Abstract
We have previously demonstrated that fibulin-7 (Fbln7) is expressed in teeth by pre-odontoblast and odontoblast cells, localized in the basement membrane and dentin matrices, and is an adhesion molecule for dental mesenchyme cells and odontoblasts. Fbln7 is also expressed in blood vessels by endothelial cells. In this report, we show that a recombinant C-terminal Fbln7 fragment (Fbln7-C) bound to Human Umbilical Vein Endothelial Cells (HUVECs) but did not promote cell spreading and actin stress fiber formation. Fbln7-C binding to HUVECs induced integrin clustering at cell adhesion sites with other focal adhesion molecules, and sustained activation of FAK, p130Cas, and Rac1. In addition, RhoA activation was inhibited, thereby preventing HUVEC spreading. As endothelial cell spreading is an important step for angiogenesis, we examined the effect of Fbln7-C on angiogenesis using in vitro assays for endothelial cell tube formation and vessel sprouting from aortic rings. We found that Fbln7-C inhibited the HUVEC tube formation and the vessel sprouting in aortic ring assays. Our findings suggest potential anti-angiogenic activity of the Fbln7 C-terminal region.
Keywords: Fibulin-7 fragment, endothelial cells, angiogenesis
1. Introduction
The fibulins comprise a family of seven secreted glycoproteins associated with basement membranes, elastic fibers, and other matrices. Fibulin family proteins contain three structural domains: a tandem repeat of epidermal growth factor (EGF)-like module in the middle portion of the protein, a C-terminal fibulin-type module characteristic of the family, and a variable N-terminal domain which differs among the members [1]. Fibulins mediate cell-to-cell and cell-to-matrix communication and provide stabilization of the extracellular matrix (ECM) during organogenesis and vasculogenesis [1–3]. Fibulins have been implicated in the modulation of cell morphology, growth, adhesion, and motility and act as both tumor-suppressors and oncogenic factors [4]. Some studies suggest that fibulins may play a role in the regulation of vascular growth during development, in lesions of injured blood vessels, in angiogenesis, and in cancer [3]. Fibulins have been demonstrated to act as angiogenesis inhibitors [5–7]; however, the mechanism of their anti-angiogenesis activity is unknown. We previously identified fibulin-7 as a cell adhesion molecule for dental mesenchyme cells and odontoblasts that interacts with ECM proteins [8]. Fbln7 is also expressed in blood vessels by endothelial cells (data not shown), but its role in vascular development and endothelial cells function remains unknown.
Angiogenesis, the formation of new vessels from preexisting vasculature, is essential for several physiologic processes, including organ development, wound healing, and reproduction [9]. It is a multistep process that involves endothelial cell activation, degradation of the basement membrane, endothelial cell invasion, proliferation, migration, lumen formation, and stabilization of the new vessels. A balance of pro- and anti-angiogenic molecules regulates this process. In pathological conditions, uncontrolled angiogenesis can occur, resulting in various diseases and cancer. Therefore, inhibiting angiogenesis is a powerful tool for preventing these pathologies.
Here, we report that the C-terminal region of the Fbln7 protein, Fbln7-C, binds to Human Umbilical Vein Endothelial Cells (HUVECs) but does not promote cell spreading. The Fbln7-C binding to HUVECs induced an accumulation of actin and integrin clustering at cell adhesion sites, together with other focal adhesion molecules, and sustained activation of FAK, p130Cas, and Rac1. In addition, RhoA activation was inhibited, thereby preventing HUVEC spreading. We found that Fbln7-C inhibited the HUVEC tube formation and the vessel sprouting in aortic ring assays. Taken all together, Fbln7-C serves as an adhesion substrate for endothelial cells, and interferes with the endothelial cell ability to sprout, suggesting a potential role inhibiting angiogenesis.
2. Materials and methods
2.1. Expression and purification of Fbln7 recombinant proteins
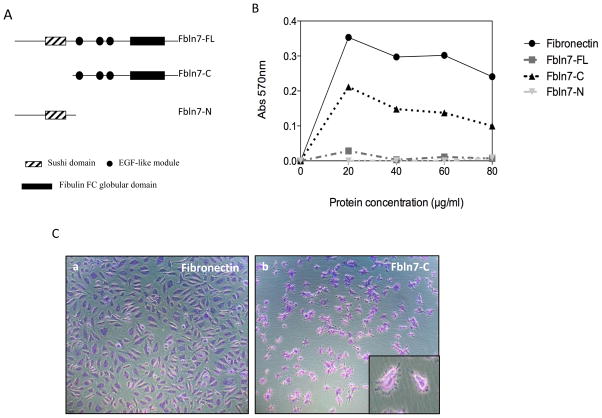
Recombinant near full-length Fbln7 (Fbln7-FL, amino acid residues 75–440, 42 kDa), recombinant Fbln7-C (amino acid residues 135–440, 33 kDa), and recombinant Fbln7-N (amino acid residues 41–139, 11 kDa) proteins (Fig. 1A) were produced and purified, as previously described [8].
Fig. 1.
(A) Diagram showing the domain structures of the recombinant proteins used. (B) HUVEC cell adhesion to fibronectin, Fbln7-FL, Fbln7-N and Fbln7-C. HUVEC cells were able to adhere to Fbln7-C but not to Fbln7-FL, nor to Fbln7-N. (C) HUVEC cells adhered to Fbln7-C but were not able to properly spread on it. The cells showed a stellate-like abnormal morphology (Cb). The insert in Cb shows a closer image of the cells plated on Fbln7-C.
2.2. Cell culture
HUVECs were purchased from Cambrex (USA) and maintained according to the recommendations of the manufacturer. The cells between passages 4 and 8 were used for the experiments.
2.3. Cell adhesion
Assays were performed in 96-well, flat-bottom microtiter plates (Immunolon 2HB, Dynex Technologies, USA). The wells were coated overnight at 4°C with 50 μL of various amounts of Fbln7-FL, Fbln7-C, Fbln7-N, and fibronectin (Sigma-Aldrich, USA). 3 × 104 cells per well were plated and incubated for 2 hours at 37°C in a humidified atmosphere of 5% CO2. Attached cells were fixed and stained for 10 min with 0.2% crystal violet in 20% methanol. The cells were dissolved in 1% sodium dodecyl sulfate (SDS) and the absorbance at 570 nm was measured.
2.4. Cell immunofluorescence
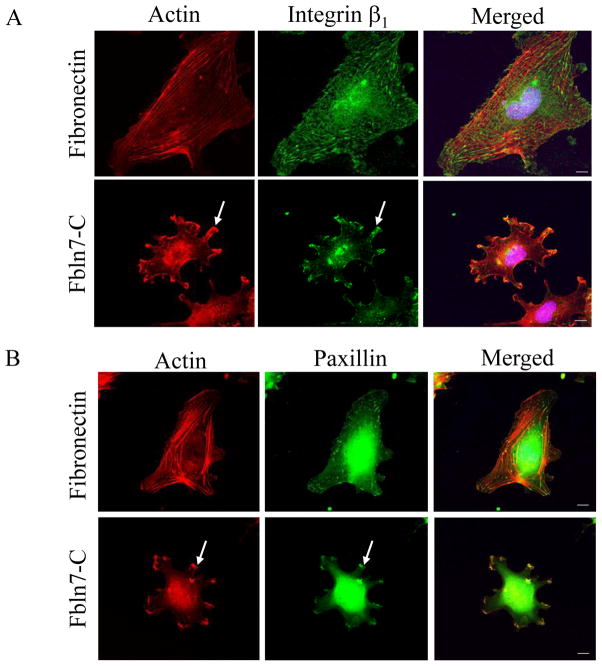
Glass chamber slides were coated with fibronectin at 5 μg/ml or Fbln7-C at 20 μg/ml overnight at 4°C. 104 cells per well were plated and allowed to attach for 2 hours, and fixed with pre-warmed 4% PFA for 10 minutes. The slides were incubated with the β1 integrin (1:50) primary antibody (kind gift from Kenneth Yamada, NIH) or paxillin antibody (1:50) (Merck, IRL) at 4°C overnight. Mouse-IgG-Alexa Fluor 488 was used as secondary antibody. Actin was detected by staining with phalloidin-rhodamine (Life Technologies, USA). Images were taken using a Zeiss LSM 510 NLO META confocal microscope.
2.5. Small GTPases activation assay
Cell culture dishes were coated with fibronectin at 5 μg/ml or Fbln7-C at 20 μg/ml overnight at 4°C. HUVECs were plated and allowed to attach for 2 hours. Rac1 activity was assayed using the Rac1 Activation Assay Kit from Cytoskeleton Inc. (USA). Cell lysates were incubated with PAK-PBD agarose beads at 4°C for 1 hour. After washing, the proteins attached to the beads were solubilized by boiling in a LDS-sample buffer with β-mercaptoethanol. SDS-polyacrylamide gel electrophoreses (PAGE) was performed, and the proteins were detected by immunoblotting using a Rac1 antibody (Merck, IRL). RhoA activity was assayed using the RhoA Activation Assay Kit from Cytoskeleton. Cell lysates were incubated with rhotekin RBD agarose beads at 4°C for 1 hour. After washing, the proteins attached to the beads were solubilized by boiling in a LDS-sample buffer with β-mercaptoethanol. SDS-PAGEs were performed, and the proteins were detected by immunoblotting using a RhoA antibody (Abcam, USA).
2.6. Western blotting
Cell culture dishes were coated with fibronectin at 5 μg/ml or Fbln7-C at 20 μg/ml overnight at 4°C. HUVECs were plated for 2 hours and lysed. The cell lysates were boiled in a LDS-sample buffer with β-mercaptoethanol. The samples were loaded on 4%–12% polyacrylamide Bis-Tris gel (Life Technologies, USA). The primary antibodies used in Western blotting were FAK (Merck, IRL), phospho-FAK (Tyr397; Life Technologies, USA), p130Cas (Merck, IRL), and phospho-p130Cas (Y165; Cell Signaling, US). Secondary antibodies were the rabbit IgG-HRP and mouse IgG-HRP (GE, England). A SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, USA) was used to detect proteins. The intensity of the bands was measured and quantified using the ImageJ software. The intensity of phospho-FAK and phospho-p130Cas was normalized using the intensity of the total FAK and total p130Cas bands, respectively.
2.7. Activation of Rho GTPase by CN03
CN03 (Cytoskeleton Inc., USA) is a cell-permeable recombinant bacterial cytotoxic necrotizing factor (CNF) that activates Rho GTPase constitutively through deamidation [10]. Cells plated on fibronectin or Fbln7-C were treated with CN03 at different concentrations (5, 7.5, and 10 μg/ml) for 5 hours. After treatment, the cells were immunostained as described above.
2.8. Endothelial cell tube formation assay
The HUVEC tube formation assay was performed using the In Vitro Angiogenesis Tube Formation Assay Kit from Trevigen Inc. (Gaithersburg, MD, USA) following the manufacturer’s instructions and a published method [11]. Briefly, a 96-well plate was coated with 50 μL of growth factor reduced BME (Basement Membrane Extract) or Matrigel. Cells were diluted in EBM-2 (Endothelial Basal Media) in the presence or absence of the recombinant Fbln7-C protein at 10 μg/ml. 1.5 × 104 cells were added to each well and incubated at 37°C for 12 hours. Tube formation was analyzed using ImageJ software.
2.9. Mouse aortic ring assay
The aortic ring assay for angiogenesis was performed following the method described by Aplin et al. [12] with some modifications. Briefly, thoracic aortas from 6-week-old mice were dissected and sliced into 1-mm rings. Rounded drops of growth factor reduced BME were added to a 48-well plate. Single aortic rings were placed on top of each drop, and another BME drop was added. 500 μl of EBM-2 basal media containing 2% FBS, in the presence or absence of Fbln7-C at 20 μg/ml, were added to the wells, and the rings were incubated for 7 days. Sprouting was quantified following the method described by Mochizuki et al. [13].
3. Results
3.1. HUVEC adhesion and spreading on recombinant Fbln7 proteins
To explore the angiogenic activity of Fbln7 with and without the sushi domain, we prepared three recombinant proteins: near full-length Fbln7-FL, N-terminal Fbln7-N containing the sushi domain, and C-terminal Fbln7-C containing the EGF-like modules and fibulin type domain (Fig. 1A). We first examined their binding activity for HUVEC cells. HUVEC cells were plated under serum-free conditions on dishes coated with fibronectin, as a positive control, Fbln7-FL, Fbln7-N, or Fbln7-C. HUVECs attached to fibronectin and spread well (Fig. 1B, C). HUVECs were not able to attach to Fbln7-FL and Fbln7-N (Fig. 1B). However, HUVECs attached to Fbln7-C with a stellate-like morphology (Fig. 1B, C).
3.2. Abnormal focal adhesion formation of HUVECs on Fbln7-C
We studied the morphology of HUVECs on Fbln7-C. When HUVECs were plated on fibronectin as a control for 2 hours, the formation of actin stress fibers was observed using rhodamine phalloidin staining, and focal adhesions were visualized by β1 integrin and paxillin staining (Fig. 2). However, when HUVECs were plated on Fbln7-C, the attached cells show a stellate-like morphology, and actin stress fibers did not form (Fig. 2). We found that β1 integrin, paxillin (Fig. 2), and other focal adhesion molecules, such vinculin, pFAK, and Rac1 (data not shown), accumulated in clusters at the periphery of the cells attached to Fbln7-C.
Fig. 2.
Accumulation of β1 integrin (A) and paxillin (B) in protrusions of HUVEC cells on Fbln7-C (20 μg/ml). Focal adhesions and actin stress fibers were well formed when the cells were plated on the fibronectin (5 μg/ml) substrate. On the Fbln7-C substrate, actin was accumulated at the tip of the protrusions, and β1 integrin and paxillin accumulated in clusters (arrows). Scale bar, 10 μm.
3.3. Fbln7-C causes sustained activation of Rac1, increased FAK and p130Cas phosphorylation, and inhibits RhoA activation
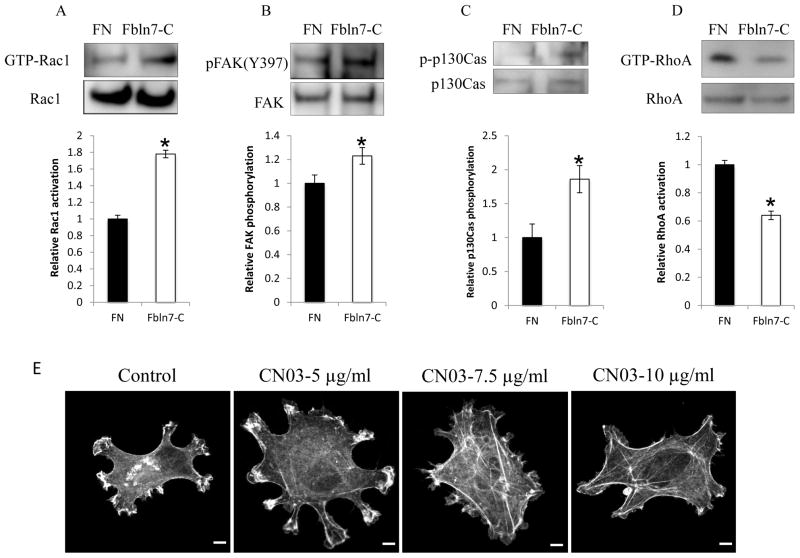
The Rho family of small GTPases, such as RhoA, Rac1, and Cdc42, regulate intracellular actin remodeling [14]; therefore, we investigated the Rho-GTPases activity in HUVECs on Fbln7-C. HUVECs were plated on either Fbln7-C or fibronectin for 2 hours. After the incubation, cell lysates were mixed with PAK-PBD beads, and the active Rac1 protein was pulled down. We found that the level of Rac1 activity (GTP-Rac1) was higher in cells on Fbln7-C substrate compared with the cells on fibronectin (Fig. 3A). These results suggest that Rac1 activation is sustained when the cells attach to Fbln7-C.
Fig. 3.
Sustained activation of Rac1 GTPase (A), increased phosphorylation of FAK (B) and p130Cas (C), and deficient activation of RhoA GTPase (D) upon attachment to Fbln7-C (20 μg/ml). Data represent mean ± s.e. (n = 3); *p < 0.05. (E) Treatment with the RhoA activator (CN03) rescued the phenotype of HUVECs on the Fbln7-C substrate. CN03 treatment induced the formation of stress fibers in a dose-dependent manner, as was shown by phalloidin staining. Scale bar = 0.25 μm for control, 5-μg/ml CN03, and 7.5-μg/ml CN03. 0.5 μm for 10-μg/ml CN03.
We next looked at the phosphorylation state of FAK, a molecule upstream of Rac1. We found increased phosphorylation of FAK when cells were plated on Fbln7-C compared with fibronectin (Fig. 3B). We also examined phosphorylation of p130Cas since p130Cas is a scaffolding protein intermediate between FAK and Rac1 signaling pathways, and is phosphorylated by FAK, which subsequently leads to the activation of Rac1 [15, 16]. We found an increase in phosphorylation of p130Cas in HUVECs on Fbln7-C (Fig. 3C). These results indicated that Fbln7-C binding to HUVECs induced sustained phosphorylation of signaling molecules in the Rac1 activation pathway, which led to sustained activation of Rac1.
Because the cells on Fbln7-C are not able to form actin stress fibers, RhoA, which is required for actin-myosin contractility, may not be activated. To test this possibility, active RhoA protein was pulled down using rhotekin-RBD beads. We found that the level of RhoA activity (GTP-RhoA) was low in the cells on Fbln7-C compared with the cells on fibronectin (Fig. 3D). These results suggest that the sustained activation of Rac1 (Fig. 3A) led to a decreased activation of RhoA (Fig. 3D) and, consequently, to a defect in actin stress fiber formation and cell spreading.
3.4. The actin stress fiber formation of HUVECs on Fbln7-C is partially restored by a RhoA activator treatment
To further confirm that the defect in cell spreading of the cells on Fbln7-C is caused by a deficient RhoA activation, we induced RhoA activity levels with the activator CN03. When cells plated on Fbln7-C were treated with CN03, actin polymerization was increased, as observed by immunofluorescence staining with phalloidin (Fig. 3E). Cell spreading was increased in a dose-dependent manner, and stress fibers were observed using 7.5 and 10 μg/ml of CN03 (Fig. 3E). These results demonstrated that increased RhoA activity levels by CN03 partially rescued the defective cellular phenotype of HUVECs on Fbln7-C.
3.5. Fbln7-C blocks HUVEC capillary formation
Because Fbln7-C disrupts the actin cytoskeleton of HUVEC endothelial cells, we hypothesized that it may inhibit angiogenesis processes. To test this hypothesis, we next analyzed the effect of Fbln7-C on tube formation of HUVECs on basement membrane extract (BME), or Matrigel. It is well established that tube formation of endothelial cell on BME recapitulates some angiogenesis steps, such as cell migration, alignment, formation of tubes, and tube branching and anastomosing with adjacent tubes [17]. Kubota et al. demonstrated that endothelial cells plated on reconstituted basement membranes, rapidly attach, align, and form capillary-like tubes, consisting of a lumen and tight cell-cell contacts [18].
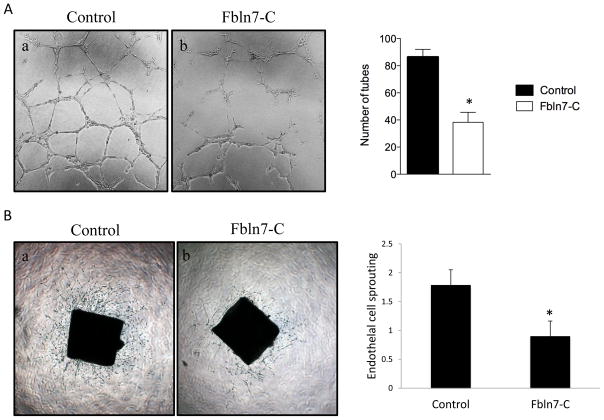
HUVECs on BME formed capillary-like tubes (Fig. 4Aa). However, Fbln7-C strongly disrupted HUVEC capillary morphogenesis (Fig. 4Ab). These results suggest that Fbln7-C inhibits endothelial cell differentiation and is a potential angiogenesis inhibitor.
Fig. 4.
Inhibition of tube formation of HUVEC endothelial cells by Fbln7-C. (A) HUVEC tube formation assay: (a) HUVEC cells formed a network of capillary-like structures when cultured on Matrigel; (b) Fbln7-C (10 μg/ml) inhibited the formation of the capillary-like network. The graph shows the quantification of capillary network formation. Data represent mean ± s.e. (n = 3); *p < 0.05. (B) Inhibition of endothelial cell sprouting by Fbln7-C (20 μg/ml) in aortic ring assays. Fbln7-C-treated rings showed significantly less sprouting compared with the control. The graph shows the quantification of endothelial cell sprouting. Data represent mean ± s.e. (n = 10); *p < 0.05.
3.6. Fbln7-C inhibited vessel sprouting in an aortic ring assay
We further tested anti-angiogenesis activity of Fbln7-C in the mouse aortic ring assay. Aortic rings from 6-week-old mice were embedded in BME sandwiches and incubated in basal media containing 2% FBS in the absence or presence of Fbln7-C at 20 μg/ml for 7 days. Fbln7-C-treated rings showed reduced numbers of vessel sprouting compared with the control (Fig. 4B). The vessels sprouting from the Fbln7-C-treated rings were shorter, thinner, and less branched than those observed with the positive control. The sprouting was scored by three blind observers (Fig. 4B, graph). This indicated that Fbln7-C affected the endothelial cells’ capacity to form new vessels.
3. Discussion
We previously identified Fbln7 as a cell adhesion molecule for dental mesenchyme cells and odontoblasts, and that the binding was mediated by integrin and heparan sulfate receptors [8]. Fbln7 is also expressed by endothelial cells, and here, we report that the Fbln7 C terminal fragment binds to endothelial cells, and this binding prevents stress fiber formation and inhibits angiogenesis processes in in vitro assays.
The assembly of focal adhesions in response to ECM molecules is gradual. Initially, nascent cell-matrix adhesions, also called focal complexes, form at the periphery as cells spread. Next, the focal complexes mature into focal adhesions and cells become stably attached [19]. On a Fbln7-C substrate, focal complexes are able to form, as demonstrated by the clustering of the integrins, FAK, vinculin, and paxillin (Fig. 2 and data not shown). However, maturation into focal adhesions does not occur, resulting in poor spreading and migration (Fig. 2 and data not shown). Inactivation of Rac1 followed by the activation of RhoA is essential for focal adhesion assembly in response to integrin-mediated adhesion [20]. When HUVECs were plated on Fbln7-C, we observed a sustained activation of Rac1 (Fig. 3A) and a deficient RhoA activation, which led to the inability to form mature focal adhesions. The abnormal phenotype of HUVECs plated on Fbln7-C resembled the phenotype of cells on laminin or fibronectin treated with an inhibitor for RhoA activity, which is characterized by a loss of stress fibers, decreased cell spreading, collapse of the cell body, and protrusion of dendritic extensions [21]. Furthermore, stress fiber formation on HUVECs on Fbln7-C was partially restored by the treatment with CN03 (Fig. 3E), a RhoA activator, further suggesting that the defective spreading of HUVECs is due to a low RhoA activity.
Our results indicated that upon Fbln7-C ligation, the cells attached and Rac1 was activated (Fig. 3A). This Rac1 activation was sustained without a complete shift to RhoA activation, leading to impaired acto-myosin contractility and cell spreading. Altered focal adhesions in the HUVECs on Fbln7-C contributed to the stellate-like morphology of these cells. When the cells adhere to the extracellular matrix, functional cellular actin and myosin remodeling is required to generate tension across the cells. This tension leads to focal adhesion maturation, composition, and localization [22]. Nascent focal adhesions are formed at the periphery of cells, recruit FAK, paxillin, and other proteins and initiate the anchoring of actin stress fibers. The conversion of nascent to mature focal adhesions is dependent on further force generation [23]. Recent studies have identified differential recruitment of proteins to focal adhesions in the presence or absence of blebbistatin, an ATPase inhibitor that prevents force generation by type II myosin [24]. Interestingly, RhoA-activating proteins are absent in the immature focal adhesions of blebbistatin-treated cells, whereas Rac1 activators are enriched. A similar mechanism is likely to occur when cells are attached to Fbln7-C.
How Fbln7-C unbalances the switch between Rac1 and RhoA activation is still unknown. It is possible that Fbln7-C binds to several cell surface receptors, resulting in the prevention of RhoA activation. In this regard, it has been shown that cadherin and growth factor receptor signaling pathways block RhoA activation [19]. Also, syndecan-4 is a component of focal adhesions and acts as a co-receptor in cell adhesion to many extracellular matrix ligands, modifying the integrin-mediated responses [25]. It would be interesting to study the ability of Fbln7-C to bind to these cell-surface receptors. We have previously shown that Fbln7 binds to integrins and heparan-sulfate receptors [8]. The binding to both or more receptors is likely to modify integrin signaling and to cause the observed sustained activation of Rac1, and to prevent the RhoA activation.
Our results of the HUVEC tube formation assay show that Fbln7-C inhibits capillary-like structure formation of endothelial cells (Fig. 4A). We also found that Fbln7-C reduces vessel sprouting in the mouse aortic ring assay (Fig. 4B), indicating that Fbln7-C is a potential inhibitor of angiogenesis. Fbln7-C is the C-terminal fragment of Fbln7 that contains the three EGF-like modules and the fibulin-type module (Fig. 1A). Fbln7-C lacks the N-terminal complement control protein domain (or sushi domain). It is possible that in the Fbln7-FL protein conformation, the active inhibitory site is masked. After degradation of the protein, the fragment might be released and act as an inhibitor. EGF-like modules are implicated in protein-protein interactions, and members of the fibulin family bind ECM proteins through the EGF tandem array [26]. It has been reported that proteolysis of some extracellular matrix proteins releases active fragments or unmasks cryptic sites that are involved in several biological processes, such as angiogenesis, inflammation, wound healing, tumor growth, and metastasis [27]. Some of these anti-angiogenic fragments are highly expressed in cancer and other pathological conditions, whereas their expression levels are reduced or absent in normal tissues [28–31]. It is conceivable that a C-terminal proteolytic fragment with anti-angiogenic activity, similar to Fbln7-C, may be generated from Fbln7 under certain pathological conditions. In preliminary studies, we found that the Fbln7 recombinant protein was enzymatically processed by cathepsins, which are often associated with pathological conditions (data not shown). Therefore, a Fbln7-C-like fragment may be released from Fbln7 by proteolytic degradation and bind to endothelial cells and blocks angiogenesis by inhibiting endothelial cell differentiation and migration. Since the fibulin-type module is a common domain in the fibulin family proteins, and some fibulins, such as fibulin-3 and fibulin-5, have shown anti-angiogenic activity [32, 33], the fibulin-type module may contain the active site for the anti-angiogenic activity. The EGF-like motifs may also contribute to this activity by forming proper structural folding for the active site.
Highlights of findings.
A C-terminal fragment (Fbln7-C) of fibulin-7 binds to endothelial cells HUVECs.
Fbln7-C does not promote cell spreading and stress fiber formation.
Fbln7-C induces integrin clustering and sustained activation of Rac1.
Fbln7-C inhibits HUVEC tube formation and vessel sprouting in aortic ring assays.
The C-terminal region of Fbln7 may function as an anti-angiogenic factor.
Acknowledgments
We thank Deborah Philp and Vira Artym for their valuable suggestions, and Kenneth Yamada for the β1 integrin antibody. This work was supported by the Intramural Research Program of the NIDCR, NIH, USA (Y.Y.) and by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) supported program for the Strategic Research Foundation at private universities 2011–2015 in Japan (E.A-H. and S.V.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Vega S, Iwamoto T, Yamada Y. Cell Mol Life Sci. 2009;66:1890–1902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu ML, Tsuda T. Birth Defects Res C Embryo Today. 2004;72:25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- 3.Argraves WS, Greene LM, Cooley MA, Gallagher WM. EMBO Rep. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher WM, Currid CA, Whelan LC. Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Law EW, Cheung AK, Kashuba VI, Pavlova TV, Zabarovsky ER, Lung HL, Cheng Y, Chua D, Lai-Wan Kwong D, Tsao SW, Sasaki T, Stanbridge EJ, Lung ML. Oncogene. 2012;31:728–738. doi: 10.1038/onc.2011.272. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Palmsten K, MacDonald B, Kieran MW, Potenta S, Vong S, Kalluri R. Exp Biol Med (Maywood) 2008;233:155–162. doi: 10.3181/0706-RM-167. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Lab Invest. 2007;87:818–827. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- 8.de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, Fukumoto S, Yamada Y. The Journal of biological chemistry. 2007;282:30878–30888. doi: 10.1074/jbc.M705847200. [DOI] [PubMed] [Google Scholar]
- 9.Chung AS, Ferrara N. Annu Rev Cell Dev Biol. 2010 doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Nature. 1997;387:725–729. [Google Scholar]
- 11.Arnaoutova I, Kleinman HK. Nat Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 12.Aplin AC, Fogel E, Zorzi P, Nicosia RF. Methods Enzymol. 2008;443:119–136. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki M, Philp D, Hozumi K, Suzuki N, Yamada Y, Kleinman HK, Nomizu M. Arch Biochem Biophys. 2007;459:249–255. doi: 10.1016/j.abb.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 15.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Mayer BJ. BMC cell biology. 2008;9:50. doi: 10.1186/1471-2121-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnaoutova I, George J, Kleinman HK, Benton G. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 18.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sastry SK, Burridge K. Experimental cell research. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 20.Hotchin NA, Hall A. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming YM, Frame MC, Houslay MD. J Cell Sci. 2004;117:2377–2388. doi: 10.1242/jcs.01096. [DOI] [PubMed] [Google Scholar]
- 22.Geiger B, Spatz JP, Bershadsky AD. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 23.Parsons JT, Horwitz AR, Schwartz MA. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couchman JR, Woods A. J Cell Sci. 1999;112(Pt 20):3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- 26.Tran H, VanDusen WJ, Argraves WS. The Journal of biological chemistry. 1997;272:22600–22606. doi: 10.1074/jbc.272.36.22600. [DOI] [PubMed] [Google Scholar]
- 27.Ricard-Blum S, Ballut L. Front Biosci. 2011;16:674–697. doi: 10.2741/3712. [DOI] [PubMed] [Google Scholar]
- 28.Feldman AL, Pak H, Yang JC, Alexander HR, Jr, Libutti SK. Cancer. 2001;91:1525–1529. doi: 10.1002/1097-0142(20010415)91:8<1525::aid-cncr1161>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.O’Reilly MS. Exs. 1997;79:273–294. [PubMed] [Google Scholar]
- 30.Dong Z, Kumar R, Yang X, Fidler IJ. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 32.Albig AR, Neil JR, Schiemann WP. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 33.Albig AR, Schiemann WP. DNA Cell Biol. 2004;23:367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]