Abstract
Repeated exposure to drugs of abuse produces forms of experience-dependent plasticity including behavioral sensitization. Although a single exposure to many addicting substances elicits locomotor sensitization, there is little information regarding the motivational effects of such single exposures. This study demonstrates that a single cocaine exposure enhances both rewarding and aversive forms of opioid place conditioning. Rats were given a single injection of cocaine (15 mg/kg i.p.) in their home cage at different times before conditioning. This treatment enhanced conditioned place preference (CPP) to morphine (2 × 10 mg/kg s.c.) if training began 1 or 5 but not 10 days after the cocaine injection. A single cocaine exposure also enhanced conditioned place aversion (CPA) to the κ-opioid receptor agonist U69593 (2 × 0.16 mg/kg s.c.). Compared to morphine CPP, U69593 CPA was delayed and persistent. It was not observed at 1 day but appeared if the conditioning began 5 or 10 days after the cocaine injection. Although the cocaine-induced enhancements of both morphine CPP and U69593 CPA followed different time courses, suggesting different mechanisms, both effects were blocked by injection of the N-methyl-d-aspartate receptor antagonist MK-801 (0.5 nmol bilaterally) into the ventral tegmental area, immediately before the cocaine injection. Thus, through a circuit involving the ventral tegmental area, a single cocaine exposure enhanced both μ-opioid receptor reward and κ-opioid receptor aversion.
Chronic exposure to drugs of abuse produces enduring changes in neural circuits that underlie the addictive process. One consequence of this experience-dependent plasticity is a progressive increase in drug response on reexposure to the drug. Termed drug sensitization, the phenomenon is typically assessed as an enhancement in locomotor activity. Robust locomotor sensitization can be produced by psychostimulants (1), opiates (2), or ethanol (3), and cross-sensitization can occur between different drugs (4, 5).
Whereas early studies of sensitization focused specifically on the progressive increase in locomotor activity (1), many researchers have since proposed that sensitization contributes to drug reward, and this process has been incorporated into several influential theoretical models of drug addiction. For example, sensitization processes have been proposed to contribute to impulsivity (6) or changes in incentive-salience state for cues associated with the drug (7). These proposals are supported by the observation that drug self-administration is enhanced after repeated drug administrations. For example, monkeys previously exposed to methamphetamine initiated self-administration to the stimulant at lower doses than drug-naïve subjects (8). Moreover, preexposure to amphetamine was sufficient to turn initial nonresponders into reliable self-administering rats (9). Finally, rats previously exposed to amphetamine exhibited higher break points than untreated rats to obtain the drug on a progressive ratio schedule of reinforcement (10). Although these and many other preclinical studies have established an important role for the sensitization process in drug addiction, direct investigation of the process in human addicts is made difficult by technical limitations. Nevertheless, there are anecdotal clinical observations and some controlled studies that suggest that a sensitization-like process can be produced by psychostimulants in human subjects (11).
Although sensitization to drug reward is usually assessed after repeated drug treatment, even a single psychostimulant exposure can induce locomotor sensitization (12–16). Moreover, this single drug exposure-induced locomotor sensitization is associated with changes in neural circuits that underlie reward and motivation. For example, locomotor sensitization induced by a single cocaine exposure is matched by transient changes in glutamate receptor function in putative dopaminergic neurons in the ventral tegmental area (VTA) (16). A variety of drugs of abuse produce this type of change with a single exposure (17). The simplicity of the manipulation and the robust behavioral change produced by single psychostimulant exposure make it an attractive paradigm for exploring the relationship of sensitization to other reward-related behaviors.
One well studied reward-related behavior is the place conditioning paradigm. This paradigm offers advantages for understanding the contribution of the processes underlying sensitization to motivation and reward (18). Importantly, subjects are tested in a drug-free state, and the model can reveal both reward and aversion. These considerations are important for understanding how repeated drug administration affects motivational systems. In the current experiments, we chose to study two opioid peptides that characteristically have opposing motivational actions. μ-Opioid receptor (MOR) agonists, such as morphine, produce conditioned place preference (CPP), whereas selective κ-opioid receptor (KOR) agonists, such as U69593, produce a conditioned place aversion (CPA) (19).
Although there are some differences in the sites of action for these two opioid effects, the VTA is a critical locus for dopaminergic neurons implicated in both preference and aversion. For example, both MOR-mediated preference and KOR-mediated aversion can be elicited by direct injection of receptor selective ligands into the VTA (20), and the acquisition of both morphine CPP and U69593 CPA are blocked by 6-hydroxydopamine (6-OHDA) lesions or microinjection of D-1 dopamine antagonists into the nucleus accumbens (21). In agreement with drug self-administration studies, the place conditioning paradigm has been used to provide evidence supporting sensitization of reward after repeated drug administration (22–25). However, whether a single exposure to cocaine can produce cross-sensitization of morphine CPP has not been examined. Furthermore, little is known about whether manipulations producing sensitization can enhance place aversion.
In the present study, we examined (i) the hypothesis that a single cocaine treatment augments drug-cue associations formed in place conditioning for both the rewarding MOR agonist morphine and the aversive KOR agonist U69593, (ii) the time course for this sensitization of place conditioning, and (iii) the role of the VTA in this process.
Methods
Subjects. Male Sprague–Dawley rats (Charles River Breeding Laboratories) weighing between 250 and 300 g were housed individually in a colony room maintained on a 12L:12D cycle (lights on at 6 a.m.). All experiments occurred during the light portion of the cycle. Food and water were available ad libitum.
Place Conditioning. Animals were trained in one of four identical three-chamber place conditioning boxes (Med Associates). Two distinct environments (28 × 21 × 21 cm) that differed in color, lighting, and floor texture were separated by a smaller central neutral gray environment (12 × 21 × 21 cm). During an initial baseline test, rats were placed in the center room and allowed to freely explore the apparatus for 30 min. Total times spent in each of the environments were automatically recorded by infrared beam breaks. After the baseline test, rats were randomly assigned to groups for pretreatment before place conditioning (see below). Depending on the experiment, different protocols were used for place conditioning. Rats had conditioning sessions over 2–4 training days. During each training day, a rat was conditioned with one drug–environment and one vehicle–environment pairing separated by at least 5 h. During each pairing, a rat was injected with drug or vehicle and immediately confined to one environment for 30 min. Groups were counterbalanced for drug order (morning, 9 a.m., or afternoon, 2 p.m.), drug side, and drug box assignment. No significant differences were observed between groups for any of these variables. One day after the final training session, a 30-min postconditioning test was run in the same manner as the initial baseline test.
Cocaine Pretreatment at Various Times Before Beginning Place Conditioning. After a baseline preference test, separate groups of rats were pretreated in their home cage with a single injection of cocaine (15 mg/kg i.p., volume 1 ml/kg) 1, 5, or 10 days before place conditioning training. A control group was injected with an equivalent volume of saline 1 day before training.
VTA Microinjection Studies. In microinjection studies, MK-801 (0.5 nmol in 0.5 μl bilaterally) or vehicle was administered into the VTA (or into a lateral off-site control region) 2 min before the systemic cocaine injection. These experiments were done to examine the effect of N-methyl-d-aspartate receptor blockade on cocaine enhancement of opioid place conditioning. In the controls for studies examining the effect of intra-VTA MK-801 microinjections on place conditioning in the absence of cocaine, a 4-day training protocol was used to match the magnitude of place conditioning observed with cocaine pretreatment.
Surgical Procedures. Animals were anesthetized with isoflurane vaporized in O2 at a flow rate of 1.0 liter/min and placed in a stereotaxic frame. The scalp was shaved and scrubbed with a betadine/2% H2O2 solution, and a midline incision was made. The skin was retracted, and four holes were drilled for implantation of sterile miniature skull screws. Separate holes were then drilled bilaterally for microinjection guide cannula. Guide cannulae were stereotaxically aimed at the VTA (anterioposterior, –5.5; lateral, 1.0; vertical, –6.5) or off-site control (anterioposterior, –5.5; lateral, 3.8; vertical, –5.50). Cannulae were fitted with dummy probes to prevent obstruction and contamination and secured with dental cement. The wound was then treated with 2% bacitracin and 2% xylocaine topical ointments and sutured closed with 3-0 vicryl sutures.
Microinjection Procedures. Obturators were removed, and drug injections were made by using a 33-gauge stainless-steel hypodermic tubing lowered to 2 mm below the end of guide cannulae. A total volume of 0.5 μl per side was delivered over 2 min. Injectors were held in place for an additional 60 s to allow diffusion of the drug into the surrounding brain tissue.
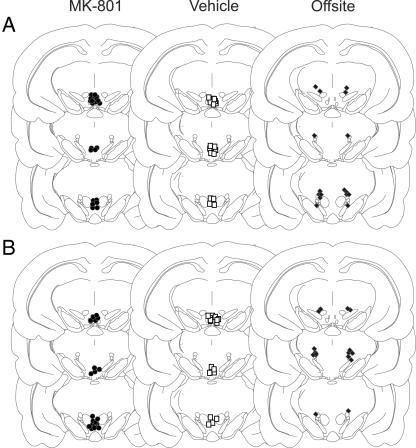
Histology. Cannulae placements were verified after completion of behavioral testing (Fig. 1). Rats were deeply anesthetized and perfused. VTA sections were stained with neutral red to verify cannula placement. In some cases, alternate VTA sections were stained with tyrosine hydroxylase antibody.
Fig. 1.
Localization of cannula tips aimed at the VTA or a lateral off-site control for morphine place conditioning (A) and U69593 place conditioning (B). (Left) MK-801 injection sites aimed at the VTA (•). (Center) Vehicle injection sites aimed at the VTA (□). (Right) MK-801 injection sites aimed at a lateral off-site control region (♦). Reconstructions are based on the stereotaxic atlas of Paxinos and Watson (46). Slices shown (top to bottom) are 5.20, 5.60, and 5.80 mm posterior to Bregma.
Drugs. Cocaine was prepared in saline and injected at a dose of 15 mg/kg i.p. Place conditioning was induced with morphine or U69593. Morphine was prepared in saline and injected at a dose of 10 mg/kg. U69593 (Research Biochemicals, Natick, MA) was prepared in a solution of 20% propylene glycol in saline and injected at a dose of 0.16 mg/kg. These doses were similar to those used in previous studies examining opioid place conditioning (5, 26). Conditioning drugs or their vehicles were administered s.c. in a volume of 1 ml/kg, immediately before placement in a conditioning environment. MK-801 was prepared in sterile water, and 0.5 nmol in 0.5 μl (per side) was microinjected directly into the VTA. This dose is similar to that shown in a previous study to block sensitization by intra-VTA MK-801 (15).
Data Analysis. A single-factor ANOVA followed by Duncan's test was used to determine the significance of differences between various treatments on place conditioning. Significance for all tests was set at P < 0.05. Data were calculated by subtracting the total time spent in the vehicle chamber from the total time spent in the drug chamber on the test day after conditioning. Therefore, a positive conditioning score reflects CPP, and a negative conditioning score indicates CPA. Data are as mean conditioning score (in seconds) ±1 SEM.
Results
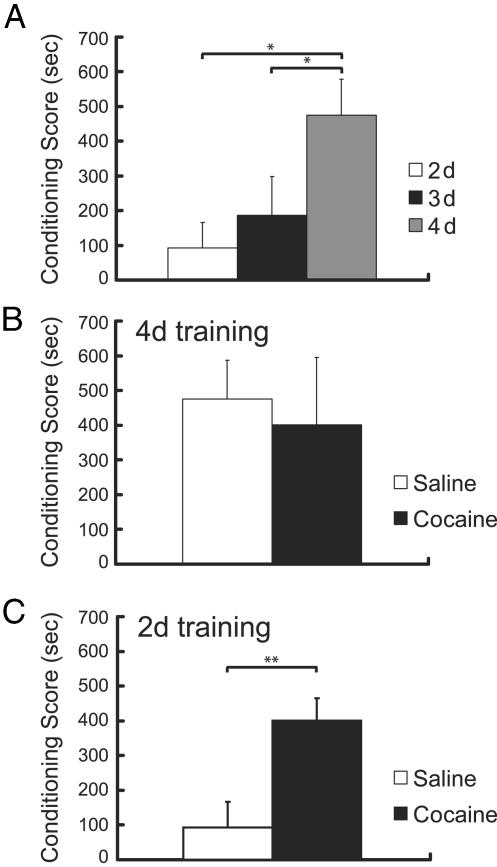
Magnitude of Place Conditioning Is Dependent on the Number of Drug–Environment Pairings. Across experiments, baseline measures for the mean time spent in the drug- and vehicle-paired environments were 662.52 ± 11.58 s and 650.53 ± 11.94 s, respectively, confirming that an unbiased place conditioning procedure was used. In cocaine-naïve rats, the magnitude of CPP was dependent on the number of drug–environment pairings [F(2,25) = 4.08; P < 0.05]. As illustrated in Fig. 2A, rats receiving four morphine–environment pairings demonstrated significantly greater preference for the morphine-paired environment than rats conditioned with only two or three drug–environment pairings (P < 0.05).
Fig. 2.
(A) In cocaine-naïve rats, there was a significant effect of number of drug–environment pairings on the magnitude of place conditioning. Rats receiving four drug–environment pairings (gray) demonstrated greater preference for the morphine-paired environment than rats that had been conditioned with only two (white) or three (black) drug–environment pairings (n = 8–12 per group). (B) Place conditioning induced by four drug–environment pairings was unaffected by pretreatment with a single cocaine exposure, indicating a ceiling effect on the conditioned responding (n = 7 per group). (C) A single cocaine injection enhanced submaximal place conditioning induced with two drug–environment pairings (n = 12–13 per group). *, P < 0.05; **, P < 0.01.
A Single Cocaine Pretreatment Enhances Morphine-Induced CPP. To determine whether a single cocaine exposure impacts subsequent place conditioning, we tested the effect of a single cocaine injection given in the home cage 1 day before initiating morphine CPP training with four drug–environment pairings. When using this paradigm, the magnitude of CPP was unaffected by the cocaine pretreatment [Fig. 2B; F(1,12) = 0.1, ns]. To examine the possibility that this result was due to a ceiling effect, we repeated the experiment with only two morphine training trials so that rats not pretreated with cocaine would have lower levels of CPP. Using this protocol, we were able to demonstrate a robust and significant enhancement of CPP by cocaine compared to vehicle treatment 1 day before initiating the 2-day place preference training protocol [Fig. 2C; F(1,23) = 10.05, P < 0.01 compared to saline-pretreated rats].
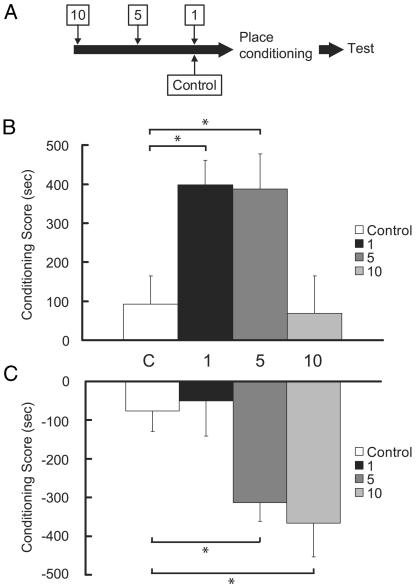
Cocaine-Induced Enhancement of Morphine CPP Is Transient. The synaptic changes observed in the VTA after a single cocaine injection are present at 1 and 5 days but not at 10 days after cocaine injection (16). We examined whether a similar time course occurs for the enhancement of morphine CPP. Separate groups of rats began morphine place conditioning 1, 5, or 10 days after a single cocaine injection (Fig. 3A). We observed a significant time-dependent effect on the sensitization of morphine reward [Fig. 3B; F(3,42) = 5.02, P < 0.01]. Pair-wise comparisons confirmed that rats which began CPP training 1 or 5 days after the cocaine injection had significantly greater conditioning scores than did rats which began training 10 days post-cocaine or were injected with saline (control) before place conditioning (P < 0.05). Thus, the time course for sensitization of morphine reward induced by a single cocaine injection parallels the changes in glutamate receptor function observed in the VTA.
Fig. 3.
(A) Separate groups of rats began opioid place conditioning with two drug–environment pairings 1, 5, or 10 days after a single cocaine injection. (B) A single cocaine injection induced a transient time-dependent effect on the magnitude of morphine CPP. Rats that began training 1 (black) or 5 (dark gray) days after the cocaine injection had significantly greater conditioning scores than did rats that began training 10 (light gray) days post-cocaine or were injected with saline (control, white) before place conditioning (n = 9–13 per group). (C) In contrast to its effect on morphine CPP, sensitization of U69593 CPA was delayed and prolonged. The ability of a single cocaine injection to augment the induction of a U69593 CPA was not observed at 1 day (black) but appeared if the conditioning began 5 (dark gray) or 10 (light gray) days after the cocaine injection (n = 10–11 per group). *, P < 0.05.
A Single Cocaine Pretreatment also Enhances U69593 CPA but with a Different Time Course than That for Morphine CPP. In common with morphine place preference, CPA to KOR agonists seems to be a dopamine-dependent process (21). Therefore, we examined whether CPA to the KOR agonist U69593 is also modulated by previous cocaine exposure. By using a similar submaximal conditioning paradigm (i.e., two drug–environment pairings), separate groups of rats began U69593 CPA training 1, 5, or 10 days after a single cocaine injection. The magnitude of the CPA to U69593 was significantly greater in rats that had received a single cocaine injection compared to preconditioning baseline [F(3,39) = 4.86, P < 0.01]. However, in contrast to its effect on morphine CPP, the enhancement of U69593 CPA by a single cocaine injection was delayed and prolonged (Fig 3C). It was not observed at 1 day but appeared if the conditioning began 5 days after the cocaine injection (significantly different from rats pretreated with saline or cocaine 1 day before conditioning, P < 0.05) and was still present if U69593 training began 10 days after cocaine exposure (significantly different from rats pretreated with saline or cocaine 1 day before conditioning, P < 0.05).
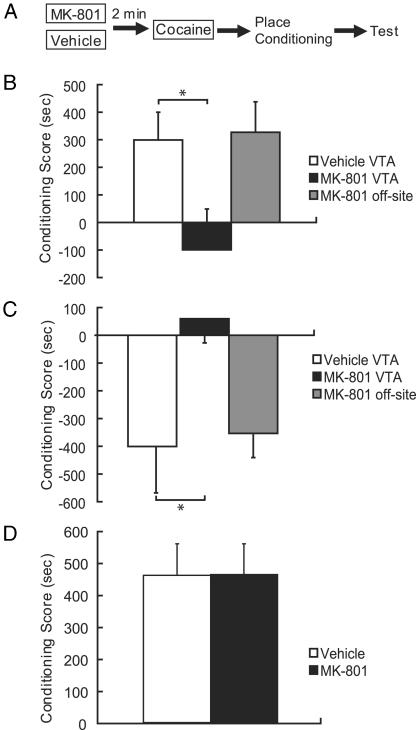
An Intra-VTA MK-801 Injection Blocks Single Cocaine-Induced Enhancement of both Morphine CPP and U69593 CPA. The VTA is a key site for induction of drug sensitization, and its dopaminergic projection to the nucleus accumbens is necessary for both morphine CPP and KOR CPA. Thus, we investigated the effect of MK-801 injected directly into the VTA immediately before cocaine treatment (Fig. 4A). Separate groups of rats were pretreated with intra-VTA MK-801 2 min before systemic cocaine. Based on the time course for enhancement of place conditioning (Fig. 3 B and C), rats began training for either morphine CPP (1 day later) or U69593 CPA (5 days later) after pretreatment. Intra-VTA MK-801 completely blocked the cocaine-induced enhancement of preference and aversion. The sensitized CPP response to morphine that was evident in rats injected with intra-VTA vehicle before cocaine was completely blocked in rats injected with intra-VTA MK-801 before cocaine [Fig. 4B; F(2,25) = 3.80, P < 0.05]. Similarly, rats injected with intra-VTA vehicle before systemic cocaine demonstrated significantly greater aversion than rats who were injected with intra-VTA MK-801 2 min before the cocaine injection [Fig. 4C; F(1,20) = 5.95, P < 0.05]. Animals injected with MK-801 into an area lateral to the VTA were similar to vehicle-injected animals and showed no reduction in either the cocaine-enhanced preference to morphine or aversion to U69593 (Fig. 4 B and C, ns).
Fig. 4.
(A) Separate groups of rats were pretreated with intra-VTA MK-801 or vehicle 2 min before systemic cocaine. Rats began place conditioning with two drug–environment pairings for either morphine (1 day later) or U69593 (5 days later) after pretreatment. (B) The enhanced CPP response to morphine that was evident in rats injected with intra-VTA vehicle (white bars) before cocaine was blocked in rats injected with intra-VTA MK-801 (black bars; n = 8–10 per group). (C) Similarly, this MK-801 pretreatment blocked the cocaine-induced sensitization of KOR place aversion (n = 8–11 per group). (D) Intra-VTA injections of MK-801 or vehicle alone did not alter the induction of a morphine CPP in the absence of cocaine, suggesting that the blockade of CPP and CPA produced by MK-801 was specific to the process underlying the cocaine-sensitizing effect (n = 6 per group). *, P < 0.05.
It is important to acknowledge that rather than selectively blocking cocaine-induced sensitization of a conditioned response, the effects of intra-VTA MK-801 treatment could have been the result of a more general deficit in learning. To address this potential confound, we tested whether MK-801 treatment could impair subsequent opioid place conditioning independent of the cocaine effect. However, because only a minimal place conditioning effect is induced with two drug–environment pairings (Fig. 2 A), our ability to observe a deficit produced by MK-801 would be limited by a potential floor effect. Therefore, we tested the effect of intra-VTA MK-801 treatment on CPP induced with four drug–environment pairings, which, as shown in Fig. 2 A, produces a large CPP in the absence of cocaine. To further increase the sensitivity of our protocol to a cocaine-independent MK-801 effect, the antagonist was administered twice: before the first and the third conditioning days. Conditioning scores on test day were similar in all rats whether they were pretreated with intra-VTA vehicle or MK-801 before the first and third conditioning days [Fig. 4D; F(1,10), ns]. This finding supports the conclusion that the reduction of place conditioning by MK-801 depends on a process initiated by cocaine and is not due to some delayed effect of MK-801 on place conditioning.
Discussion
Previous studies have demonstrated that sensitization induced by repeated drug injections is associated with enhanced self-administration of rewarding drugs (8–10). These studies offer face validity to the relevance of sensitization processes to drug addiction. However, changes in drug self-administration behavior that follow repeated drug administration are complicated by the acute CNS effects of the self-administered drugs during testing, and the confound that increased drug intake could represent either an enhanced or reduced level of drug elicited reward (27). Furthermore, drug self-administration studies may be insensitive to treatments that concomitantly induce or enhance opposing motivational changes. Our findings demonstrate that a single cocaine exposure produces changes that have profound bidirectional effects on subsequent opioid reward as assayed with the place conditioning paradigm. Under conditions that produced submaximal place conditioning in control rats, a single cocaine exposure enhanced morphine CPP. This finding confirms and extends those of Shippenberg et al. (5), who showed that repeated cocaine administration enhances morphine CPP. Our findings demonstrate that this sensitized response can be induced by a single cocaine exposure in a transient manner through a process requiring the VTA. We also show that a single cocaine treatment enhances CPA elicited by the KOR agonist, U69593. It is interesting that a MOR-mediated preference and a KOR-mediated aversion can also be enhanced by a single exposure to restraint stress (28). In contrast to morphine CPP, the enhanced U69593 CPA was delayed and prolonged; it was absent at 1 day but was present when CPA training began 5 or 10 days after cocaine. In common with the enhanced morphine CPP, the induction of the enhanced CPA was VTA-dependent.
The different time course for the enhancement of morphine CPP and U69593 CPA suggests that they have distinct mechanisms. The transient time course for induction of the augmented morphine CPP paralleled the time course observed for the change in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/N-methyl-d-aspartate receptor ratio recorded within the VTA after a single cocaine injection (16). Such synaptic changes could contribute to an enhanced excitability of dopamine neurons in the VTA and, in turn, may account for the observed increases in dopamine release that correlate with psychostimulant sensitization. Our findings are consistent with the view that enhanced preference for a morphine-paired environment after a single exposure to cocaine is the result of increased dopamine release. In fact, MOR agonists applied in the VTA increase dopamine release in the accumbens (29) presumably by disinhibiting dopamine neurons (30). Additionally, MOR agonists produce CPP when injected directly into the VTA (20, 31), an effect that can be blocked by 6-hydroxy-dopamine lesions in the accumbens (21). Thus, although other explanations are possible, several lines of investigation, including the current study, are consistent with the view that cocaine enhancement of morphine CPP is the result of morphine causing a greater activation of VTA dopaminergic neurons in sensitized animals.
Although the mesolimbic dopamine system is often implicated in reward, there is evidence that the same system is also involved in mediating behavioral responses to aversive stimuli. Acute exposure to aversive stimuli activates putative dopaminergic midbrain neurons and increases dopamine release in the accumbens (32). Additionally, rats trained to avoid footshock display elevated levels of extracellular dopamine in the accumbens during the instrumental response (33). This avoidance response was reportedly reduced by 6-hydroxy-dopamine lesions of the accumbens. Some studies have directly shown a dual role for dopaminergic systems in both reward and aversion. For example, a selective D1 dopamine antagonist microinjected into the nucleus accumbens impairs the acquisition of both a morphine CPP and a U69593 CPA (21). In addition, studies with dopamine D2L receptor knockout mice have shown impaired acquisition of both morphine CPP and morphine-induced withdrawal CPA (34). Thus, under certain conditions, mesolimbic dopaminergic systems engaged by drugs of abuse for reward learning are also required for aversive learning.
In contrast to sensitization of morphine reward, cocaine's enhancement of U69593 CPA did not emerge until 5 days after the cocaine injection. This delayed onset may reflect a gradual increase in KOR function or in levels of the endogenous KOR agonist dynorphin. Such changes have been observed after repeated exposure to cocaine (35, 36) and may serve as a regulatory adaptation to psychostimulant challenge. Indeed, administration of the endogenous KOR agonist dynorphin A (1–17) attenuates the rewarding effect of cocaine (37). One possible target for this altered KOR signaling could be VTA dopamine neurons, which are hyperpolarized by U69593 (38).
That cocaine exposure activates neural systems that can enhance both reward and aversion is consistent with observations that, under some circumstances, cocaine can produce CPA (39, 40). Furthermore, rats show a preference for an environment paired with the immediate onset of cocaine (1.0 mg/kg i.v.) but show an aversion to the same environment if there is a delay of 15 min before they are placed in that environment (41). These opposing reward and aversion systems engaged by cocaine administration appear to be active concomitantly. This idea follows from studies in which rats are trained to run a straight alley to a goal box for cocaine. With repeated administration of cocaine, rats take progressively longer to enter the goal box where they receive the cocaine. In fact, rats display a progressive “approach–avoidance” pattern of running in which they run up to the verge of the goal box but retreat toward the start box instead of entering it. They may repeat this approach–retreat behavior several times before finally entering to receive the reward. This behavior is similar to that observed in rats approaching a goal box in which they have concurrently received food and shock (42). Together with the current observations, these studies indicate that cocaine concomitantly activates neural circuits producing both appetitive and aversive behaviors.
Although the enhancement of CPP and CPA followed different time courses, both processes reflect drug–experience-dependent plasticity. Several lines of investigation implicate the VTA as a key site for induction of drug sensitization. First, acute exposure to drugs of abuse induces dopamine release in VTA target areas, an effect that is enhanced with repeated drug administration (43). Second, locomotor sensitization can be induced by injecting psychostimulants (amphetamine or cocaine) or the selective dopamine reuptake inhibitor GBR-12909 directly into the VTA (44). Third, administration of an N-methyl-d-aspartate receptor antagonist (MK-801) directly into the VTA during cocaine pretreatment blocks the induction of locomotor sensitization (15). Intriguingly, in the present studies, both the sensitized preference and aversion effects were blocked by microinjection of an N-methyl-d-aspartate receptor antagonist into the VTA immediately before cocaine administration. Importantly, this N-methyl-d-aspartate receptor antagonist blockade was site-specific to the VTA because MK-801 injected into a lateral off-site control did not block the enhancement of place conditioning by cocaine. Furthermore, although it has been shown that glutamate antagonists microinjected into the VTA block the acquisition of morphine CPP (45), we show that MK-801 injection 24 h before training has no lingering effect on CPP. Thus, a manipulation that blocks the induction of behavioral and neural components of drug sensitization also blocks single cocaine exposure-induced enhancement of opioid place conditioning.
In summary, a single cocaine injection induces processes that enhance both an MOR place preference and a KOR place aversion. The enhanced CPP had a time course that parallels observed changes in synaptic function in the VTA, consistent with increased dopaminergic neuron firing (16). This transient effect may contribute to the enhancement of morphine-cue associations. However, the enhanced place aversion was not apparent until at least 5 days after the cocaine injection. We suggest that this effect is due to a delayed and prolonged change in endogenous KOR systems that enhances the aversive effect of systemically administered KOR agonists. This MOR opposing action of the KOR system could be considered a compensatory effect that serves to regulate the addictive power of psychostimulant drugs.
Acknowledgments
This work was supported by State of California funds for medical research on alcohol and substance abuse through the University of California, by National Institute on Drug Abuse Grants 01949 (to H.L.F.) and 015686 (to G.O.H.) and by DAMD17-03-1-0059 awarded by the U.S. Army Medical Research Acquisition Activity.
Abbreviations: CPA, conditioned place aversion; CPP, conditioned place preference; KOR, κ-opioid receptor; MOR, μ-opioid receptor; VTA, ventral tegmental area.
References
- 1.Post, R. M. & Rose, H. (1976) Nature 260, 731–732. [DOI] [PubMed] [Google Scholar]
- 2.Babbini, M. & Davis, W. M. (1972) Br. J. Pharmacol. 46, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masur, J. & Boerngen, R. (1980) Pharmacol. Biochem. Behav. 13, 777–780. [DOI] [PubMed] [Google Scholar]
- 4.Bonate, P. L., Swann, A. & Silverman, P. B. (1997) Life Sci. 60, 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Shippenberg, T. S., LeFevour, A. & Thompson, A. C. (1998) Eur. J. Pharmacol. 345, 27–34. [DOI] [PubMed] [Google Scholar]
- 6.Koob, G. F. & Le Moal, M. (2001) Neuropsychopharmacology 24, 97–129. [DOI] [PubMed] [Google Scholar]
- 7.Robinson, T. E. & Berridge, K. C. (1993) Brain Res. Rev. 18, 247–291. [DOI] [PubMed] [Google Scholar]
- 8.Woolverton, W. L., Cervo, L. & Johanson, C. E. (1984) Pharmacol. Biochem. Behav. 21, 737–741. [DOI] [PubMed] [Google Scholar]
- 9.Piazza, P. V., Deminiere, J. M., le Moal, M. & Simon, H. (1990) Brain Res. 514, 22–26. [DOI] [PubMed] [Google Scholar]
- 10.Vezina, P., Lorrain, D. S., Arnold, G. M., Austin, J. D. & Suto, N. (2002) J. Neurosci. 22, 4654–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sax, K. W. & Strakowski, S. M. (2001) J. Addict. Dis. 20, 55–65. [DOI] [PubMed] [Google Scholar]
- 12.Post, R. M. & Weiss, S. R. (1988) NIDA Res. Monogr. 88, 217–238. [PubMed] [Google Scholar]
- 13.Vanderschuren, L. J., De Vries, T. J., Wardeh, G., Hogenboom, F. A. & Schoffelmeer, A. N. (2001) Eur. J. Neurosci. 14, 1533–1538. [DOI] [PubMed] [Google Scholar]
- 14.Vanderschuren, L. J., Schmidt, E. D., De Vries, T. J., Van Moorsel, C. A., Tilders, F. J. & Schoffelmeer, A. N. (1999) J. Neurosci. 19, 9579–9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alesdatter, J. E. & Kalivas, P. W. (1993) Behav. Pharmacol. 4, 645–651. [PubMed] [Google Scholar]
- 16.Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. (2001) Nature 411, 583–587. [DOI] [PubMed] [Google Scholar]
- 17.Saal, D., Dong, Y., Bonci, A. & Malenka, R. C. (2003) Neuron 37, 577–582. [DOI] [PubMed] [Google Scholar]
- 18.Bardo, M. T. & Bevins, R. A. (2000) Psychopharmacology 153, 31–43. [DOI] [PubMed] [Google Scholar]
- 19.Mucha, R. F. & Herz, A. (1985) Psychopharmacology 86, 274–280. [DOI] [PubMed] [Google Scholar]
- 20.Bals-Kubik, R., Ableitner, A., Herz, A. & Shippenberg, T. S. (1993) J. Pharmacol. Exp. Ther. 264, 489–495. [PubMed] [Google Scholar]
- 21.Shippenberg, T. S., Bals-Kubik, R. & Herz, A. (1993) J. Pharmacol. Exp. Ther. 265, 53–59. [PubMed] [Google Scholar]
- 22.Shippenberg, T. S. & Heidbreder, C. (1995) J. Pharmacol. Exp. Ther. 273, 808–815. [PubMed] [Google Scholar]
- 23.Shippenberg, T. S., Heidbreder, C. & Lefevour, A. (1996) Eur. J. Pharmacol. 299, 33–39. [DOI] [PubMed] [Google Scholar]
- 24.Gaiardi, M., Bartoletti, M., Bacchi, A., Gubellini, C., Costa, M. & Babbini, M. (1991) Psychopharmacology 103, 183–186. [DOI] [PubMed] [Google Scholar]
- 25.Lett, B. T. (1989) Psychopharmacology 98, 357–362. [DOI] [PubMed] [Google Scholar]
- 26.Mueller, D., Perdikaris, D. & Stewart, J. (2002) Behav. Brain Res. 136, 389–397. [DOI] [PubMed] [Google Scholar]
- 27.Schenk, S., Partridge, B. & Shippenberg, T. S. (2001) Neuropsychopharmacology 24, 441–450. [DOI] [PubMed] [Google Scholar]
- 28.del Rosario Capriles, N. & Cancela, L. M. (2002) Behav. Brain Res. 132, 159–169. [DOI] [PubMed] [Google Scholar]
- 29.Spanagel, R., Herz, A. & Shippenberg, T. S. (1992) Proc. Natl. Acad. Sci. USA 89, 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, S. W. & North, R. A. (1992) J. Neurosci. 12, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nader, K. & van der Kooy, D. (1997) J. Neurosci. 17, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, C. W., Sullivan, R. M. & Gratton, A. (2003) Neuroscience 116, 285–293. [DOI] [PubMed] [Google Scholar]
- 33.McCullough, L. D., Sokolowski, J. D. & Salamone, J. D. (1993) Neuroscience 52, 919–925. [DOI] [PubMed] [Google Scholar]
- 34.Smith, J. W., Fetsko, L. A., Xu, R. & Wang, Y. (2002) Neuroscience 113, 755–765. [DOI] [PubMed] [Google Scholar]
- 35.Spangler, R., Unterwald, E. M. & Kreek, M. J. (1993) Mol. Brain Res. 19, 323–327. [DOI] [PubMed] [Google Scholar]
- 36.Hurd, Y. L. & Herkenham, M. (1992) Mol. Brain Res. 16, 97–104. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y., Butelman, E. R., Schlussman, S. D., Ho, A. & Kreek, M. J. (2004) Psychopharmacology, in press.
- 38.Margolis, E. B., Hjelmstad, G. O., Bonci, A. & Fields, H. L. (2003) J. Neurosci. 23, 9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlezon, W. A., Jr., Thome, J., Olson, V. G., Lane-Ladd, S. B., Brodkin, E. S., Hiroi, N., Duman, R. S., Neve, R. L. & Nestler, E. J. (1998) Science 282, 2272–2275. [DOI] [PubMed] [Google Scholar]
- 40.Pliakas, A. M., Carlson, R. R., Neve, R. L., Konradi, C., Nestler, E. J. & Carlezon, W. A., Jr. (2001) J. Neurosci. 21, 7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ettenberg, A., Raven, M. A., Danluck, D. A. & Necessary, B. D. (1999) Pharmacol. Biochem. Behav. 64, 507–512. [DOI] [PubMed] [Google Scholar]
- 42.Geist, T. D. & Ettenberg, A. (1997) Pharmacol. Biochem. Behav. 57, 145–150. [DOI] [PubMed] [Google Scholar]
- 43.Kalivas, P. W. & Stewart, J. (1991) Brain Res. Rev. 16, 223–244. [DOI] [PubMed] [Google Scholar]
- 44.Cornish, J. L. & Kalivas, P. W. (2001) Behav. Brain Res. 126, 205–209. [DOI] [PubMed] [Google Scholar]
- 45.Harris, G. C. & Aston-Jones, G. (2003) Neuropsychopharmacology 28, 73–76. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos, G. & Watson, C. (1998) The Rat Brain in Stereotaxic Coordinates (Academic, San Diego), 4th Ed.