squares) or adults (n = 5, colored squares) of three different T. infestans strains from Argentina (blue line), Bolivia (red line) and Chile (green line). Each group of animals was made of 3 guinea pigs. Additionally, three guinea pigs were exposed to a set of nymphs (n = 10, white squares) and adult triatomines (n = 10, grey squares) from Peru (grey lines). Guinea pigs were exposed weekly to triatomines and for a period of 10 weeks. Animals were bled 5 days after each exposure and all sera were analyzed by ELISA using either crude saliva of nymphs or adults. From each group of guinea pig sera (n = 3) the mean optical density (O.D.490 nm) was calculated after subtracting the O.D. of the negative control (pre-exposure). The results here presented show the final mean optical densities (O.D.490 nm) of two independent ELISA assays. (B–E) Logistic models describing the relationship between the sum of feeding nymphal and/or adult triatomines (number of triatomines used at each feeding event plus the number of triatomines already fed in previous events) and the corresponding IgG antibody level of guinea pigs to saliva of the Peruvian (B, D), Argentinean (C, E, black graphs), Bolivian (C, E, blue graphs) and Chilean T. infestans strains (C, E, green graphs).
Anti-saliva IgG levels of guinea pigs challenged with only nymphs of either the Argentinean, the Bolivian or the Chilean T. infestans strain increased significantly slower (max. mean O.D.490 nm range = 0.238–0.766) compared to the antibody levels of guinea pigs exposed to adults only (max. mean O.D.490 nm range = 0.574–1.669; Wilcoxon signed-rank test, p<0.001); these guinea pigs reached lower antibody levels in general. Although some guinea pigs were exposed to a set of nymphal and adult triatomines from Peru, significant differences in the IgG antibody response were detected when using either only nymphal or adult Peruvian saliva in ELISA assays (Wilcoxon signed-rank test, p<0.001). Moreover, IgG antibody responses of animals exposed to adult triatomines of all strains differed significantly (Friedman test, p<0.001, Figure 3A) but no significant differences appeared between the immune response of guinea pigs exposed to nymphs of the different T. infestans strains (Friedman test, p>0.05). Overall, the sum of feeding nymphal and/or adult triatomines on guinea pigs (number of triatomines used at each feeding event plus the number of triatomines already fed in previous events) of all T. infestans strains correlated significantly and strongly, positively with the IgG antibody level of guinea pigs over the experimental period of triatomine exposure (for statistics please see Table S3). But a linear relationship between the number of biting triatomines and the corresponding IgG antibody response was only measurable until the 7th or 8th week of triatomine exposure (35–40 fed bugs) when using the T. infestans strains from Argentina, Bolivia and Chile (Figure 3C and E) and until the 6th or 7th week of triatomine exposure (120–140 fed bugs) when using Peruvian T. infestans (Figure 3B and D). Afterwards, the relationship between the IgG antibody response and the number of biting triatomines saturated.
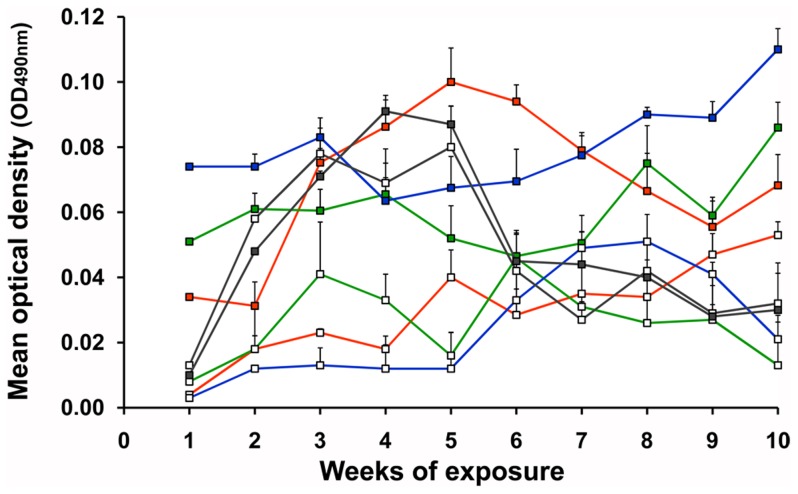
IgM antibodies of all guinea pigs to crude saliva of nymphal (5th instar) and/or adult T. infestans were detectable after the first exposure to triatomines (max. mean O.D.490 nm = 0.074, Figure 4). Compared to IgG antibody responses (Figure 3A), IgM responses of guinea pigs to triatomine saliva were very low and they only fluctuated slightly during the serial exposure to triatomine bites (max. mean O.D.490 nm range = 0.004–0.110). No significant differences between the IgM responses were detected using either nymphs and/or adults of all strains in the exposure study (Wilcoxon signed-rank test, p>0.05, Figure 4), and the overall sum of feeding nymphal and/or adult triatomines of all strains were not correlated with IgM antibody levels (Spearman rank correlation test, p>0.05).
Figure 4. IgM antibody response of guinea pigs to saliva of T. infestans.
Eighteen guinea pigs were either exposed to nymphs (white squares) or adults (colored squares) of three different T. infestans from Argentina (blue line), Bolivia (red line) and Chile (green line). A second group of three guinea pigs was exposed to a set of nymphal (n = 10, white squares) and adult (n = 10, colored squares) T. infestans from Peru (grey line). Sera from all exposure events were tested either with crude saliva of nymphs or adults in ELISA assays to monitor the development of the IgM antibody response in guinea pigs. Mean optical densities (O.D.490 nm) of each exposure subgroup (3 guinea pig sera) were calculated after subtracting the O.D.s of the negative controls (pre-exposures). The final mean O.D.s presented in this graph were calculated from two independent ELISA assays.
Immunogenic salivary antigens of T. infestans
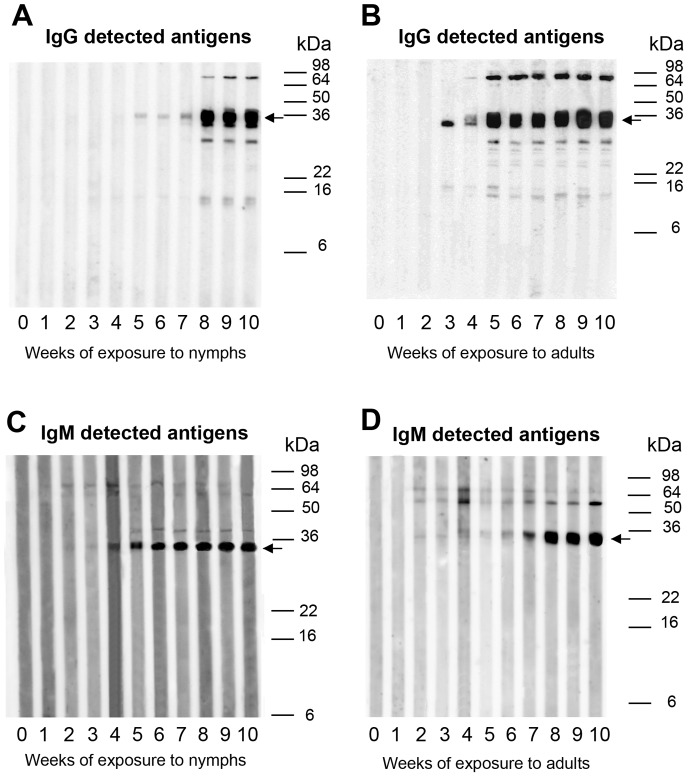
Guinea pig sera were used to detect immunogenic salivary proteins. Figure 5 represents an example of developmental stage-specific antigens of the Bolivian T. infestans strain. Antigen reactions of all four strains are summarized in Table S2. IgG reacting antigens were mainly detected from the third week of triatomine exposure (Figure 5A and B), but some bands were already visible on Western blots from the second week of triatomine exposure using sera of guinea pigs exposed to the Peruvian strain (data not shown). More salivary proteins of adults than of 5th instar nymphs were recognized by IgG antibodies (Figures 5A and B, Table S2). In contrast to IgG reactions, IgM antibodies bound to salivary proteins already after the first or second week of triatomine exposure (Figure 5C and D). Only a few proteins were detected in nymphal and adult saliva using IgM antibodies, and no antigens below 30 kDa of the Bolivian T. infestans strain reacted with IgM antibodies.
Figure 5. Immunogenic salivary antigens of T. infestans.
Sera of guinea pigs that were weekly exposed to nymphal (A, C) or adult T. infestans (B, D) of the Bolivian strain over a period of 10 weeks (1–10) were used in Western blot experiments to detect IgG (A, B) and IgM (C, D) reacting antigens. A serum from a guinea pig prior to the exposure to bug bites was used as a negative control (0) in the Western blot experiments to demonstrate the specificity of the IgG and IgM anti-saliva T. infestans responses. Arrows mark the 35 kDa salivary protein that was detected in all Western blots by IgG and IgM antibodies.
Strain-specific variations in the recognition of salivary proteins by antibodies were also evident in Western blots (Table S2). Using IgG secondary antibodies, most antigens were detected for the Chilean strain (nymphal antigens = 12, adult antigens = 15) while only 8 proteins of adult saliva from Argentinean triatomines were immunogenic (Table S2). IgM reactions with saliva of the Argentinean, Chilean and Peruvian T. infestans strains were similar to the reactions with saliva of the Bolivian strain; less proteins reacted with IgM antibodies but a few proteins with molecular weights <30 kDa were detected. The differences observed in the antibody response of the Peruvian T. infestans strain when using only nymphal or adult saliva in ELISA assays were also reflected in the Western blots; IgG or IgM antibodies did not bound entirely to the same salivary proteins using either nymphal or adult saliva. Despite the differences in developmental-stage and -strain specific antigens of T. infestans, a 35 kDa antigen was detected by sera of almost all guinea pigs using both IgG (19 out of 21) and IgM (18 out of 21) antibodies (Table S2).
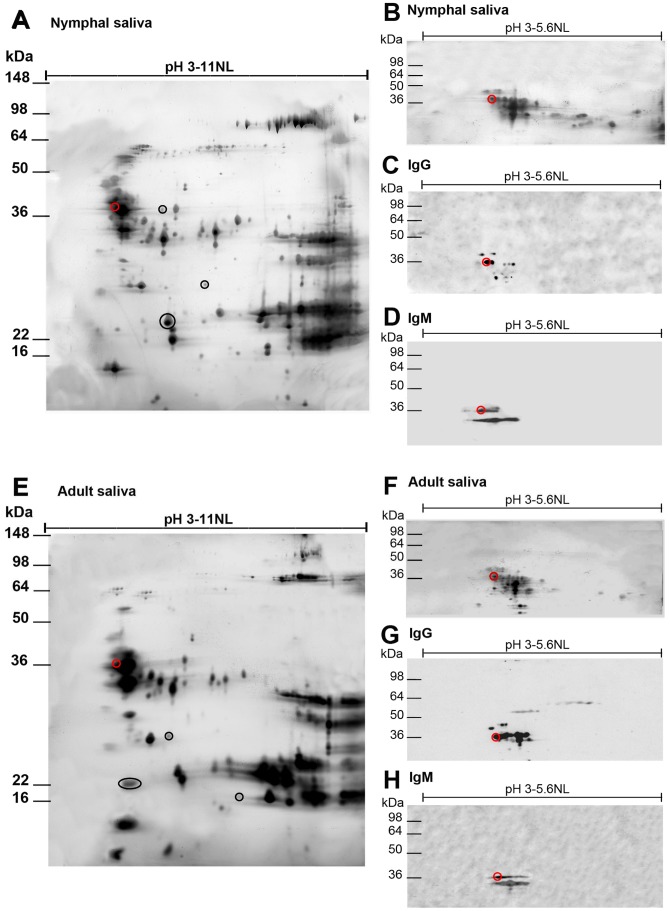
In order to further characterize the 35 kDa antigen of T. infestans that may be a potential triatomine exposure marker, proteins were separated by 2D SDS PAGE, using a nonlinear pH range of 3–11 (Figures 6A, E and Figures S1, S2, S3A, E), and used for Western blots. In accordance with Figure 5, Figure 6 represents an example of 2D salivary protein patterns and Western blots of saliva of 5th instar nymphs (A–D) and adults (E–H) of T. infestans from Bolivia. The 2D salivary protein profiles of the Chilean, Argentinean and Peruvian T. infestans strain are presented in the Figures S1, S2, S3. Although T. infestans proteins appeared almost along the entire pH range, concentrations of proteins were focused at pH 3.8–4.6 and 8.4–11.0. 2D gel analyses revealed much more precisely and detailed the differences in the salivary profile between the 5th instars and adults of T. infestans as shown in the Figures 6A and E and Figures S1, S2, S3A and E (some examples of nymphal- or adult-specific protein spots are marked).
Figure 6. Characterization of the 35T. infestans.
Salivary proteins of T. infestans were analyzed by 2D gel electrophoresis and 2D Western blotting in order to identify the candidate exposure marker protein of 35 kDa. The figure presents an overview of nymphal (5th instar, A) and adult (females and males, E) salivary proteins of the Bolivian T. infestans. Developmental stage specific salivary proteins in nymphal (A) and adult (E) saliva are marked with black circles. In order to improve the protein separation, crude saliva of nymphs (B) and adults (F) were isoelectric focused in the nonlinear pH range of 3–5.6. Focused proteins were blotted onto nitrocellulose and tested for their immunogenicity using a guinea pig serum from the 5th week of triatomine exposure. IgG (C, G) and IgM antibody reactions (D, H) with nymphal (C, D) and adult (G, H) T. infestans salivary proteins were analyzed. The candidate exposure marker antigen of 35 kDa detected by IgG and IgM antibodies is marked with a red circle.
Experimental guinea pig sera from the fifth week of exposure were used to detect the candidate exposure marker antigen of 35 kDa. Since this antigen had a pH of 4.1 and thus accumulated in the 2D gels with other T. infestans proteins of the same pH, salivary proteins of nymphs and adults were isoelectrically focused at a pH range of 3–5.6 for a better separation of the salivary proteins (Figures 6B, F and Figures S1, S2, S3B, F) and blotted onto nitrocellulose. A maximum of 9 and 12 salivary protein spots of about 35 kDa in nymphal and adult saliva of the Bolivian T. infestans strain, respectively, were recognized by IgG antibodies (Figure 6C, G). In contrast, IgM antibodies reacted with less protein spots of 35 kDa; a maximum of 4 spots from nymphal saliva of the Bolivian T. infestans strain appeared to be immunogenic and 6 salivary protein spots were recognized by IgM antibodies in adult saliva (Figure 6D, H). Less salivary proteins of 35 kDa from the saliva of the other three T. infestans strains were immunogenic (Figures S1, S2, S3C, G). A maximum of 4 protein spots were detected using IgG antibodies, while only maximal 2 spots were detectable with IgM antibodies (Figure S1, S2, S3, D, H) using sera of guinea pigs exposed to nymphs or adults of other strains of T. infestans. Of all salivary proteins detected by IgG and IgM antibodies in 2D gel analyses, only one salivary protein of about 35 kDa reacted with both types of immunoglobulins using sera of guinea pigs exposed to nymphs and/or adults of all T. infestans strains.
Discussion
Multinational Chagas disease control initiatives in Latin American countries have greatly reduced the transmission of T. cruzi since their establishment in the 1990s [53], [54]. Success in Chagas disease control depends mainly on the elimination of triatomines, especially T. infestans [55]–[57]. Although insecticide-based vector control has been very effective and reduced infestation rates throughout Latin America, such as in Chile where domestic populations of T. infestans were reduced by 96% between 1982 and 1998 [58], the resistance of T. infestans to insecticides [59], [60] and the low effectiveness of insecticides in peridomestic environments, has become a wide-spread problem [61], [62]. Moreover, after successful elimination of triatomine populations conspecific triatomine species can re-infest triatomine-free houses [63], [64]. To detect re-infesting triatomines artificial shelter units or biosensor boxes have been already used, but they are of limited sensitivity for the detection of triatomines [65]–[67]. Thus, highly sensitive surveillance methods are required that can especially detect low level re-infestations of triatomines [55]. A promising epidemiological tool to detect such levels is the use of anti-triatomine saliva antibodies from triatomine hosts that react with salivary antigens of these bugs [37]–[39]. These salivary antigens may be useful markers of triatomine exposure. In the present study we analyzed IgG and IgM antibody responses of guinea pigs that are typical peridomestic hosts of triatomine bugs, to the saliva of different developmental stages and strains of T. infestans in order to detect candidate T. infestans exposure markers.
Several studies focused on the characterization of immunogenic salivary antigens as potential markers of exposure to detect hematophagous arthropods using sera of different host species [e.g. 14,16,21,37]. Depending on the host species different salivary antigens of T. infestans were detected as immunogenic, e.g. 12–59 kDa proteins were recognized by chicken sera, 14–120 kDa proteins by mice sera and 13–81 kDa as well as 8–105 kDa antigens were recognized by sera of Chagas disease patients and non-Chagas disease patients living in triatomine infested areas, respectively [37], [68], [69]. However, in all these studies developmental stage and/or strain-specific differences in salivary proteins were not examined. Our results here emphasize the importance of these covariates on the humoral immune response of the vertebrate hosts. The immunogenicity of salivary proteins differed between developmental stages of T. infestans as especially demonstrated for the Peruvian 5th instars and adults. Despite the variability of recognized proteins from the developmental stages and strains of T. infestans, a salivary antigen of 35 kDa could be identified as candidate exposure marker using guinea pig sera that was shared by all developmental stages and strains of T. infestans using both IgG and IgM antibodies.
Previously, rTiSP14.6, a 14.6. kDa recombinant salivary protein of T. infestans, was evaluated as a suitable exposure marker to detect low-level infestation of T. infestans using chicken sera [37]–[39]. An immunogenic salivary protein of about 14 kDa also reacted in Western blot experiments with sera of guinea pigs exposed to T. infestans, but rTiSP14.6 was not sufficient in reacting with guinea pig sera in immunoassays [39]. Although in previous studies the same immunogenic protein bands were detected in Western blot experiments using sera of exposure experiments with different host species [37], [68], [69], it does not automatically mean that the actual same proteins were detected. Thus, triatomine exposure markers developed for a certain host species may not be applicable for another species and they need to be tested and evaluated individually with sera of the candidate host species. Therefore, our study focused on the humoral immune response of guinea pigs that are important in the epidemiology of Chagas disease. Guinea pigs are usually kept for consumption and trade in different South American countries such as in Peru [70], and they are sometimes maintained inside human dwellings which increases the risk of triatomine exposure and Chagas disease transmission, respectively [43]. Infestation levels of T. infestans can be very high in guinea pig enclosures compared to other animal enclosures, and the presence of animals inside of households during the night can increase the vector abundance about 5-fold [43]. Furthermore, high densities of guinea pigs may allow the re-emergence of T. cruzi after vector control interventions as assumed in an entomological study of a peri-rural area, southwest of Arequipa, in Peru, where 27% infested T. infestans households contained T. cruzi-positive triatomines, but no new parasite infections were detected in humans [71]. Intervention strategies to reduce T. cruzi transmission rate in animals, in turn, were already proposed and/or applied, including the keeping of animals outside of the houses at night [72], the prevention of triatomine contact with animals by using material for animal enclosures that do not provide hideouts for triatomines [43], [72], [73] or the use of impregnated nets that cover animal enclosures [25], [74]. The efficiency of these interventions needs to be evaluated. Recently anti-triatomine saliva immunoassays were used to examine if guinea pig enclosures with impregnated nets protected the animals against triatomine bites by measuring the antibody level of the guinea pigs against crude saliva of T. infestans [25]. However, the study could not completely rule out that cross reacting IgG antibodies to orthologous salivary proteins of other hematophagous arthropods were also detected in the assays when using crude triatomine saliva. Thus, the application of a recombinant salivary antigen of T. infestans as a marker of exposure instead of crude saliva will increase the specificity and sensitivity of such immunological tests for Chagas disease intervention measurements. The usefulness of exposure markers in insecticide treated netting studies was demonstrated in the application of the recombinant A. gambiae salivary antigen gSG6-P1 to evaluate the efficacy of insecticide treated nets (ITNs) in a malaria-endemic area using human sera [24]. The authors verified the better specificity and sensitivity of gSG6-P1 compared to crude mosquito saliva in immunoassays when comparing human anti-gSG6-P1 and human anti-saliva A. gambiae antibody reactions before and after the introduction of ITNs.
A triatomine exposure marker could be also very useful in epidemiologic surveys to complete entomological data in areas where T. cruzi transmission is low, especially after vector control measures against triatomines, and the risk of parasite transmission is newly assessed. Vector control in Chagas disease surveillance relies on regularly inspections of sprayed households and the participation of the local communities in notifying about the presence of bugs [75]. However, sustained surveillance measures are often not implemented in endemic Chagas disease countries. High-throughput blood sampling of humans and/or animals on a regular basis to measure not only T. cruzi prevalences but also exposure to bug bites may improve Chagas disease control in detecting especially newly establishing triatomine infestation and parasite transmission foci. Nevertheless, a triatomine exposure marker would be not suitable for correlating T. cruzi prevalences and vector bites given the complexity of the relationship between vector exposure and parasite infection as occurring, for example, in Arequipa, Peru. Although the majority of the inhabitants and animals are frequently exposed to T. infestans in Arequipa, T. cruzi infection is very clustered and sparse throughout the city [76], [77].
For vector control purposes, a sensitive exposure marker should be useable shortly after insecticide sprayings to promptly detect re-establishing triatomine populations. Analyses of the IgG antibody responses in guinea pigs revealed a strong correlation between the antibody response and the number of biting triatomines. Higher numbers of bugs elicit a higher antibody response in the triatomine host which was reflected in the antibody responses of guinea pigs exposed to the Peruvian T. infestans strain compared to other T. infestans strains. However, due to the saturation effect of the IgG antibody response after a couple of exposure events it is possible to distinguish between a low and high antibody response and thus to distinguish indirectly between highly and lowly infested triatomine sites but a concrete determination of triatomine numbers that fed on triatomine hosts is not possible. Furthermore, anti-triatomine saliva IgG antibodies persist in guinea pigs and chickens up to five months after the last exposure to bugs [37]. Thus, IgG reacting exposure markers are not applicable in areas where control measures were recently carried out. Instead, IgM reacting antigens may be an alternative because IgM antibody responses are detectable only a few weeks after the last exposure to bug bites [38]. The use of IgM antibodies instead of IgG antibodies as immunological tool is generally not considered in studies that characterize the antibody responses of hosts to salivary proteins of hematophagous arthropods for the development of an exposure marker.
Even though recombinant salivary proteins increase the specificity and sensitivity in immunoassays compared to saliva, antibodies that cross react with salivary proteins of different hematophagous arthropods will still counteract the specificity of immunological tests. This cross reactivity can be minimized if species specific salivary proteins are selected as candidate exposure markers instead of highly conserved salivary proteins across the hematophagous arthropod genera which can be verified by sequence similarity analysis using public genetic sequence databases such as GenBank [36]. However, candidate exposure markers should be tested experimentally with sera of hosts that were challenged with the bites of hematophagous arthropods other than the candidate arthropod species to verify certainly the specificity of the antigen [36], [39] if the occurrence of other hematophagous arthropods cannot be ruled out [35]. An alternative approach to minimize cross reactivity may be the use of peptides instead of an entire recombinant protein. Several peptides deriving from the recombinant Anopheles exposure marker gSG6 were synthetized and one out of five peptides (gSG6-P1) were proven to detect reliably Anopheles exposure [29]. In the latter approach cross reactivity was ruled out by similarity searches of the peptides using GenBank, but peptide specificity was not experimentally verified.
Cross reacting antibodies may be, however, a useful tool if they are elicited by salivary proteins of different triatomine species. Thus, an exposure marker would not be only useful for the detection of a single triatomine species but could be used as a universal marker for the detection of different triatomine species. We tested in Western blots if the 35 kDa candidate marker protein of T. infestans is recognized by IgG antibodies of guinea pigs exposed to Triatoma dimidiata, Triatoma brasiliensis, Triatoma sordida, Triatoma vitticeps, Panstrongylus megistus and Rhodnius prolixus. Our analysis revealed that the candidate marker protein is recognized by sera of guinea pigs exposed to 3 out of 6 triatomine species (T. dimidiata, T. brasiliensis, T. sordida, data not shown). However, its final reactivity and sensitivity need to be confirmed after the candidate salivary protein of T. infestans is identified molecularly, heterologously expressed and the recombinant candidate protein retested with guinea pig sera.
In summary, salivary antigens are increasingly considered as an epidemiological tool to measure the exposure to hematophagous arthropods, however, developmental stage- and strain-specific variations of in the antibody responses of their hosts are often neglected in the development of an exposure marker. Our study revealed a strong variability not only in the salivary protein profiles between the different T. infestans strains but also among the different developmental stages. This variability was reflected in the varying humoral immune response of guinea pigs and resulted in the detection of different salivary antigens depending on the developmental stage and strain of T. infestans. Despite the variability of salivary antigens, an antigen of 35 kDa reacted with almost all guinea pig sera using both IgG and IgM secondary antibodies in immunoassays. This protein may be a useful marker of T. infestans exposure marker in monitoring programs of Chagas disease control campaigns.
Supporting Information
2D salivary profiles and 2D western blot analyses of nymphal and adult T. infestans from Argentina. Saliva of 5th instar nymphs (A) and adults (females and males, E) of Argentinean T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H), the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Argentinean T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
2D salivary profile and western blot analyses of nymphal and adult T. infestans from Chile. Saliva of 5th instar nymphs (5th instar, A) and adults (females and males, E) of Chilean T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H) T. infestans saliva, the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Chilean T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
2D salivary profile and western blot analyses of nymphal and adult T. infestans from Peru. Saliva of 5th instar nymphs (5th instar, A) and adults (females and males, E) Peruvian T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H) T. infestans saliva, the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Peruvian T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
Origin of different Triatoma infestans strains.
(PDF)
Immunogenic salivary antigens of four different T. infestans strains.
(PDF)
Correlation between sum of feeding T. infestans and IgG antibody response of guinea pigs.
(PDF)
Acknowledgments
We thank Christine Krüger, Essen University Hospital, Germany for her training in guinea pig bleeding and Petra Rozkošná for the maintenance of the triatomine colonies. We also want to acknowledge Patrik Kilian's and Petra Rozkošná's assistance in the guinea pig bleedings and the help of Jan Erhart in performing the animal experiments.
Funding Statement
Funding for this study was received from the Grant Agency of the Czech Republic (Grant No. P302/11/P798), the Ministry of Education, Youth and Sports of the Czech Republic (KONTAKT II grant no. LH12002), the Academy of Sciences of the Czech Republic (grant no. Z60220518) and the National Institutes of Health (grant no. 5K01 AI079162-05). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berry AA, Nyunt MM, Plowe CV (2011) Malaria: clinical and epidemiological aspects. In: Kaufmann SHE, Rouse BT, Sacks DL, editors. Immune response to infection. Washington, DC: ASM Press. pp. 633–641. [Google Scholar]
- 2. Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. [DOI] [PubMed] [Google Scholar]
- 3. Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet 379: 461–473. [DOI] [PubMed] [Google Scholar]
- 4. Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 5. Gubler DJ (1998) Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis 4: 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalluri S, Gilruth P, Rogers D, Szczur M (2007) Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: a review. Plos Pathog 3: 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beugnet F, Marie JL (2009) Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol 163: 298–305. [DOI] [PubMed] [Google Scholar]
- 8. Otranto D, Wall R (2008) New strategies for the control of arthropod vectors of disease in dogs and cats. Med Vet Entomol 22: 291–302. [DOI] [PubMed] [Google Scholar]
- 9. Ribeiro JMC (1995) Blood-feeding arthropods - live syringes or invertebrate pharmacologists. Infect Agent Dis 4: 143–152. [PubMed] [Google Scholar]
- 10. Andrade BB, Texeira CR, Barral A, Barral-Netto M (2005) Haematophagous arthropod saliva and host defense system: a tale of tear and blood. An Acad Bras Cienc 77: 665–693. [DOI] [PubMed] [Google Scholar]
- 11. Francischetti IMB, Sá-Nunes A, Mans BJ, Santos IM, Ribeiro JMC (2009) The role of saliva in tick feeding. Front Biosci 14: 2051–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fontaine A, Diouf I, Bakkali N, Missé D, Pagès F, et al. (2011) Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors 4: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz BS, Ribeiro JM, Goldstein MD (1990) Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am J Epidemiol 132: 58–66. [DOI] [PubMed] [Google Scholar]
- 14. Rohoušová I, Ozensoy S, Ozbel Y, Volf P (2005) Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology 130: 493–499. [DOI] [PubMed] [Google Scholar]
- 15. Marzouki S (2011) Characterization of the antibody response to the saliva of Phlebotomus papatasi in people living in endemic areas of cutaneous leishmaniasis. Am J Trop Med Hyg 85: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vlková M, Rohoušová I, Hostomská J, Pohanková L, Zidková L, et al. (2012) Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi . PLoS Negl Trop Dis 6: e1719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Orlandi-Pradines E, Almeras L, de Senneville LD, Barbe S, Remoué F, et al. (2007) Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect 9: 1454–1462. [DOI] [PubMed] [Google Scholar]
- 18. Doucoure S, Mouchet F, Cornelie S, DeHecq JS, Rutee AH, et al. (2012) Evaluation of the human IgG antibody response to Aedes albopictus saliva as a new specific biomarker of exposure to vector bites. PLoS Negl Trop Dis 6: e1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogden NH, Casey ANJ, Lawrie CH, French NP, Woldehiwet Z, et al. (2002) IgG responses to salivary gland extract of Ixodes ricinus ticks vary inversely with resistance in naturally exposed sheep. Med Vet Entomol 16: 186–192. [DOI] [PubMed] [Google Scholar]
- 20. Kashino SS, Resende J, Sacco AM, Rocha C, Proenca L, et al. (2005) Boophilus microplus: the pattern of bovine immunoglobulin isotype responses to high and low tick infestations. Exp Parasitol 110: 12–21. [DOI] [PubMed] [Google Scholar]
- 21. Sanders ML, Glass GE, Scott AL, Schwartz BS (1998) Kinetics and cross-species comparisons of host antibody responses to lone star ticks and American dog ticks (Acari: Ixodidae). J Med Entomol 35: 849–856. [DOI] [PubMed] [Google Scholar]
- 22. Cross ML, Cupp MS, Cupp EW, Galloway AL, Enriquez FJ (1993) Modulation of murine immunological responses by salivary-gland extract of Simulium vittatum (Diptera, Simuliidae). J Med Entomol 30: 928–935. [DOI] [PubMed] [Google Scholar]
- 23. Cross ML, Cupp MS, Cupp EW, Ramberg FB, Enriquez FJ (1993) Antibody-responses of Balb/C mice to salivary antigens of hematophagous black flies (Diptera, Simuliidae). J Med Entomol 30: 725–734. [DOI] [PubMed] [Google Scholar]
- 24. Drame PM, Poinsignon A, Besnard P, Le Mire J, Dos-Santos MA, et al. (2010) Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg 83: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwarz A, Juarez JA, Richards J, Rath B, Machaca VQ, et al. (2011) Anti-triatomine saliva immunoassays for the evaluation of impregnated netting trials against Chagas disease transmission. Int J Parasitol 41: 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gidwani K, Picado A, Rijal S, Singh SP, Roy L, et al. (2011) Serological markers of sand fly exposure to evaluate insecticidal nets against visceral leishmaniasis in India and Nepal: a cluster-randomized trial. PLoS Negl Trop Dis 5: e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barral A, Honda E, Caldas A, Costa J, Vinhas V, et al. (2000) Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg 62: 740–745. [DOI] [PubMed] [Google Scholar]
- 28. Lombardo F, Ronca R, Rizzo C, Mestres-Simon M, Lanfrancotti A, et al. (2009) The Anopheles gambiae salivary protein gSG6: an anopheline-specific protein with a blood-feeding role. Insect Biochem Mol Biol 39: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poinsignon A, Cornelie S, Mestres-Simon M, Lanfrancotti A, Rossignol M, et al. (2008) Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. Plos One 3: e2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poinsignon A, Cornelie S, Ba F, Boulanger D, Sow C, et al. (2009) Human IgG response to a salivary peptide, gSG6-PI, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malaria J 8: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizzo C, Ronca R, Fiorentino G, Verra F, Mangano V, et al. (2011) Humoral response to the Anopheles gambiae salivary protein gSG6: a serological indicator of exposure to Afrotropical malaria vectors. Plos One 6: e17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poinsignon A, Samb B, Doucoure S, Drame PM, Sarr JB, et al. (2010) First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop Med Int Health 15: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 33. Sanders ML, Jaworski DC, Sanchez JL, DeFraites RE, Glass GE, et al. (1998) Antibody to a cDNA-derived calreticulin protein from Amblyomma americanum as a biomarker of tick exposure in humans. Am J Trop Med Hyg 59: 279–285. [DOI] [PubMed] [Google Scholar]
- 34. Alarcon-Chaidez F, Ryan R, Wikel S, Dardick K, Lawler C, et al. (2006) Confirmation of tick bite by detection of antibody to Ixodes calreticulin salivary protein. Clin Vaccine Immunol 13: 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Souza AP, Andrade BB, Aquino D, Entringer P, Miranda JC, et al. (2010) Using recombinant proteins from Lutzomyia longipalpis saliva to estimate human vector exposure in visceral leishmaniasis endemic areas. PLoS Negl Trop Dis 4: e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teixeira C, Gomes R, Collin N, Reynoso D, Jochim R, et al. (2010) Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis 4: e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwarz A, Sternberg JM, Johnston V, Medrano-Mercado N, Anderson JM, et al. (2009) Antibody responses of domestic animals to salivary antigens of Triatoma infestans as biomarkers for low-level infestation of triatomines. Int J Parasitol 39: 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwarz A, Medrano-Mercado N, Billingsley PF, Schaub GA, Sternberg JM (2010) IgM-antibody responses of chickens to salivary antigens of Triatoma infestans as early biomarkers for low-level infestation of triatomines. Int J Parasitol 40: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 39. Schwarz A, Helling S, Collin N, Teixeira CR, Medrano-Mercado N, et al. (2009) Immunogenic salivary proteins of Triatoma infestans: development of a recombinant antigen for the detection of low-level infestation of triatomines. PLoS Negl Trop Dis 3: e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, et al. (2011) Cavia porcellus as a model for experimental infection by Trypanosoma cruzi . Am J Pathol 179: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, et al. (2007) Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology 134: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gürtler RE, Cecere MC, Lauricella IA, Petersen RM, Chuit R, et al. (2005) Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural Northwestern Argentina. Am J Trop Med Hyg 73: 95–103. [PMC free article] [PubMed] [Google Scholar]
- 43. Levy MZ, Bowman NM, Kawai V, Waller LA, Cornejo del Carpio JG, et al. (2006) Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerg Infect Dis 12: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rohoušová I, Volfová V, Nová S, Volf P (2012) Individual variability of salivary gland proteins in three Phlebotomus species. Acta Trop 122: 80–86. [DOI] [PubMed] [Google Scholar]
- 45. Barbosa SE, Diotaiuti L, Braga EM, Pereira MH (2004) Variability of the salivary proteins of 20 Brazilian populations of Panstrongylus megistus (Hemiptera: Reduviidae: Triatominae). Acta Trop 92: 25–33. [DOI] [PubMed] [Google Scholar]
- 46. Barbosa SE, Diotaiuti L, Soares RP, Pereira MH (1999) Differences in saliva composition among three Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae). Acta Trop 72: 91–98. [DOI] [PubMed] [Google Scholar]
- 47. Volf P, Tesarová P, Nohýnkova E (2000) Salivary proteins and glycoproteins in phlebotomine sandflies of various species, sex and age. Med Vet Entomol 14: 251–256. [DOI] [PubMed] [Google Scholar]
- 48. Guarneri AA, Diotaiuti L, Gontijo NF, Gontijo AF, Pereira MH (2003) Blood-feeding performance of nymphs and adults of Triatoma brasiliensis on human hosts. Acta Trop 87: 361–370. [DOI] [PubMed] [Google Scholar]
- 49. Meiser CK, Piechura H, Meyer HE, Warscheid B, Schaub GA, et al. (2010) A salivary serine protease of the haematophagous reduviid Panstrongylus megistus: sequence characterization, expression pattern and characterization of proteolytic activity. Insect molecular biology 19: 409–421. [DOI] [PubMed] [Google Scholar]
- 50. Pavlicek A, Hrda S, Flegr J (1999) Free-Tree–freeware program for construction of phylogenetic trees on the basis of distance data and bootstrap/jackknife analysis of the tree robustness. Application in the RAPD analysis of genus Frenkelia. Folia biologica 45: 97–99. [PubMed] [Google Scholar]
- 51. Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Computer applications in the biosciences : CABIOS 12: 357–358. [DOI] [PubMed] [Google Scholar]
- 52.Team RC (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- 53. Hashimoto K, Yoshioka K (2012) Review: surveillance of Chagas disease. Adv Parasitol 79: 375–428. [DOI] [PubMed] [Google Scholar]
- 54. Yamagata Y, Nakagawa J (2006) Control of Chagas disease. Adv Parasitol 61: 129–165. [DOI] [PubMed] [Google Scholar]
- 55. Abad-Franch F, Santos WS, Schofield CJ (2010) Research needs for Chagas disease prevention. Acta Trop 115: 44–54. [DOI] [PubMed] [Google Scholar]
- 56. Dias JCP, Silveira AC, Schofield CJ (2002) The impact of Chagas disease control in Latin America - a review. Mem Inst Oswaldo Cruz 97: 603–612. [DOI] [PubMed] [Google Scholar]
- 57. Schofield CJ, Jannin J, Salvatella R (2006) The future of Chagas disease control. Trends Parasitol 22: 583–588. [DOI] [PubMed] [Google Scholar]
- 58. Dias JCP (2007) Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusional Chagas disease. Historical aspects, present situation, and perspectives. Mem Inst Oswaldo Cruz 102: 11–18. [DOI] [PubMed] [Google Scholar]
- 59. Lardeux F, Depickere S, Duchon S, Chavez T (2010) Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia. Trop Med Int Health 15: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 60. Picollo MI, Vassena C, Santo Orihuela P, Barrios S, Zaidemberg M, et al. (2005) High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from Northern Argentina. J Med Entomol 42: 637–642. [DOI] [PubMed] [Google Scholar]
- 61. Gürtler RE, Canale DM, Spillmann C, Stariolo R, Salomon OD, et al. (2004) Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull World Health Organ 82: 196–205. [PMC free article] [PubMed] [Google Scholar]
- 62. Cecere MC, Gurtler RE, Canale D, Chuit R, Cohen JE (1997) The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev Panam Salud Publica 1: 273–279. [DOI] [PubMed] [Google Scholar]
- 63. Guhl F, Pinto N, Aguilera G (2009) Sylvatic triatominae: a new challenge in vector control transmission. Mem Inst Oswaldo Cruz 104: 71–75. [DOI] [PubMed] [Google Scholar]
- 64. Gürtler RE (2009) Sustainability of vector control strategies in the Gran Chaco Region: current challenges and possible approaches. Mem Inst Oswaldo Cruz 104: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gürtler RE, Chuit R, Cecere MC, Castanera MB (1995) Detecting domestic vectors of Chagas disease: a comparative trial of six methods in north-west Argentina. Bull World Health Organ 73: 487–494. [PMC free article] [PubMed] [Google Scholar]
- 66. De Marco RJ, Gurtler RE, Salomon OD, Chuit R (1999) Small-scale field trial of a sensing device for detecting peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae) in northwestern Argentina. J Med Entomol 36: 884–887. [DOI] [PubMed] [Google Scholar]
- 67. Vazquez-Prokopec GM, Ceballos LA, Salomon OD, Gurtler RE (2002) Field trials of an improved cost-effective device for detecting peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae) in rural Argentina. Mem Inst Oswaldo Cruz 97: 971–977. [DOI] [PubMed] [Google Scholar]
- 68. Volf P, Grubhoffer L, Hosek P (1993) Characterization of salivary-gland antigens of Triatoma infestans and antigen-specific serum antibody-response in mice exposed to bites of T. infestans . Vet Parasitol 47: 327–337. [DOI] [PubMed] [Google Scholar]
- 69. Nascimento RJ, Santana JM, Lozzi SP, Araujo CN, Teixeira AR (2001) Human IgG1 and IgG4: the main antibodies against Triatoma infestans (Hemiptera: Reduviidae) salivary gland proteins. Am J Trop Med Hyg 65: 219–226. [DOI] [PubMed] [Google Scholar]
- 70. Bayer AM, Hunter GC, Gilman RH, del Carpio JGC, Naquira C, et al. (2009) Chagas disease, migration and community settlement patterns in Arequipa, Peru. PLoS Negl Trop Dis 3: e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Delgado S, Castillo Neyra R, Quispe Machaca VR, Ancca Juarez J, Chou Chu L, et al. (2011) A history of Chagas disease transmission, control, and re-emergence in peri-rural La Joya, Peru. PLoS Negl Trop Dis 5: e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gürtler RE, Chuit R, Cecere MC, Castanera MB, Cohen JE, et al. (1998) Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages in northwest Argentina: environmental, demographic, and entomologic associations. Am J Trop Med Hyg 59: 741–749. [DOI] [PubMed] [Google Scholar]
- 73. Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, et al. (2011) Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl Trop Dis 5: e1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Levy MZ, Quispe-Machaca VR, Ylla-Velasquez JL, Waller LA, Richards JM, et al. (2008) Impregnated netting slows infestation by Triatoma infestans . Am J Trop Med Hyg 79: 528–534. [PMC free article] [PubMed] [Google Scholar]
- 75. Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE (2007) Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci U S A 104: 16194–16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Levy MZ, Small DS, Vilhena DA, Bowman NM, Kawai V, et al. (2011) Retracing micro-epidemics of Chagas disease using epicenter regression. PLoS computational biology 7: e1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Delgado S, Ernst KC, Pumahuanca ML, Yool SR, Comrie AC, et al. (2013) A country bug in the city: urban infestation by the Chagas disease vector Triatoma infestans in Arequipa, Peru. International journal of health geographics 12: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D salivary profiles and 2D western blot analyses of nymphal and adult T. infestans from Argentina. Saliva of 5th instar nymphs (A) and adults (females and males, E) of Argentinean T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H), the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Argentinean T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
2D salivary profile and western blot analyses of nymphal and adult T. infestans from Chile. Saliva of 5th instar nymphs (5th instar, A) and adults (females and males, E) of Chilean T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H) T. infestans saliva, the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Chilean T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
2D salivary profile and western blot analyses of nymphal and adult T. infestans from Peru. Saliva of 5th instar nymphs (5th instar, A) and adults (females and males, E) Peruvian T. infestans were isoelectrically focused in the nonlinear pH range of 3–11. Black circles indicate nymphal (A) or adult (E) specific salivary proteins. In order to improve the protein separation, saliva of nymphs (B) and adults (F) were isoelectrically focused in the nonlinear pH range of 3–5.6 and blotted onto nitrocellulose. Comparing IgG (C, G) and IgM antibody reactions (D, H) with salivary proteins of nymphs (C, D) and adults (G, H) T. infestans saliva, the candidate exposure marker antigen of 35 kDa was recognized by IgG and IgM antibodies of guinea pig serum from the 5th week of exposure to the Peruvian T. infestans strain. This protein is marked with a red circle in the different panels.
(PDF)
Origin of different Triatoma infestans strains.
(PDF)
Immunogenic salivary antigens of four different T. infestans strains.
(PDF)
Correlation between sum of feeding T. infestans and IgG antibody response of guinea pigs.
(PDF)