Abstract
Induction of specific immunological unresponsiveness by feeding protein antigens is termed oral tolerance and may be a potential therapy for autoimmune diseases. Whereas oral tolerance therapy may be both simple and effective, the requirement for large amounts of protein will limit clinical testing of autoantigens, which are difficult to produce. We have previously demonstrated transgenic plant production and direct oral delivery of a β cell autoantigen murine GAD67 to prevent autoimmune diabetes in nonobese diabetic mice. Mucosal adjuvants such as cholera toxin B subunit may lower the level of autoantigen required, but the development of neutralizing mucosal antibody responses may limit usefulness in enhancing long-term oral tolerance. IL-4, being an endogenous protein, would avoid this result and possibly enhance oral tolerance but has not been tested as a mucosal adjuvant. In this study, human GAD65 (hGAD65), as well as murine IL-4, was expressed in transgenic plants for feeding trials. Both IL-4 and hGAD65 plant tissue were required to protect nonobese diabetic mice from diabetes, and no benefit was found if either was used alone. Combined therapy enhanced levels of IgG1 anti-GAD antibodies, increased splenocyte IL-4/IFN-γ cytokine responses, and produced protective regulatory T cells. These results demonstrate that orally administered plant IL-4 remains biologically active and is synergistic when given with hGAD65 in inducing robust oral immune tolerance. Using transgenic plants expressing IL-4 and GAD65 may be a novel clinical approach to the prevention of human type 1 diabetes by oral tolerance.
Oral administration of protein antigens can result in diminished peripheral immune responses to a subsequent systemic challenge with the same antigen, in a process known as oral immune tolerance (1). The basis for the development of such a regulatory system in mammals may be related to balancing protective mucosal antibody responses to pathogens and attenuating potentially harmful allergic responses to newly encountered food proteins. Oral tolerance has also been viewed as a potential therapeutic strategy for preventing and treating autoimmune diseases such as diabetes when specific autoantigens such as glutamic acid decarboxylase (GAD) have been postulated. With greater patient acceptance of oral rather than systemic therapies (e.g., by injection) and antigen-specific effects without toxic effects of general immunosuppression, oral tolerance remains an attractive strategy that merits further clinical testing. Indeed, in animal models, oral administration of autoantigens has been shown to prevent spontaneous autoimmune disease, including the nonobese diabetic (NOD) mouse model of type 1 diabetes (reviewed by Weiner in ref. 2). However, clinical trials of oral tolerance in human disease such as rheumatoid arthritis, uveitis, and multiple sclerosis have had variable results. This finding may be related to issues of uncertainty in primary autoantigen identification, changing specificities due to epitope spreading of responses, insufficient dosing of antigen, or the unavailability of suitable mucosal adjuvants to allow or enhance tolerizing effects in longer term administration (2, 3). Another serious limitation in the clinical application of oral tolerance strategies will be the potentially huge cost of producing autoantigens, particularly if repeated regular doses are required to maintain beneficial effects.
The use of plants as an expression system or “bioreactor” for the production of mammalian antigenic proteins for clinical use offers several advantages, not the least of which is high production capacity with near unlimited scale up. Being eukaryotes, plants can also perform posttranslational modifications required to assemble functional recombinant proteins such as formation of disulfide bonds and proper folding (4). Because protein purification costs can eliminate the economic advantage of any production system, an additional advantage of transgenic plants for oral tolerance is that plants can also become effective delivery systems without extensive purification. Plant expression also largely eliminates concerns regarding potential pathogens that could be transmitted to humans. Also, augmented immune responses to plant-produced vaccines may suggest increased stability for plant-expressed recombinant proteins to gastrointestinal degradation, and collectively these features make plants an ideal expression and delivery system for oral tolerance (5, 6).
We have previously demonstrated that a diabetes-associated beta cell autoantigen, mouse GAD67, can be produced in transgenic tobacco and potato, and that NOD mice were protected from diabetes when given GAD67 plant tissue (7). Protection was due to inhibition of autoreactive GAD-specific T lymphocytes with immune deviation to Th2 T cell subsets. However, oral GAD67 did not produce regulatory cells capable of suppressing diabetogenic T cells (unpublished data), suggesting other factors might be required to enhance oral tolerance. Mucosal adjuvants such as cholera toxin B subunit (CTB) may enhance oral tolerance to coadministered antigens by targeting small amounts of protein antigens to specialized antigen-presenting cells of the gut-associated lymphoid tissue (8). However, oral administration of the bacterial toxin CTB as a mucosal adjuvant or carrier molecule for conjugated antigens can induce neutralizing antibody mucosal responses, which could limit its usefulness in oral tolerance, particularly if prolonged or repeated administration is required to initiate or maintain tolerance.
Because adjuvants facilitate tolerance induction and reduce required quantities of coadministered autoantigens, we were interested in finding effective mucosal adjuvants suitable for long-term administration of oral antigen therapies for autoimmune diseases using plant-based systems. The immunoregulatory cytokine IL-4 strongly promotes Th2 immune responses, which could augment tolerance to autoantigens such as GAD (9). Consistent with this concept, attenuated expression of IL-4 has been suggested to contribute to autoimmune diabetes (10) whereas overexpression of IL-4 in pancreas has prevented insulitis and diabetes in NOD mice (11). Cytokines can resist gastrointestinal degradation and retain biological effect when administered orally because oral IFN-α can attenuate autoimmune diabetes by a non-antigen-specific mechanism (12). IL-4 is unlikely to induce neutralizing antibody responses, being an endogenous protein, but would not be an ideal candidate as a mucosal adjuvant to enhance responses with coadministered antigen unless its function was maintained after oral delivery. As with any cytokine, given the quantities needed for oral delivery, high production costs must also be addressed.
The present study was designed to test whether transgenic tobacco plants could produce biologically active IL-4 that resisted gastrointestinal degradation and was thus suitable for oral administration and whether oral IL-4 could enhance oral tolerance to the human diabetes-associated autoantigen GAD65. Human GAD65 (hGAD65) was selected to test because this is the major isoform in human islets and thus is possibly linked to human diabetes. As well, it has been shown to be effective in preventing diabetes in NOD mice by many routes of administration (13, 14). Because there is no species crossreactivity, mouse IL-4 was selected for studies in the NOD mouse model.
Here, we report that transgenic tobacco plants can express biologically active mouse IL-4 (mIL-4) as well as hGAD65 in a form suitable for direct dietary supplementation. Prediabetic NOD mice given dietary supplementation with mIL-4 plant tissue along with hGAD65 were protected from both insulitis and diabetes whereas mice receiving mIL-4 or hGAD65 plant tissue alone were not protected. Although plant-expressed mIL-4 can resist intestinal degradation and retains function, it had no effect on diabetes without coadministered hGAD65. These results demonstrate that orally delivered plant-derived mIL-4 and hGAD65 are synergistic in inducing beneficial immune responses. Transgenic plant expressing IL-4 and GAD65 may provide a practical and novel approach for the prevention of human type 1 diabetes by oral tolerance.
Materials and Methods
Construction of Plant Expression Vectors for mIL-4 and hGAD65. mIL-4 is a glycoprotein of 19 to 21 kDa (15). The cDNA encoding a mature form of mIL-4 was a gift from R. Levy (Stanford University). To construct the plant vector expressing mIL-4, the coding sequence of the mIL-4 gene was amplified by PCR and fused to peanut peroxidase signal peptide (16). Primers used were 5′-AATTCGAGCATATCCACGGATGCGAC-3′ and 5′-ATAGGTACCCGAGTAATCCATTTGCATGATGC-3′. To facilitate isolation of the recombinant product from plant cells, an mIL-4 gene with a C-terminal His tag was also created. The reconstituted mIL-4 genes were then used to replace the GUS gene in plasmid pTRL2-GUS (17). The resulting mIL-4 expression cassettes were isolated and cloned into the binary plant vector pBIN19 (18), and then transferred into the Agrobacterium tumefaciens LBA4404 (19).
To construct a plant vector expressing hGAD65, the GAD cDNA was isolated by RT-PCR. Total RNA was prepared from a human brain pathology specimen (obtained by permission from the London Health Sciences Centre) as a template source. First-strand cDNA was generated by using random oligo(dT) primers (GIBCO/BRL). The human GAD65 cDNA was amplified by PCR using the following primers: 5′-AATTCCATGGCATCTCCGGGCTCTGGC-3′ and 5′-ATAATCTAGATTATAAATCTTGTCCAAGGCG TTC-3′, with underlined NcoI and XbaI sites being inserted to facilitate subsequent cloning. After confirmation by DNA sequencing, the isolated hAD65 cDNA was cloned into pTRL2-GUS (17) and then into pBIN19 (18) as described above for mIL-4.
For production of transgenic plants, tobacco (Nicotiana tabacum cv. SR1) leaf explants were transformed with Agrobacterium harboring mIL-4 or hGAD65 plant vectors (7). Mature plants were generated from regenerated shoots and subjected to screening for the presence of the transgene by PCR amplification of transgenic tobacco genomic DNA (7), by using mIL-4- or hGAD65-specific primers. Transgene copy number was determined by Southern blot analysis of the genomic DNA after digestion with XhoI, which cuts the T-DNA [portion of the Ti (tumor-inducing) plasmid that is transferred to plant cells] only once, using 32P-labeled mIL-4 or hGAD65 probes (7). Transcriptional expression of the transgene was verified by Northern blot as described (16), using the same 32P-labeled DNA probes.
Western Blot Analysis and ELISA. Western blotting was used to analyze the production of recombinant mIL-4 (rmIL-4) and hGAD65 (rhGAD65) in plants as described (7). Quantification of plant-derived rmIL-4 was by ELISA using commercial kits (OptEIA, PharMingen) according to the manufacturer's instructions. Quantitation of rhGAD65 was completed by scanning the signal strength of the applied GAD leaf extracts and purified Escherichia coli-derived rhGAD of known concentration on the obtained blots by using a pdi white light scanner (Raytest, Wilmington, NC).
Determination of Biological Activity of Plant rmIL-4. The biological activity of plant rmIL-4 was assessed by its ability to support the proliferation of a murine IL-4-dependent mast cell line, MC/9 (American Type Culture Collection, ATCC). Dilutions of standard rmIL-4 (PharMingen), an rmIL-4 reference reagent from the National Cancer Institute (5,000 units/μg) and plant rmIL-4 purified by immobilized metal ion affinity chromatography on a chelating HiTrap column (Amersham Pharmacia) were added to quadruplicate wells of 96-well plates in a final volume of 100 μl per well. MC/9 indicator cells were washed to remove IL-4 and resuspended to a final concentration of 2 × 105 viable cells per ml in RPMI medium 1640. The cell suspension (100 μl) was added to all wells. The plates were incubated for 60 h at 37°C in 5% CO2. Sixteen hours before termination of the assay, tritiated thymidine (1 μCi/well) (1 Ci = 37 GBq) was added. At termination, the cells were harvested, and thymidine incorporation was determined by scintillation counting.
Feeding of NOD Mice with Transgenic Plant Tissues. All mice were housed according to guidelines of the Canadian Council on Animal Care. Parental colony incidence of diabetes in female NOD mice is typically 75–90% by 30 weeks but may exceed 90% in some cohorts. Fresh and frozen IL-4 and GAD65 transgenic tobacco leaf tissues were homogenized in liquid nitrogen, and the tissue homogenates were mixed in a prepared chow as a powder to deliver required nutrition and ≈1–2 μg of plant rmIL-4 and 6–8 μg of plant rhGAD65 per mouse daily. Feeding was started in 4-week old female prediabetic NOD mice. Chow was changed every 2 days to control for potential transgenic protein degradation, and changes in chow were identical for each group. Mice were caged in groups of four. Control mice received an equivalent amount of corresponding leaf tissue from emptyvector, IL-4, or GAD65 transformed tobacco. The development of diabetes was monitored by measuring glucose in urine by using TES-TAPE (Eli Lilly, Toronto) and confirmed by measuring blood glucose by using the One Touch Basic kit (Lifescan, Canada). A mouse with a blood glucose level of above 16.7 mmol/liter on two consecutive days was scored as diabetic.
Histopathological Analysis of Pancreatic Islets. Mice that had been fed (i) IL-4 with GAD65, (ii) IL-4, (iii) GAD65, or (iv) empty-vector transgenic tissue at 4 weeks of age were killed at 9 weeks of age, and the pancreata were removed. Each pancreas was fixed with 10% buffered formalin and embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin. The degree of insulitis was evaluated on a blind basis by using a standardized scoring system described by others (20).
Enzyme-Linked Immunospot (ELISPOT) Analysis. An ELISPOT assay was used for detection of IL-4 and IFN-γ secretion at the single cell level. In brief, 96-well plates were coated overnight at 4°C with 2 μg/ml IL-4- or IFN-γ-specific capture mAb (PharMingen). Splenic cell suspensions prepared from the 9-week-old plant-fed NOD mice used for histology were added to individual wells (1 × 106 cells per well) alone, or with GAD as antigen (20 μg/ml) and incubated for 72 h. Biotinylated anti-mouse IL-4 or anti-mouse IFN-γ detection mAb (1 μg/ml; PharMingen) was added, and the plates were incubated overnight. The plate-bound secondary antibody was visualized by using horseradish peroxidase and 3-amino-9-ethylcarbazole (AEC) substrate (both from Sigma). The brown-colored immunospots that corresponded to the cells that had secreted IL-4 or IFN-γ were counted manually under a dissection microscope. Results are expressed as numbers of spots per 106 splenic cells after subtraction of background spots appearing in non-antigen-stimulated cultures.
Serum IgE and anti-GAD IgG1 Antibody Determination. The levels of IgE and anti-GAD IgG1 antibodies in sera collected from 9-week-old NOD mice used to determine cytokine profiles as well as 30-week-old diabetes free mice that had received IL-4/GAD65 were determined by ELISA using commercial kits (OptEIA, PharMingen) according to the manufacturer's instructions. For IgE, ELISA serum samples at 1:10 dilution were tested. For anti-GAD IgG1, ELISA serum samples at 1:50 dilution were tested.
Adoptive Transfer of Diabetes. Splenocytes from 9-week-old NOD mice fed IL-4 and GAD65 were assessed for capacity to suppress the diabetogenic activity of splenocytes taken from an acutely diabetic donor. In brief, splenocytes (107 cells) from IL-4 plus GAD, IL-4, or GAD65 plant-fed mice were mixed with splenocytes (107 cells) from newly diabetic NOD mice and given i.v. into the tail veins of 6- to 8-week-old NOD/severe combined immunodeficient (NOD-scid) mice. NOD-scid mice receiving only 107 diabetic splenocytes were used as a positive control. Recipient mice were monitored for the development of diabetes up to 8 weeks.
Statistical Analysis. Survival analyses with Kaplan–Meier estimates were used to evaluate the difference in the incidence of insulitis and diabetes onset between the groups in NOD mice and differences determined by log rank analysis. Results were otherwise analyzed by using t tests and ANOVA for multiple comparisons. Values of P < 0.05 were held to be significant.
Results
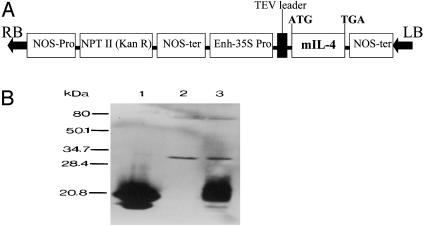
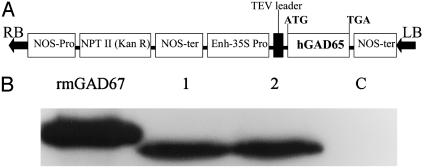
Transgenic Plants Can Express Human GAD65 and Murine IL-4. IL-4 (Fig. 1A) and GAD65 (Fig. 2A) binary vectors were used to produce transgenic tobacco plants. The presence of IL-4 or GAD65 in transformed tobacco genome was confirmed by PCR (data not shown). Southern blot analysis of genomic DNA after digestion with XhoI that cuts the T-DNA only once further revealed that they present in variable copy numbers ranging from 1 to 3 (data not shown). Northern blot analysis of total RNA from transgenic leaf tissue detected a single mRNA transcript of 430 bases expected for mIL-4 and a single mRNA transcript of 1,752 bases expected for hGAD65 (see Fig. 7, which is published as supporting information on the PNAS website). Immunoblot analysis of protein homogenates from IL-4 tobacco leaf showed a protein band of expected size recognized by mIL-4-specific mAb whereas no band was detected in extracts from control plants transformed with empty vector (Fig. 1B). The expression level of mIL-4 in leaf tissue reached up to 0.1% of total soluble protein (TSP). Similarly, immunoblot analysis of protein homogenates from GAD65 tobacco showed a band of expected size recognized by hGAD65-specific mAb (Fig. 2B). The level of hGAD65 in leaf tissue was ≈0.04% of TSP.
Fig. 1.
(A) Schematic structure of the plant expression vector for mIL-4. Enh-35S, enhanced 35S promoter with a duplicated enhancer; TEV leader, leader sequence of tobacco etch virus; NOS-ter, the nopaline synthase 3′ terminator; NPT II, neomycin phosphotransferase II gene; RB, right border; LB, left border. (B) Immunoblot analysis of mIL-4 protein from leaf extracts of transgenic tobacco. Lane 1, standard rmIL-4 (PharMingen); lane 2, extracts from empty vector-transformed tobacco; lane 3, extracts from IL-4 transgenic tobacco.
Fig. 2.
(A) Schematic structure of the plant expression vector for hGAD65. (B) Immunoblot analysis of rhGAD protein from leaf extracts of transgenic tobacco. Lanes 1 and 2, extracts from individual transgenic lines; lane C, extracts from empty vector-transformed tobacco; rmGAD67, standard GAD67 purified from E. coli.
Plant-Derived rmIL-4 Is Biologically Active. To demonstrate the biological activity of plant rhIL-4, a bioassay on the IL-4-dependent mast cell line MC/9 was performed. Proliferation of mast cells occurred with both purified plant rmIL-4 and control standard rmIL-4 at a comparable capacity (see Fig. 8, which is published as supporting information on the PNAS web site), suggesting that plant rmIL-4 is fully biologically active, which would be required for in vivo immunomodulating effect.
Combined Feeding of IL-4 and GAD65 Transgenic Plants Reduces Insulitis in NOD Mice. The destruction of pancreatic β cells of NOD mice is preceded by insulitis characterized by infiltration of mononuclear inflammatory cells (21). To determine the effect of combined feeding of IL-4 and GAD65 plants on insulitis before overt diabetes, NOD mice were given transgenic plant tissue containing both IL-4 and GAD65 or IL-4, GAD65, or empty vector alone, beginning at 4 weeks of age. At 5 weeks of supplementation, pancreata were harvested, and a minimum of 25 islets for each of four animals per group were scored. Although NOD mice from all treatment groups contained islets free of inflammation as well as moderate to severe insulitis, the percentage of the examined islets that showed severe insulitis was substantially lower in IL-4 plus GAD65 plant-fed mice than in animals fed empty vector transgenic plant (8% vs. 31%, P = 0.03; see Fig. 9, which is published as supporting information on the PNAS web site). Although the percentage of normal islets in IL-4 plus GAD65 plant-fed mice was higher compared with empty vector transgenic plant (70% vs. 45%), this difference did not reach significance (P = 0.1; see Fig. 9). No significant differences in insulitis scores were found in NOD mice fed either empty vector or IL-4 or GAD65 transgenic plant (data not shown).
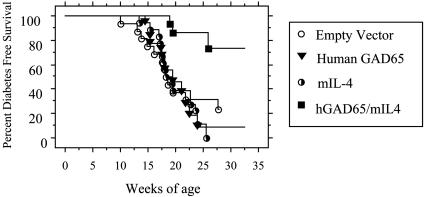
Combined Feeding of IL-4 and GAD65 Transgenic Plants Prevents Diabetes. The effect of long-term feeding of IL-4 plus GAD65 plants on diabetes prevention was investigated. Young prediabetic female NOD mice received dietary supplementation with IL-4 plus GAD65 tobacco tissue from 4 to 30 weeks of age. Previously, we have shown that mice tolerated the tobacco leaf tissue in their diets well and all had normal weight gains (7). Mice were followed for the development of overt diabetes as described (7). Twenty-six percent (4/15) of the NOD mice that received IL-4 plus GAD65 plant tissue developed diabetes by 30 weeks of age whereas 83% (15/18) of the NOD mice that received GAD65, 75% (12/16) of the NOD mice that received empty vector, and 100% (18/18) of the NOD mice that received IL-4 transgenic plant tissue alone developed diabetes by the same age (P = 0.0002) (Fig. 3). The greater percentage of mice given IL-4 alone was not different from controls or our parental colony incidence. Furthermore, the onset of disease was markedly delayed in the NOD mice fed IL-4 plus GAD plant. Several of the mice given IL-4 plus GAD65 developed diabetes. Although this result may represent incomplete protection often seen in this mouse model, it is also possible that small differences in plant tissue delivery occurred due to having four mice per cage. These results demonstrate that prevention of diabetes in NOD mice using oral GAD65 requires the coadministration of IL-4.
Fig. 3.
Combined feeding of IL-4 and GAD65 plants prevented the development of diabetes in NOD mice. Female NOD mice were fed IL-4 plus GAD (n = 15) or IL-4 (n = 18) or GAD65 (n = 18) or empty vector (n = 16) plant starting at 4 weeks of age. Mice were followed for the onset of diabetes up to 30 weeks of age. Diabetes was diagnosed when mice were hyperglycemic for two consecutive readings.
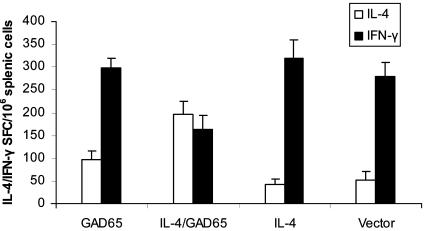
Combined Feeding of IL-4 and GAD65 Transgenic Plant Tissue Induces Th2 Immune Responses. We tested whether prevention of diabetes in NOD mice given GAD65 plus IL4 was mediated by the induction of GAD65-specific Th2 cells. Splenocyte cultures were prepared from 9-week-old NOD mice of all treated groups and analyzed for the frequency of IFN-γ and IL-4 secreting T cells by ELISPOT. As shown in Fig. 4, an increase in IL-4-producing T cells with corresponding decrease in IFN-γ-producing T cells in response to stimulation with GAD65 was detected only in mice treated with both IL-4 and GAD65 plants and not those treated with empty vector, IL-4, or GAD65 plant. Overall, the ratio of IL-4:IFNγ-producing cells was consistently higher in NOD mice treated with IL-4/GAD plants, suggesting that combined feeding of IL-4 and GAD65 plants promotes the induction or preferential differentiation of Th2-like phenotypes.
Fig. 4.
Combined feeding of IL-4 and GAD65 plants stimulated the expansion of GAD65-specific Th2 cells. Splenocytes from the same 9-week-old NOD mice used to examine the development of insulitis were pooled within a given treatment group. The number of individual GAD65-expanded T cells producing IL-4 (open bars) or IFN-γ (filled bars) was determined by ELISPOT and expressed as the mean numbers ± SD of SFC (spot-forming colonies). Four mice per group were tested in two separate experiments.
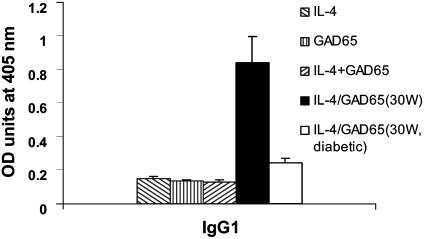
Because Th2 cells promote the production of IgG1 antibodies (22), IgG1 titers have been used to determine whether a Th2 type of response has been induced after administration of a specific antigen. Because NOD mice are unable to produce IgG2a antibody often used to indicate Th1 responses (23), we measured anti-GAD IgG1 levels to complement the ELISPOT results in NOD mice that received IL-4 plus GAD65, IL-4, GAD65, or empty vector-transgenic plant. Anti-GAD65 IgG1 titer was not increased in 9-week-old mice regardless of therapy (Fig. 5). However, by 30 weeks, protected nondiabetc NOD mice fed IL-4 plus GAD65 had high titers of anti-GAD IgG1 antibodies. In the few mice that developed diabetes by 30 weeks with IL-4 plus GAD treatment, there was no increase in anti-GAD IgG1 supporting a benefit of Th2 responses (Fig. 5).
Fig. 5.
Combined feeding of IL-4 and GAD65 plants increased IgG1 anti-GAD antibodies by 30 weeks. Sera collected from 9-week-old (hatched bars) NOD mice of each treatment group and 30-week-old protected nondiabetic (filled bar) mice fed IL-4/GAD65 and 30-week-old diabetic (open bar) mice with IL-4/GAD65 treatment were tested for anti-GAD IgG1 (Th2) by ELISA. Results are presented as the mean A405 values ± SD of grouped samples (n = 4) and are representative of two experiments.
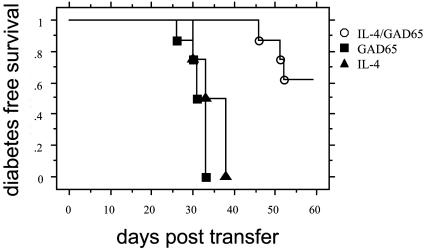
Combined Feeding of IL-4 and GAD65 Transgenic Plants Induces Regulatory Cells. To determine whether administration of IL-4 plus GAD65 plants protected NOD animals from the development of diabetes by regulatory cells, we used an adoptive transfer model. Splenocytes from 9-week-old IL-4 plus GAD65, or IL-4, or GAD65 plant-fed NOD mice were mixed with diabetogenic splenocytes and were given i.v. to 6- to 8-week-old syngeneic NOD-scid recipient mice. NOD-scid mice receiving only diabetogenic splenocytes were used as a positive control. As expected, 100% of NOD-scid recipient mice that received only diabetogenic lymphocytes developed diabetes whereas splenocytes from IL-4 plus GAD plant-fed mice were able to prevent diabetes in >50% of NOD-scid mice (Fig. 6). In contrast, splenocytes from IL-4 or GAD65 plant-fed mice were not protective, and all animals receiving them developed diabetes. These results demonstrate that administration of plant rhGAD65 together with plant rmIL-4 generated potent regulatory cells that can suppress diabetogenic T cells.
Fig. 6.
Splenocytes from IL-4/GAD plant-treated NOD mice prevented diabetes after adoptive transfer. Splenocytes (1 × 107 cells) isolated from IL-4 plus GAD65 (open circles), IL-4 (triangles), or GAD65 (squares) plant-treated mice at 9 weeks of age were mixed with diabetogenic splenocytes (1 × 107 cells) from diabetic NOD mice and transferred to 8-week-old NOD-scid mice (n = 4 for all groups). The development of diabetes in the recipients was monitored. Diabetes in mice that received only splenocytes from diabetic NOD mice was not different from IL-4 or GAD alone groups (not shown).
Feeding of IL-4 Transgenic Plants Does Not Significantly Alter Serum IL-4 Levels. Systemic administration of IL-4 is known to stimulate IgE production (24) and can be associated with induction of allergic reactions. Therefore, we tested whether administration of IL-4 plant elevated the level of IgE Ab in NOD mice. No difference in IgE levels was found in sera from mice in all treated groups (see Fig. 10, which is published as supporting information on the PNAS web site). These results demonstrate that mucosal administration of a low dose of IL-4 has no systemically detectable effect on the level of serum IgE in mice.
Discussion
Type I diabetes in humans and NOD mice is a polygenic autoimmune disease that is markedly influenced by environmental factors. In fact, concordance rates in HLA identical twins is surprisingly low (<40%) (25), suggesting that, although environmental factors may lead to disease in some individuals with permissive genetic loci, they may also attenuate disease in others. The NOD mouse is a spontaneous model that has allowed substantial insight into the development of diabetes through complex interactions between genes and the environment (26). Dietary exposure to various agents that modulate immune responses has long been speculated to be an important variable that controls the incidence of diabetes (27). Oral administration of protein antigens can result in diminished peripheral immune responses to a subsequent systemic challenge to that protein, in a process known as oral tolerance (1). In cases where inappropriate immune responses to specific “self” antigen proteins have been found that precede overt autoimmune disease, oral tolerance has been viewed as a potential therapeutic strategy. However, the potential success of this approach in autoimmune diseases depends not only on the identification of relevant autoantigens and availability of spontaneous models but also the capacity to produce autoantigens in quantities that are both sufficient and affordable to merit clinical testing.
The use of transgenic plant tissue for oral antigen immunotherapy has considerable clinical appeal, not only for efficacy but also for simplicity of production and delivery, advantages of cost, absence of contamination risk with human pathogens, and perhaps, if used in edible plants, increased patient acceptance. In the present study, we demonstrate that transgenic plants can be used to synthesize hGAD65 and biologically active mIL-4. Importantly, tissue from these plants can be used directly to prevent diabetes in NOD mice, and the mechanisms seem to include a shift in Th1/Th2 T cell subsets and, as well, the induction of regulatory T cells. Our findings that hGAD and mIL-4 expressed by plants can induce protective responses but requires coadministration to prevent diabetes have important implications. Our results demonstrate that plant rmIL-4 induces biological response after oral delivery. Moreover, it can act as a mucosal adjuvant for oral tolerance induction to the concurrently delivered autoantigen GAD65. These results also suggest that combination therapy of other autoantigens along with one or more regulatory Th2 cytokines given orally to enhance protection may represent an important new strategy in the prevention of autoimmune diseases through a form of forced manipulation of antigen-specific Th1:Th2 T cell populations (28). In contrast to systemic delivery of IL-4, which leads to numerous side effects in humans (29), oral IL-4 seemed to be well tolerated in mice. The efficacy of oral human IL-4 to induce protection in patients without systemic adverse effects remains to be tested, but these data would support this approach. The nature of resistance of IL-4 to digestive degradation may be a general feature of recombinant proteins produced in plants, perhaps through bio-encapsulation (5, 30). Thus, plants may be the ideal production and delivery system for cytokines, which are highly unstable molecules.
One of the limiting factors in using transgenic plants as an expression system for therapeutics may be that accumulation levels of transgenic proteins may be too low for an oral tolerizing effect using plant tissue directly. Previously, we had expressed mouse GAD67 in both tobacco and potato and demonstrated that NOD mice given GAD67 plant tissue alone were protected from disease (7). In the present study, we could not similarly demonstrate that oral administration of hGAD65 plant tissue alone had a beneficial effect in NOD mice. This finding may be due to an insufficient amount of GAD65 (6 to 8 μg daily) delivered to each mouse using plant tissue directly, as the expression level of hGAD65 was much lower compared with levels of mouse GAD67 in tobacco plants (0.04% vs. 0.4%). Porceddu et al. (31) have reported similar low levels of hGAD65 in transgenic tobacco. In a previous study of NOD mice using oral insulin, Zhang et al. (32) reported that the administration of 1 mg of porcine insulin was an effective oral dose; our use of relatively large amounts of GAD67 (1 mg daily) to achieve an effect was consistent with this dose range. Alternatively, oral mouse GAD67 could be more effective in tolerizing NOD mice than GAD65, although using systemic administration GAD67 and GAD65 seems to be equivalent in effectiveness (33).
In therapeutic applications, with current relatively low expression levels, it may be difficult to deliver comparable quantities of GAD65 to humans using plant tissue directly. Although increasing expression levels may be possible by alternative targeting or enhanced promoter systems, augmenting responses to low levels of autoantigen may be as effective. Mucosal adjuvants such as cholera toxin B subunit have been shown to permit the use of lower antigen amounts (30). However, the development of neutralizing mucosal antibody responses to pathogen components may limit long-term usefulness in enhancing oral tolerance to a coadministered autoantigen. We therefore selected the immunoregulatory cytokine IL-4 to express in plants for coadministration with GAD because IL-4 promotes Th2 immune responses that could augment tolerance to autoantigens. Furthermore, as an endogenous protein to which we are tolerant, IL-4 orally would not be expected to elicit neutralizing antibody responses.
The oral administration of cytokines for therapies has been suggested (29). Type I IFN has been shown to resist digestive enzymes present in the gut (29). Recently, oral IFN-α has been studied in type I diabetes. IFN-α delivered orally suppressed disease and increased mitogen-induced IL-4 and IL-10 production in spleen cells from treated mice, raising the possibility that the protective effect may involve induction of protective Th2 cytokines (12). In early uncontrolled clinical trials with oral IFN-α, newly diagnosed type I diabetic patients showed some preservation in beta cell function over time (34). Although coadministration of an autoantigen was not tested and the substantial cost of sustained cytokine therapy was not discussed, these data support the feasibility of oral cytokine therapy in type I diabetes.
Our results suggest that protection from disease in IL-4 plus GAD65 plant-fed mice involves active tolerance and the induction of regulatory GAD-specific Th2 cells. Several lines of evidence support this notion. Adoptive cotransfer experiments showed that splenocytes from IL-4/GAD65 plant-fed mice were capable of inhibiting the transfer of diabetes by splenocytes from newly diabetic NOD mice to NOD-scid mice. In mice protected with GAD67 alone (1 mg/day) in our previous work, we could not demonstrate protection in adoptive transfer experiments (unpublished data), suggesting that high levels of autoantigen may have resulted in deletion of GAD-reactive cells and did not promote the generation of regulatory cells. The emergence of Th2 cells in IL-4/GAD65-treated mice was evident from cytokine analysis of splenocytes, which showed a higher frequency of IL-4-secreting GAD65-reactive cells, with a concomitant decrease of IFN-γ-secreting GAD65-reactive cells as compared with control plant-fed mice. Finally, there was a significant increase in titers of IgG1 anti-GAD antibodies. Similar emergence of Th2 reactivity has been reported in NOD mice after administration of GAD as well as other islet antigen. Tian et al. (35) observed that intranasal administration of GAD65 or GAD65 peptides induced high levels of IgG1 anti-GAD65 antibodies, reduced IFN-γ with increased IL-4 expression in response to GAD65, and induced regulatory cells. Similar results were obtained in NOD mice treated with nasal insulin B chain (36). Collectively, these data suggest that the amount of antigen, mode of delivery, and coadministration of Th2 cytokines may have profound effects on directing the mechanism of tolerance.
The precise mechanism by which IL-4 enhances oral tolerance remains unknown. Because IL-4 plays a pivotal role in T cell differentiation into Th2 lineages (22), mucosal administration of exogenous IL-4 could create an IL-4-enriched microenvironment in the gut that could promote the emergence of Th2 cells directly. Alternatively, orally administered IL-4 might have inhibited the development of autoreactive Th1 responses in gut-associated lymphoid tissue or Peyer's patches. Interestingly the benefit of IL-4 in combination with autoantigen has been shown in several models using DNA vaccination approaches. Vaccination of NOD mice with plasmid DNA encoding GAD was effective in preventing disease only if the plasmid also contained DNA encoding IL-4 (37), but again issues of systemic exposure to IL-4 remain in this approach. Oral administration of IL-4 at the dose used in the present study did not significantly alter serum IgE levels although more extensive testing would be required to exclude a potential effect.
The identification of relevant autoantigens is of considerable clinical importance because they represent potential therapeutic targets for tolerogenic regimens. There are numerous reports of disease protection in NOD mice by tolerizing strategies based on GAD (13, 14, 35, 36). The expression of GAD within islet tissue seems to be important because antisense silencing of GAD can prevent disease and confer resistance of islets to diabetes after transplantation (38). However, there have been recent reports questioning the importance of GAD in diabetes (39). Elimination of detectable numbers of GAD-reactive T cells by expressing a modified form of GAD under control of the invariant chain promoter within the thymus results in NOD mice with insulitis and diabetes that occurs with the same kinetics as wild-type NOD mice. The basis for these discrepancies is not known, but the validity of models that have alterations in GAD responsiveness initiated from birth need to be carefully considered because they may not represent precisely the clinical progression of diabetes and GAD responsiveness in patients. Additionally, it is possible that, in the absence of GAD-responding T cells, the aggressiveness of autoimmune response to “non-GAD” autoantigens may also be increased by progressive priming of T cells, thus accounting for the lack of attenuation of kinetics (40). Importantly, because oral tolerance may be derived from the induction of regulatory T cells and “dominant” tolerance, and the site of oral tolerance induction has not been established, the loss of T cells that can respond to a primary autoantigen such as GAD may in fact have a significant detrimental effect on the subsequent induction of specific regulatory cells. Testing the ability of these GAD-tolerant mice to develop GAD-regulatory cells after oral administration of plant GAD65/IL4 may provide insight into this controversy. However, although the issue of primary autoantigens in diabetes continues to be studied, antigen-specific intervention protocols for the treatment of patients at risk using oral GAD remain reasonable.
In summary, there is growing evidence to support oral tolerance induction as a feasible therapeutic modality for the treatment of autoimmune diseases. Using transgenic plants expressing IL-4 and GAD65 may be a novel and practical approach to the prevention of type 1 diabetes by oral tolerance.
Supplementary Material
Acknowledgments
This work was supported by the Multiorgan Transplant Program of the London Health Sciences Centre, the Agriculture and Agri-Food Canada Matching Program, and the Natural Sciences and Engineering Research Council.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GAD, glutamic acid decarboxylase; NOD, nonobese diabetic; hGAD65, human GAD65; mIL-4, mouse IL-4; rmIL-4, recombinant mIL-4; rhGAD65, recombinant hGAD65; ELISPOT, enzyme-linked immunospot; scid, severe combined immunodeficient; T-DNA, portion of the Ti (tumor-inducing) plasmid that is transferred to plant cells.
References
- 1.Mowat, A. (1987) Immunol. Today 8, 93–98. [DOI] [PubMed] [Google Scholar]
- 2.Weiner, H. (1997) Annu. Rev. Med. 48, 341–351. [DOI] [PubMed] [Google Scholar]
- 3.Weiner, H. (1997) Immunol. Today 18, 335–343. [DOI] [PubMed] [Google Scholar]
- 4.Kusnadi, A. R., Nikolov, Z. L. & Howard, J. A. (1997) Biotechnol. Bioeng. 56, 473–485. [DOI] [PubMed] [Google Scholar]
- 5.Walmsley, A. M. & Arntzen, C. A. (2000) Curr. Opin. Biotechnol. 11, 126–129. [DOI] [PubMed] [Google Scholar]
- 6.Streatfield, S. J. & Howard, J. A. (2003) Int. J. Parasitol. 33, 479–493. [DOI] [PubMed] [Google Scholar]
- 7.Ma, S., Zhao, D., Yin, Z., Mukherjee, M., Singh, B., Qin, H., Stiller, C. R. & Jevnikar, A. M. (1997) Nat. Med. 3, 793–796. [DOI] [PubMed] [Google Scholar]
- 8.Sun, J. B., Rask, C. & Olsson, T. (1996) Proc. Natl. Acad. Sci. USA 93, 7196–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inobe, J., Slavin, A. J., Komagata, Y., Chen, Y., Liu, L. & Weiner, H. L. (1998) Eur. J. Immunol. 28, 2780–2790. [DOI] [PubMed] [Google Scholar]
- 10.Delovitch, T. L. & Singh, B. (1997) Immunity 7, 727–738. [DOI] [PubMed] [Google Scholar]
- 11.Mueller, R., Krahl, T. & Sarvetnick, N. (1996). J. Exp. Med. 184, 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broad, S., Darcan, S., Malone, M., Pappolla, M. & Nelson, L. (1998) Diabetologia 41, 1227–1232. [DOI] [PubMed] [Google Scholar]
- 13.Tisch, R., Yang, X. D., Singer, S. M., Liblau, R. S., Fugger, L. & McDevitt, H. O. (1993) Nature 366, 72–75. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman, D. L., Clare-Salzler, M., Tian, J., Forsthuber, T., Ting. G. S., Robinson, P., Atkinson, M. A., Sercarz, E. E., Tobin, A. J. & Lehmann, P. (1993) Nature 366, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, F., Yokota, T., Otsuka, T., Meyerson, P., Villaret, D., Coffman, R., Mosmann, T., Rennick, D., Roehm, N., Smith, C., et al. (1986) Proc. Natl. Acad. Sci. USA 83, 2061–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lige, B., Ma, S., Zhao, D. & van Huystee, R. B. (1998) Plant Sci. 136, 159–168. [Google Scholar]
- 17.Carrington, J. C & Freed, D. D. (1990) J. Virol. 64, 1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bevan, M. W. (1984) Nucleic Acids Res. 12, 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoekema, A., Hirsch, P. R., Hooykaas, P. J. J. & Schilperoort, R. A. (1983) Nature 303, 179–180. [Google Scholar]
- 20.Charlton, B. & Mandel, T. E. (1988) Diabetes 37, 1108–1112. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson, M. A. & Maclaren, N. K. (1994) N. Engl. J. Med. 331, 1428–1436. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann, T. R. & Coffman, R. L. (1989) Annu. Rev. Immunol. 7, 145–173. [DOI] [PubMed] [Google Scholar]
- 23.Martin, R. M., Brady, J. L. & Lew, A. M. (1998) J. Immunol. Methods 212, 187–192. [DOI] [PubMed] [Google Scholar]
- 24.Ryan, J. J. (1997) J. Allergy Clin. Immunol. 99, 1–5. [DOI] [PubMed] [Google Scholar]
- 25.Kaprio, J., Tuomilehto, J., Koskenvuo, M., Romanov, K., Reunanen, A., Eriksson, J., Stengard, J. & Kesaniemi, Y. A. (1992) Diabetologia 35, 1060–1067. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson, M. A. & Leiter, E. H. (1999) Nat. Med. 5, 601–604. [DOI] [PubMed] [Google Scholar]
- 27.Scott, F. W., Rowsell, P., Wang, G.-S., Kolb, H. & Flohé, S. (2002) Diabetes 51, 73–78. [DOI] [PubMed] [Google Scholar]
- 28.Slavin, A. J., Maron, R. & Weiner, H. L. (2001) Int. Immunol. 13, 825–833. [DOI] [PubMed] [Google Scholar]
- 29.Rollwagen, F. M. & Baqar, S. (1996) Immunol. Today 171, 979–996. [DOI] [PubMed] [Google Scholar]
- 30.Arakawa, T., Chong, D. K. X. & Langridge, W. H. R. (1998) Nat. Biotechnol. 16, 292–297. [DOI] [PubMed] [Google Scholar]
- 31.Porceddu, A., Falorni, A., Ferradini, N., Cosentino, A., Calcinaro, F., Faleri, C., Cresti, M., Lorenzetti, F., Brunetti, P. & Pezzotti, M. (1999) Mol. Breeding 5, 553–560. [Google Scholar]
- 32.Zhang, Z. J., Davidson, L., Eisenbarth, G. & Weiner, H. L. (1991) Proc. Natl. Acad. Sci. USA 88, 10252–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott, J. F., Qin, H., Bhatti, S., Smith, D. K., Singh, R. K., Dillon, T., Lauzon. J. & Singh, B. (1994) Diabetes 43, 1494–1499. [DOI] [PubMed] [Google Scholar]
- 34.Brod, S. A., Atkinson, M., Lavis, V. R., Brosnan, P. G., Hardin, D. S., Orlander, P. R., Nguyen, M. & Riley, W. J. (2001) J. Interferon Cytokine Res. 21, 1021–1030. [DOI] [PubMed] [Google Scholar]
- 35.Tian, J., Atkinson, M. A., Clare-Salzler, M., Herschenfeld, A., Forsthuber, T., Lehmann, P. V. & Kaufman, D. L. (1996) J. Exp. Med. 183, 1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maron, R., Melican, N. S. & Weiner, H. L. (1999) J. Autoimmunol. 12, 251–258. [DOI] [PubMed] [Google Scholar]
- 37.Tisch, R., Wang, B., Weaver, D. J., Liu, B., Bui, T., Arthos, J. & Serreze, D. V. (2001) J. Immunol. 166, 2122–2132. [DOI] [PubMed] [Google Scholar]
- 38.Yoon, J. W., Yoon, C. S., Lim, H. W., Huang, Q. Q., Kang, Y., Pyun, K. H., Hirasawa, K., Sherwin, R. S. & Jun, H. S. (1999) Science 284, 1183–1187. [DOI] [PubMed] [Google Scholar]
- 39.Jaeckel, E., Klein, L., Martin-Orozco, N. & von Boehmer, H. (2003) J. Exp. Med. 197, 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amrani, A., Verdaguer, J., Serra, P., Tafuro, S., Tan, R. & Santamaria, P. (2000) Nature 406, 739–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.