Abstract
Spatio-temporal control of RhoA GTPase is critical for regulation of cell migration, attachment to extracellular matrix, and cell–cell adhesions. Activation of RhoA is mediated by guanine nucleotide exchange factors (GEFs), a diverse family of enzymes that are controlled by multiple signaling pathways regulating actin cytoskeleton and cell migration. GEFs can be regulated by different mechanisms. Growing evidence demonstrates that phosphorylation serves as one of the predominant signals controlling activity, interactions, and localization of RhoGEFs. It acts as a positive and a negative regulator, and allows for regulation of RhoGEFs by multiple signaling cascades. Although there are common trends in phosphorylation-mediated regulation of some RhoGEF homologs, the majority of GEFs utilize distinct mechanisms that are dictated by their unique structure and interaction networks. This diversity enables multiple signaling pathways to use different RhoGEFs for regulation of a single central—RhoA. Here, we review current examples of phosphorylation-mediated regulation of GEFs for RhoA and its role in cell migration, discuss mechanisms, and provide insights into potential future directions.
Keywords: RhoA, RhoGEF, phosphorylation, kinase, cell migration
Introduction
Small GTPase RhoA functions as a bi-molecular switch that cycles between active GTP-bound state and inactive GDP-bound state. Interaction of activated RhoA with downstream effectors initiates events critical in regulation of cell migration. Activated RhoA stimulates formation of actin stress fibers, focal adhesion (FA) maturation, and increases contractility of actin cytoskeleton.1,2 Its activation in the rear of a migrating cell has been proposed to be critical for tail retraction.1-3 Interestingly, activated RhoA has also been detected at the front of a migrating cell, where it may participate in the regulation of membrane protrusions and contacts with extracellular matrix.1,3,4 RhoA-mediated contractility of actin cytoskeleton also influences cell–cell adhesions.2,5,6 Thus, spatiotemporal control of RhoA activity determines the location and timing of specific morphological processes occurring in a migrating cell.
RhoA activity is regulated by several distinct families of enzymes. RhoA is negatively regulated by Rho GTPase-activating protein (RhoGAPs) that accelerate the hydrolysis of GTP to GDP, thus inactivating RhoA.7 RhoA bound to GDP is maintained in this inactive state by interacting with Rho guanine nucleotide dissociation inhibitors (RhoGDIs) that sequester inactive RhoA within the cytosol.8 Positive regulation of RhoA is mediated by guanine nucleotide exchange factors (GEFs) that promote dissociation of GDP from inactive RhoA allowing it to bind GTP, which activates RhoA.9 Therefore, activation of RhoA-mediated pathways is primarily achieved through regulation of RhoGEFs.
RhoGEFs belong to a large family of proteins that are characterized by Dbl homology (DH) domain, which is primarily responsible for catalyzing the exchange of GDP for GTP. Members within this family of proteins also contain a C-terminal Pleckstrin homology (PH) domain immediately adjacent to the DH domain. Studies have shown that the DH-PH domains together have greater GEF activity in comparison to only the DH domain.10-12 RhoGEFs employ multiple strategies to regulate activity of DH-PH domains. Growing evidence indicates that in many cases control of GEF activity is achieved by phosphorylation of these RhoGEFs. In some cases phosphorylation functions as a positive regulator of GEF activity, and in others it leads to inactivation of RhoGEFs. Mechanisms of regulation also vary: phosphorylation either directly regulates activity of RhoGEFs or leads to recruitment of proteins that modulate the RhoGEF’s activity. For some RhoGEFs, phosphorylation is the primary regulatory event, whereas in other cases it appears to act in parallel with other regulatory mechanisms. In all of these cases, RhoGEFs serve as critical signaling nodes coupling multiple kinase-mediated signaling cascades to regulation of RhoA. In the current review, we discuss recent evidence of phosphorylation-mediated regulation of RhoGEF activity and its role in regulation of cell migration.
Phosphorylation of Vav Directly Stimulates GEF Activity
Regulation of Vav by phosphorylation is one of the most well-characterized mechanisms demonstrating direct activation of its GEF activity through phosphorylation-mediated conformational changes. The DH-PH domains of Vav demonstrates higher activity toward Rac1 and Cdc42 GTPases, but can also activate RhoA in vitro.13-15 Furthermore, Vav has also been reported to activate all three Rho GTPase in vivo.15,16 Experimental evidence suggests that Vav-mediated activation of RhoA is required for Vav’s potent cell transforming activity.15,17 There are three isoforms of Vav: Vav1, Vav2, and Vav3. Vav1 is primarily expressed in hematopoietic cells, where as Vav2 and Vav3 are more widely expressed. Vav regulates variety of cellular processes such as T-cell activation, phagocytoses, superoxide production in neutrophils, cancer cell proliferation, and cell migration.18-24 Current evidence indicates that Vav2-RhoA signaling plays a significant role in regulation of cancer cell migration and invasion.23,24 It is interesting to note that Vav2 activation downstream of growth factor receptors, such as transforming growth factor β-receptor (TGFβ-R) and epidermal growth factor receptor (EGFR), regulates RhoA activation in variety of tissues, including in different solid tumors.24-27 EGFR-mediated activation of Vav2-RhoA signaling has been reported to delay EGFR internalization and degradation in HeLa cells.26 However, the role Vav2-RhoA signaling in regulation of EGFR internalization and degradation in other cancer cell type, and its effect on cancer cell biology is yet to be determined.
Vav has eight well-characterized domains, which include: calponin homology (CH), acidic region (Ac), DH, PH, zinc finger, Src homology 3 (SH3), SH2, and SH3 domains (Fig. 1A).28 Current evidence demonstrates that the five N-terminal domains play a role in regulation of GEF activity by the DH domain, whereas the C-terminal SH3-SH2-SH3 domains mediate cellular localization of Vav. It is well known that Src and Syk family of kinases play a critical role in regulating Vav’s GEF activity. In its inactive form, Vav assumes an autoinhibited closed conformation where the N-terminal domains interact with DH and PH domains, thus inhibiting its GEF activity (Fig. 1B).28 Initially, it was described that Src and Syk phosphorylates Vav on Tyr-174 found in the Ac region. Phosphorylation of Tyr-174 in turn resulted in disengagement of the N-terminal peptide from its DH domain inhibitory contacts, allowing substrate access to the Vav DH domain.29 However, as we now know, this is only one part of Vav regulation as the previous study utilized a truncated Vav1 with residues 170–375, which only contained part of the Ac region and the DH domain. A recent study by Yu et al. revealed how CH, Ac, DH, PH, and zinc finger domains (CADPZ) of Vav interact with each other to negatively regulate its basal GEF activity.28 The study elegantly shows how the CADPZ domains function together to constrain interaction of the inhibitory helix from the C-terminal Ac region with the DH domain. The constraint arising from intramolecular interactions between the CADPZ domains is relieved through multistep phosphorylation of Vav, where Tyr-142 and Tyr-160 in the Ac region are initially phosphorylated, possibly destabilizing the modulatory interactions (Fig. 1B). This results in exposure of the inhibitory helix that can now be readily phosphorylated on Tyr-174, culminating in the opening of the GTPase binding interface on the DH domain (Fig. 1B).28 The multistep phosphorylation system provides tight control of Vav activity, ensuring that the main regulatory Tyr-174 amino acid can be phosphorylated only when Src activity reaches a certain level sufficient to phosphorylate all three tyrosines. Since Tyr-142 and Tyr160 are more accessible to phosphorylation, then it is possible that a tyrosine phosphatase keeps basal phosphorylation level of these residues low until Src activity is elevated. Activation of a tyrosine phosphatase also could be used to reverse processes initiated by Src and Syk. Tyrosine phosphatases PTP-PEST, PTPN22, and Shp1 have been shown to dephosphorylate Vav.30-32 PTP-PEST negatively regulates membrane protrusions by dephosphorylating Vav, and Shp1-mediated dephosphorylation of Vav inhibits cytotoxicity of natural killer cells.30,32 Thus, reversible control of Vav phosphorylation provides a mechanism for dynamic regulation of Vav-mediated processes.
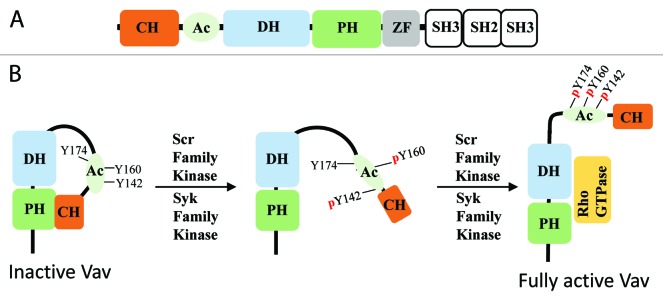
Figure 1. Multi-step phosphorylation-mediated regulation of Vav. (A) Schematic representation of the domain structures of Vav. (B) Vav activation is achieved via initial phosphorylation of Tyr-142 and Tyr-160 in the Ac region, resulting in disruption of the CH domain interaction with the PH domain. It is hypothesized that this allows for exposure of Tyr-174. Subsequent phosphorylation of this residue results in conformation change that allows RhoGTPase to access the DH domain.
Regulation of RhoGEFs by Tyrosine Phosphorylation
Due to its unique domain structure, the mechanism for phosphorylation-mediated regulation of Vav cannot be extrapolated to other RhoGEFs. However, several RhoGEFs have been shown to be phosphorylated on tyrosine residues and in most cases it leads to activation of the GEF. However, the mechanisms of activation appear to be different even among close homologs.
RH-RhoGEF subfamily of RhoA-specific GEFs is comprised of p115RhoGEF (p115), PDZ-RhoGEF (PRG), and Leukemia-associated RhoGEF (LARG) (Fig. 2). It is well established that this family of proteins are regulated specifically by Gα12/13 hetero-trimeric G-proteins.33 Therefore, RH-RhoGEFs provide a direct functional link from activated G-protein-coupled receptors (GPCRs) coupled to Gα12/13 to RhoA signaling. RH-RhoGEFs share three common domain structures (Fig. 2). They contain the tandem DH-PH domains that catalyze GDP/GTP exchange specifically on RhoA. The distinction of the RH-RhoGEF subfamily comes from the N-terminal regulator of G-protein signaling homology (RH) domain. Structural and biochemical experiments have established that GTP-bound Gα12/13 subunits make direct contacts with the RH-domain, and that this interaction positively regulates RhoGEF activity.34-38 It has also been shown that the RH domain of p115 and LARG also can function as negative regulator of Gα12/13 by increasing its intrinsic hydrolysis rate of GTP to GDP.33,37,39 While interactions of RH-RhoGEFs with Gα12/13 is believed to be the primary and the most well-characterized mode of regulation, several reports also indicate that additional control could be provided by phosphorylation of tyrosine residues.
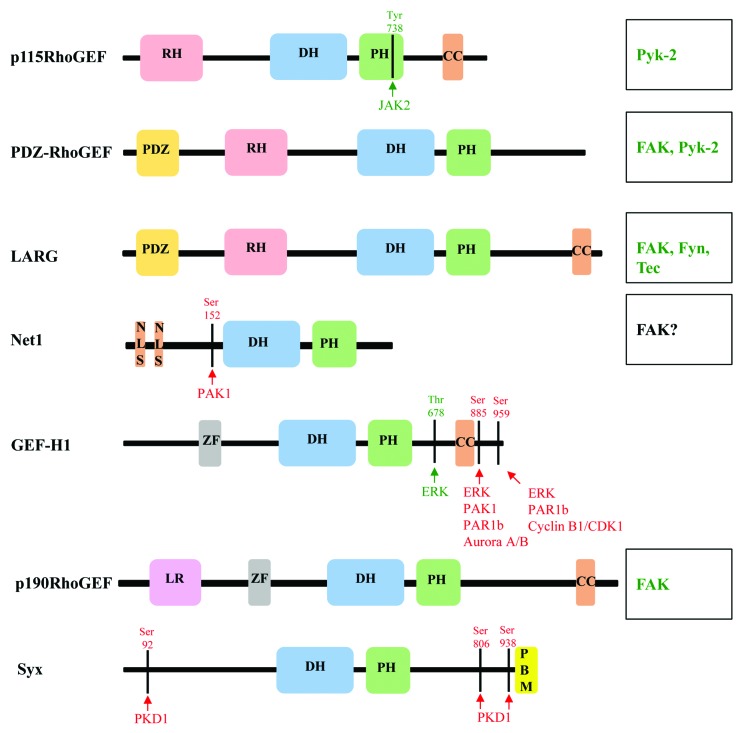
Figure 2. Domain structures and phosphorylation sites of RhoA-specific GEFs. Schematic diagram of the domain structure of RhoGEFs, and depiction of phosphorylation sites and kinases that mediate activation (green) and inhibition (red) of RhoGEFs. Kinases that are known to phosphorylate and regulate RhoGEF function where phosphorylation sites have not been identified are listed on the right (see text for detail). CC, coiled-coil; DH, Dbl homology; LR, leucine rich; NLS, nuclear localization signal; PDZ, post-synaptic density 95; disk large, zona occludens-1; PBM, PDZ binding motif; PH, Pleckstrin homology; RH, RGS homology; and ZF, Zinc finger-like binding domain.
The smallest member of RH-RhoGEF subfamily, p115, is ubiquitously expressed with highest expression observed in hematopoietic cells. Activation of angiotensin II receptor type-I (AT-1) in vascular smooth muscle cells (VSMCs) leads to phosphorylation of p115 on Tyr-738 by Janus Kinase2 (JAK2). Evidence indicates that phosphorylation Tyr-738 is sufficient for positive regulation of GEF activity of p115, independent from Gα13 binding to p115.40 AT-1-mediated phosphorylation of p115 and activation of p115-RhoA signaling was reported to contribute to increased vascular remodeling.40 The mechanism of phosphorylation-mediated activation has not been determined. However, the location of the phosphorylated residue in the PH domain of p115RhoGEF is unusual, suggesting a potentially novel mechanism for regulation of RH-RhoGEFs. Tyr-738 is buried in the hydrophobic pocket of the PH domain (Fig. 3A). Phosphorylation of this tyrosine would dramatically change the environment in the pocket and should result in conformational changes in the PH domain. Another intriguing question is how does JAK-2 access this Tyr-738 residue? Either this particular PH domain has very dynamic structure allowing Tyr-738 to be exposed at least transiently, or there is an alternative conformation that has not yet been determined. Interestingly, Tyr-738 is located within a motif highly conserved among RH-RhoGEFs, but it is still unknown if JAK-2 can phosphorylate LARG and PRG on that residue (Fig. 3B). p115 has also been reported to be phosphorylated by proline-rich tyrosine kinase2 (Pyk2) upon activation of protease-activated receptor-1 (PAR-1) in THP-1 cells.41 However, the phosphorylation site has not been determined and it is not clear if p115 phosphorylation by Pyk2 in this context is sufficient to increase its GEF activity. Non-the-less, evidence indicates that Pyk2 activity and p115 expression is required for thrombin-induced THP-1 cell migration and VSMC migration.41,42 Furthermore, Pyk2 and its close homolog focal adhesion kinase (FAK) can also regulate activity of two other members of RH-RhoGEF family, PRG and LARG.
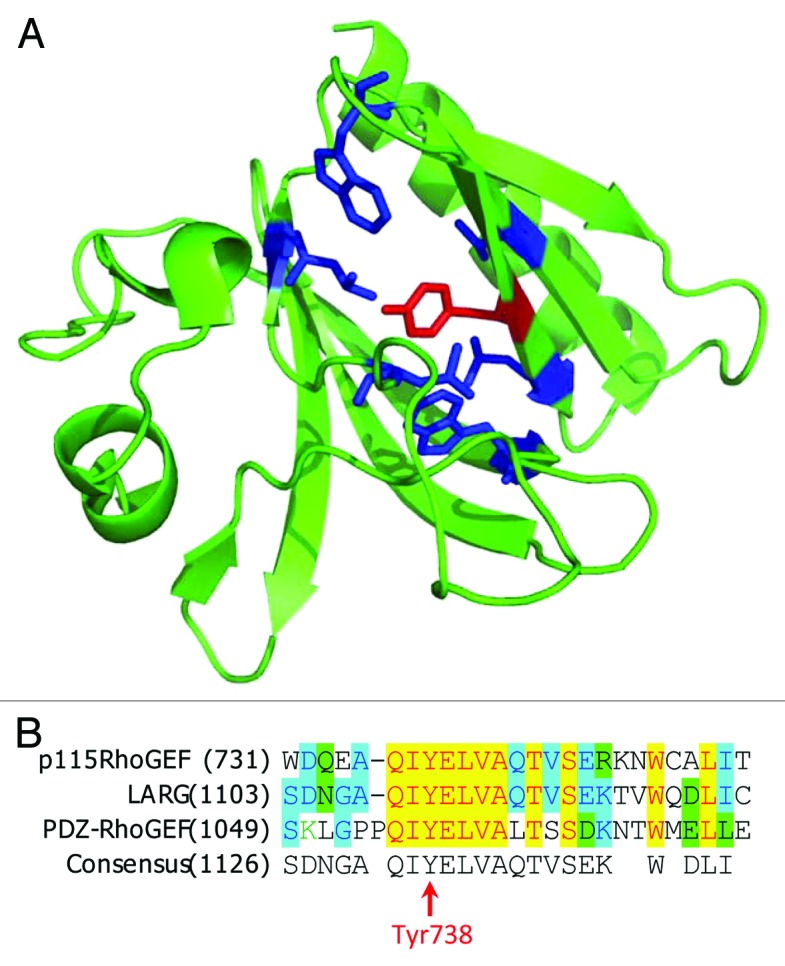
Figure 3. Phosphorylation of PH domain of p115RhoGEF by JAK2. (A) Structure of p115RhoGEF’s PH domain (PDB identifier: 3ODO). TheTyr738 residue is depicted in red and the surrounding hydrophobic residues are depicted in blue. (B) Alignment of amino acid sequences for p115RhoGEF, LARG, and PDZ-RhoGEF.
PRG and LARG are ubiquitously expressed with higher expression of PRG observed in the brain. PRG was the first RH-RhoGEF reported to be regulated by tyrosine phosphorylation. Both PRG and LARG are tyrosine phosphorylated by FAK upon PAR-1 activation in HEK293T cells. The report provides evidence that the tyrosine phosphorylation occurs in the C terminus of PRG and LARG, and that this phosphorylation positively regulates GEF activity.43 Furthermore, existing data demonstrate that PRG and LARG tyrosine phosphorylation is sufficient for positive regulation of its GEF activity independently of Gα12 or Gα13.43 Current evidence suggests that C-terminal domains of LARG and PRG mediate their homo- and hetero-dimerization, leading to inhibition of GEF activity.44,45 Thus, it is possible that phosphorylation of the C-terminal portion of LARG and PRG disrupts inhibitory dimerization, and leads to their activation.44,45 Importantly, p115 does not have the similar sequence homology in its C-terminal fragment, suggesting that this mode of regulation is unique for PRG and LARG. In the initial study, the impact of GEF phosphorylation on cell migration was not assessed. However, a study by Iwanicki and colleagues showed that indeed the interaction between FAK and PRG at focal adhesions is critical for trailing-edge retraction in fibroblasts upon LPA stimulation.46 PRG phosphorylation has also been implicated in cancer cell migration. In prostate cancer cells, circumstantial evidence implicated FAK-mediated phosphorylation of PRG downstream of bombesin-2 (BB-2) receptor in regulating prostate cancer cell migration.47 Thus, collective evidence suggests that FAK-mediated regulation of PRG and potentially LARG could represent an alternative mechanism for regulation of cell migration via RH-RhoGEFs downstream of GPCR activation.
Several other tyrosine kinases have been shown to phosphorylate PRG and LARG. Pyk2 phosphorylates PRG providing positive regulation of its GEF activity.48,49 Furthermore, Pyk2-mediated tyrosine phosphorylation of PRG downstream of AT-1 receptor regulates Rho-ROCK cascade in vascular smooth muscle cells leading to increased migration.49 Given the homology between Pyk2 and FAK, it is possible that Pyk2 regulates PRG via the same mechanism as FAK. However, the site(s) of phosphorylation on PRG by Pyk2 have not been mapped. It has also been shown that LARG is tyrosine phosphorylated by Fyn, a Src family kinase, in response to tensional force resulting in positive regulation of its GEF activity and targeting of LARG to FA.50 Suzuki et al. have also identified Tec tyrosine kinase to phosphorylate LARG. This study utilizing in vitro reconstitution assays and cell-based assays demonstrated that LARG phosphorylation by Tec makes LARG receptive to activation by Gα12 both in vitro and in vivo.37 This suggests an interesting mechanism where Tec-mediated phosphorylation of LARG provides an additional mode of control-enhancing GEF activation by GPCR signaling. However, the phosphorylation sites and the details of this potential regulation still remain to be determined.
Another RhoGEF known to be regulated by tyrosine phosphorylation is p190RhoGEF, also known as Rgnef. It is a ubiquitously expressed multi-domain-containing GEF (Fig. 2).51,52 p190RhoGEF has been shown to directly interact with FAK, and this interaction helps localize p190RhoGEF to focal adhesions. Biochemical studies have revealed that the interaction of FAK with p190RhoGEF is important for its function. The interaction between p190RhoGEF and FAK has been shown to occur via the coiled-coil domain of p190RhoGEF, which contains the residues 1292–1301, and the focal adhesion targeting (FAT) domain of FAK.53 Laminin stimulation of Neuro-2a cells promoted FAK-p190RhoGEF complex formation, which enhances p190RhoGEF tyrosine phosphorylation resulting in positive regulation of its GEF activity.53 Additional studies have also reported the importance of FAK-p190RhoGEF complex in regulation of FA formation and fibronectin-stimulated motility of mouse embryonic fibroblasts.54 In a pathological condition, evidence also supports the role of FAK-p190RhoGEF complex in regulation of colon cancer cell migration and invasion downstream of gastrin binding to cholecystokinin receptor-2.55 Furthermore, recent studies have illuminated the mechanisms by which p190RhoGEF may regulate cancer cell migration and invasion through the use of novel Rho biosensors. These studies elegantly demonstrate that p190RhoGEF plays a critical role in formation of leading edge protrusions and invadopodia through spatial regulation of RhoC (a Rho isoform), which leads to regulation of directional cell migration and invasion.56,57 These reports suggest that p190RhoGEF interaction with FAK is important for proper localization of p190RhoGEF and regulation of its GEF activity. However, what is unknown is the role of tyrosine phosphorylation of p190RhoGEF by FAK in regulating its activity. Thus, it would be of great interest to identify the tyrosine phosphorylation site(s) of p190RhoGEF and assess if phosphorylation is sufficient to regulate its GEF activity independent of its interactions with FAK.
Regulation of GEFs by Ser/Thr Kinases
While tyrosine kinases appear to predominantly activate RhoGEFs, phosphorylation by Ser/Thr kinases can be both activating and inhibitory. In most cases, phosphorylation on Ser or Thr residues either disrupts or enhances interactions with other proteins that regulates sub-cellular localization of RhoGEFs and influences their GEF activity. Below we discuss several examples of such regulation.
Guanine nucleotide exchange factor-H1 (GEF-H1) is regulated via protein–protein interaction and through protein phosphorylation. It has been reported that GEF-H1 regulates cell motility through spatial control of RhoA activation at the leading edge without having any appreciable effect on overall RhoA activation.58 This spatial regulation of RhoA activation in turn regulates focal adhesions at the leading edge in HeLa cells.58 GEF-H1 was originally identified as microtubule-associated nucleotide exchange factor.59 GEF-H1 directly interacts with either microtubules or microtubule-associated proteins (MAPs) via its N-terminal zinc finger-like domain and through its C-terminal coiled-coil domain (Fig. 2).59,60 This interaction has been shown to negatively regulate its GEF activity.60 Recently, several studies have provided evidence that GEF-H1 is both positively and negatively regulated via phosphorylation. It has been demonstrated that extracellular signal-regulated kinase (ERK) phosphorylates GEF-H1 on Thr-678 in HeLa S3 and HT1080 cells increasing its GEF activity.61 Similar results were obtained upon stimulation of LLC-PK and MDCK cells with TNFα.62 An independent study also demonstrated that GEF-H1 is activated by ERK1 downstream of FAK-Raf signaling pathway in rat fibroblasts upon application of force.50 Intriguingly, a recent study shows that ERK-mediated phosphorylation of GEF-H1 on Ser-959 inhibits its activity and regulates tumor cell migration and invasiveness.63 It has been reported that PAK-1 phosphorylates GEF-H1 on Ser-885 stimulating interaction of GEF-H1 with 14-3-3 protein. This interaction localizes GEF-H1/14-3-3 complex to microtubules, thus inhibiting its GEF activity.64 Similarly, Aurora A/B kinase and cyclin B1/cdk1 phosphorylate GEF-H1 on Ser-885 and Ser-959, respectively, thus negatively regulating its activity.65 Partioning–defective 1b (PAR1b) kinase can also phosphorylate both Ser-885 and Ser-959 on GEF-H1 in COS-7 cells. Similar to PAK-1-mediated phosphorylation of GEF-H1 on Ser-885, PAR1b-mediated phosphorylation of Ser-885 and Ser-959 increases 14-3-3 binding to GEF-H1, and thus, decreasing its GEF activity. Existing evidence also supports the notion that phosphorylation of GEF-H1 on Ser-885 and S-959 is sufficient to inhibit its GEF activity independent of 14-3-3 interaction.66 It is worth noting that while phosphorylation in the C-terminal portion (Ser-885 and Ser-959) promotes inhibitory interactions, the activating phosphorylation (Thr-678) occurs between the PH and coiled-coil domain of GEF-H1 (Fig. 2). It still remains to be determined if phosphorylation of Thr-678 affects interaction with 14-3-3 and/or association with microtubules.
Another example of negative regulation by phosphorylation is observed with neuroepithelioma transforming gene 1 (Net1) RhoGEF. Net1 is unusual among the RhoGEFs in that in its N terminus it contains multiple nuclear localization signals (NLS) (Fig. 2).67 It is thought that the NLS on Net1 plays a critical role in negative regulation of RhoA activation and stress fiber formation.68 An isoform of Net1 known as Net1A lacks two NLS, and thus, is less regulated through subcellular localization.69 It is believed that cytoplasmic redistribution of Net1 is sufficient for its GEF activation.68 However, there is evidence that supports the existence of additional regulatory elements that control Net1 activity in the cytoplasm. A report by Alberts et al. indicates that PAK1 activation downstream of Rac1 leads to phosphorylation of Net1 on Ser-152 and Ser-153 in vitro.70 Indeed, overexpression of constitutively active Rac1 increases Pak1-mediated phosphorylation of Net1 on Ser-152 in HEK293 cells, resulting in decreased Net1 RhoGEF activity.70 However, the mechanism by which phosphorylation of Net1 on Ser-152 inhibits its activity is not known. Much like other RhoGEFs, Net1A has also been shown to be important in cancer cell migration and invasion. It has been demonstrated that Net1A co-localize with FAK and promotes focal adhesion maturation in breast cancer cells.69 However, whether Net1A is phosphorylated by FAK in breast cancer cells and if that impacts its GEF activity is yet to be determined.
Synectin-binding RhoA exchange factor (Syx also known as TECH, PLEKHG5, or GEF720) is a RhoGEF that is highly expressed in the brain and endothelial cells.71-73 Syx contains DH-PH domains and a C-terminal PDZ-binding motif (Fig. 2). The PDZ-binding motif is required for Syx membrane localization through its interaction with Crumbs polarity complex.72 Cell membrane localization and Syx RhoGEF function is required for cell polarity and directional cell migration of glioblastoma multiforme cancer cells, breast cancer cells, and endothelial cells.72,74,75 It is interesting to note that in these studies with different cell types, Syx was shown to be targeted to leading edge of the cell where it mediated local RhoA-Dia activation, which was required for directional cell migration. Recently, two reports have shown that Syx is phosphorylated by protein kinase D1 (PKD1) on Ser-92, Ser-806, and Ser-938.6,76 Through mutational analysis, it was found that phosphorylation of Ser-92 and Ser-938 results in binding of Syx to 14-3-3 proteins. The authors speculate that phosphorylation of Ser-806 may induce conformational changes of Syx that may allow for dimerization of the 14-3-3 proteins bound to the N- and C-termini of Syx. This ultimately leads to conformational changes that inactivate Syx GEF activity, leading to de-stabilization cell–cell junctions.76
Concluding Remarks
Protein phosphorylation is one of the key post-translational modifications utilized in signal transduction. Thus, it is not surprising that phosphorylation is used to regulate activity of RhoGEFs. Interestingly, unlike for many other enzymes, the direct phosphorylation of the catalytic domain (DH) has not been detected so far and there is only one report demonstrating phosphorylation of PH domain in p115RhoGEF.40 As evident from current research, regulation of GEF activity is achieved by phosphorylation of residues outside the DH-PH domains affecting either intramolecular interactions or association with binding partners that modulate GEF activity. The diversity of the regulatory mechanisms enables control of different RhoGEFs through independent pathways, and allows use of combination of pathways for regulation of a single RhoGEF. This is particularly important for spatio-temporal regulation of RhoA activation, where phosphorylation and dephosphorylation of different RhoGEFs at specific subcellular locations defines local control of RhoA activity. Better understanding of these processes requires further identification and dissection of phosphorylation-driven mechanisms regulating RhoGEF activity. Recent improvements in phosphoproteomic analysis simplify identification of novel phosphorylation sites in RhoGEFs. Development of novel biosensors for RhoGEFs and application of existing biosensors for RhoA will help to identify local changes in their activity.3 It is possible to ascertain a more complete picture of kinase-mediated RhoGEF-RhoA activation in “real-time” by employing recently developed techniques that allow for temporal regulation of kinase activity in living cells.77-80 Precise control of specific kinases in living cells will be instrumental for dissection of phosphorylation-mediated regulation of RhoGEFs.
Phosphorylation-mediated regulation of RhoA signaling goes beyond modulating RhoGEF activity. Other molecular entities that regulate RhoA activity, such as GDIs and GAPs, are also controlled by phosphorylation. Evidence indicates that RhoA itself is phosphorylated on Ser-188 by cGMP-dependent protein kinase (cGK), protein kinase A (PKA), and by Ste20-related kinase (SLK).81-83 It has been shown that RhoA Ser-188 phosphorylation negatively regulates its function by promoting its association with RhoGDIs.84 Thus, phosphorylation of all components has to be taken into account in order to describe the signaling network controlling RhoA.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institutes of Health (grant R21CA159179 to Karginov AV).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/28058
References
- 1.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 2.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 4.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A. 2012;109:9905–10. doi: 10.1073/pnas.1115063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol. 2012;199:1103–15. doi: 10.1083/jcb.201207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamblin SJ, Smerdon SJ. GTPase-activating proteins and their complexes. Curr Opin Struct Biol. 1998;8:195–201. doi: 10.1016/S0959-440X(98)80038-9. [DOI] [PubMed] [Google Scholar]
- 8.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–54. doi: 10.1016/S0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Wang H, Eberstadt M, Schnuchel A, Olejniczak ET, Meadows RP, Schkeryantz JM, Janowick DA, Harlan JE, Harris EAS, et al. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell. 1998;95:269–77. doi: 10.1016/S0092-8674(00)81757-2. [DOI] [PubMed] [Google Scholar]
- 11.Kristelly R, Gao G, Tesmer JJG. Structural determinants of RhoA binding and nucleotide exchange in leukemia-associated Rho guanine-nucleotide exchange factor. J Biol Chem. 2004;279:47352–62. doi: 10.1074/jbc.M406056200. [DOI] [PubMed] [Google Scholar]
- 12.Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca(2+) sensitization pathway in smooth muscle. Structure. 2004;12:1955–65. doi: 10.1016/j.str.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–72. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 14.Heo J, Thapar R, Campbell SL. Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors. Biochemistry. 2005;44:6573–85. doi: 10.1021/bi047443q. [DOI] [PubMed] [Google Scholar]
- 15.Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–9. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 16.Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol Cell Biol. 2000;20:7160–9. doi: 10.1128/MCB.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmby TR, Abe K, Karnoub AE, Der CJ. Vav transformation requires activation of multiple GTPases and regulation of gene expression. Mol Cancer Res. 2004;2:702–11. [PubMed] [Google Scholar]
- 18.Tybulewicz VLJ. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–74. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Hall AB, Gakidis MAM, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–16. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Utomo A, Cullere X, Glogauer M, Swat W, Mayadas TN. Vav proteins in neutrophils are required for FcgammaR-mediated signaling to Rac GTPases and nicotinamide adenine dinucleotide phosphate oxidase component p40(phox) J Immunol. 2006;177:6388–97. doi: 10.4049/jimmunol.177.9.6388. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z, Liu Y, Lu S, Wang A, Lee K, Wang L-H, Revelo M, Lu S. Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer. Mol Endocrinol. 2006;20:2315–25. doi: 10.1210/me.2006-0048. [DOI] [PubMed] [Google Scholar]
- 22.Razidlo GL, Wang Y, Chen J, Krueger EW, Billadeau DD, McNiven MA. Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Dev Cell. 2013;24:573–85. doi: 10.1016/j.devcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Varas P, Coló GP, Bartolomé RA, Paterson A, Medraño-Fernández I, Arellano-Sánchez N, Cabañas C, Sánchez-Mateos P, Lafuente EM, Boussiotis VA, et al. Rap1-GTP-interacting adaptor molecule (RIAM) protein controls invasion and growth of melanoma cells. J Biol Chem. 2011;286:18492–504. doi: 10.1074/jbc.M110.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molli PR, Adam L, Kumar R. Therapeutic IMC-C225 antibody inhibits breast cancer cell invasiveness via Vav2-dependent activation of RhoA GTPase. Clin Cancer Res. 2008;14:6161–70. doi: 10.1158/1078-0432.CCR-07-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadimitriou E, Kardassis D, Moustakas A, Stournaras C. TGFβ-induced early activation of the small GTPase RhoA is Smad2/3-independent and involves Src and the guanine nucleotide exchange factor Vav2. Cell Physiol Biochem. 2011;28:229–38. doi: 10.1159/000331734. [DOI] [PubMed] [Google Scholar]
- 26.Thalappilly S, Soubeyran P, Iovanna JL, Dusetti NJ. VAV2 regulates epidermal growth factor receptor endocytosis and degradation. Oncogene. 2010;29:2528–39. doi: 10.1038/onc.2010.1. [DOI] [PubMed] [Google Scholar]
- 27.Peng F, Zhang B, Ingram AJ, Gao B, Zhang Y, Krepinsky JC. Mechanical stretch-induced RhoA activation is mediated by the RhoGEF Vav2 in mesangial cells. Cell Signal. 2010;22:34–40. doi: 10.1016/j.cellsig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Yu B, Martins IRS, Li P, Amarasinghe GK, Umetani J, Fernandez-Zapico ME, Billadeau DD, Machius M, Tomchick DR, Rosen MK. Structural and energetic mechanisms of cooperative autoinhibition and activation of Vav1. Cell. 2010;140:246–56. doi: 10.1016/j.cell.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghazadeh B, Lowry WE, Huang X-Y, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–33. doi: 10.1016/S0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 30.Sastry SK, Rajfur Z, Liu BP, Cote J-F, Tremblay ML, Burridge K. PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J Biol Chem. 2006;281:11627–36. doi: 10.1074/jbc.M600897200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006;281:11002–10. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 32.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–9. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozasa T, Hajicek N, Chow CR, Suzuki N. Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13. J Biochem. 2011;150:357–69. doi: 10.1093/jb/mvr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–4. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 35.Hajicek N, Kukimoto-Niino M, Mishima-Tsumagari C, Chow CR, Shirouzu M, Terada T, Patel M, Yokoyama S, Kozasa T. Identification of critical residues in G(α)13 for stimulation of p115RhoGEF activity and the structure of the G(α)13-p115RhoGEF regulator of G protein signaling homology (RH) domain complex. J Biol Chem. 2011;286:20625–36. doi: 10.1074/jbc.M110.201392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Singer WD, Danesh SM, Sternweis PC, Sprang SR. Recognition of the activated states of Galpha13 by the rgRGS domain of PDZRhoGEF. Structure. 2008;16:1532–43. doi: 10.1016/j.str.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki N, Nakamura S, Mano H, Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci U S A. 2003;100:733–8. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274:5868–79. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 39.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–11. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 40.Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–90. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 41.Gadepalli R, Kotla S, Heckle MR, Verma SK, Singh NK, Rao GN. Novel role for p21-activated kinase 2 in thrombin-induced monocyte migration. J Biol Chem. 2013;288:30815–31. doi: 10.1074/jbc.M113.463414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Gadepalli R, Singh NK, Kundumani-Sridharan V, Heckle MR, Rao GN. Novel role of proline-rich nonreceptor tyrosine kinase 2 in vascular wall remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2012;32:2652–61. doi: 10.1161/ATVBAHA.112.253112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chikumi H, Fukuhara S, Gutkind JS. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J Biol Chem. 2002;277:12463–73. doi: 10.1074/jbc.M108504200. [DOI] [PubMed] [Google Scholar]
- 44.Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, Gutkind JS. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–40. doi: 10.1038/sj.onc.1207012. [DOI] [PubMed] [Google Scholar]
- 45.Artamonov MV, Momotani K, Stevenson A, Trentham DR, Derewenda U, Derewenda ZS, Read PW, Gutkind JS, Somlyo AV. Agonist-induced Ca2+ sensitization in smooth muscle: redundancy of Rho guanine nucleotide exchange factors (RhoGEFs) and response kinetics, a caged compound study. J Biol Chem. 2013;288:34030–40. doi: 10.1074/jbc.M113.514596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- 47.Zheng R, Iwase A, Shen R, Goodman OB, Jr., Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25:5942–52. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- 48.Ying Z, Giachini FRC, Tostes RC, Webb RC. PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arterioscler Thromb Vasc Biol. 2009;29:1657–63. doi: 10.1161/ATVBAHA.109.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–6. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 50.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gebbink MFBG, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–13. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Horck FPG, Ahmadian MR, Haeusler LC, Moolenaar WH, Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–56. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- 53.Zhai J, Lin H, Nie Z, Wu J, Cañete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–73. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 54.Lim Y, Lim S-T, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H-G, Nam J-O, Miller NLG, Tanjoni I, Walsh C, Shi L, Kim L, Chen XL, Tomar A, Lim S-T, et al. p190RhoGEF (Rgnef) promotes colon carcinoma tumor progression via interaction with focal adhesion kinase. Cancer Res. 2011;71:360–70. doi: 10.1158/0008-5472.CAN-10-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–44. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo-Cordero JJ, Sharma VP, Roh-Johnson M, Chen X, Eddy R, Condeelis J, Hodgson L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J Cell Sci. 2013;126:3356–69. doi: 10.1242/jcs.123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nalbant P, Chang Y-C, Birkenfeld J, Chang Z-F, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070–82. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–60. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 60.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 61.Fujishiro SH, Tanimura S, Mure S, Kashimoto Y, Watanabe K, Kohno M. ERK1/2 phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem Biophys Res Commun. 2008;368:162–7. doi: 10.1016/j.bbrc.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 62.Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szászi K. GEF-H1 mediates tumor necrosis factor-α-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem. 2009;284:11454–66. doi: 10.1074/jbc.M805933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Thun A, Preisinger C, Rath O, Schwarz JP, Ward C, Monsefi N, Rodríguez J, Garcia-Munoz A, Birtwistle M, Bienvenut W, et al. Extracellular signal-regulated kinase regulates RhoA activation and tumor cell plasticity by inhibiting guanine exchange factor H1 activity. Mol Cell Biol. 2013;33:4526–37. doi: 10.1128/MCB.00585-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279:18392–400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 65.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M. Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem. 2011;286:44576–84. doi: 10.1074/jbc.M111.267021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qin H, Carr HS, Wu X, Muallem D, Tran NH, Frost JA. Characterization of the biochemical and transforming properties of the neuroepithelial transforming protein 1. J Biol Chem. 2005;280:7603–13. doi: 10.1074/jbc.M412141200. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem. 2002;277:14581–8. doi: 10.1074/jbc.M111108200. [DOI] [PubMed] [Google Scholar]
- 69.Carr HS, Zuo Y, Oh W, Frost JA. Regulation of focal adhesion kinase activation, breast cancer cell motility, and amoeboid invasion by the RhoA guanine nucleotide exchange factor Net1. Mol Cell Biol. 2013;33:2773–86. doi: 10.1128/MCB.00175-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–61. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 71.De Toledo M, Coulon V, Schmidt S, Fort P, Blangy A. The gene for a new brain specific RhoA exchange factor maps to the highly unstable chromosomal region 1p36.2-1p36.3. Oncogene. 2001;20:7307–17. doi: 10.1038/sj.onc.1204921. [DOI] [PubMed] [Google Scholar]
- 72.Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–53. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garnaas MK, Moodie KL, Liu ML, Samant GV, Li K, Marx R, Baraban JM, Horowitz A, Ramchandran R. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in Vivo. Circ Res. 2008;103:710–6. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, Feathers R, Anastasiadis PZ. The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol Cell Biol. 2013;33:4909–18. doi: 10.1128/MCB.00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu M, Horowitz A. A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol Biol Cell. 2006;17:1880–7. doi: 10.1091/mbc.E06-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ngok SP, Geyer R, Kourtidis A, Storz P, Anastasiadis PZ. Phosphorylation-mediated 14-3-3 protein binding regulates the function of the rho-specific guanine nucleotide exchange factor (RhoGEF) Syx. J Biol Chem. 2013;288:6640–50. doi: 10.1074/jbc.M112.432682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qiao Y, Molina H, Pandey A, Zhang J, Cole PA. Chemical rescue of a mutant enzyme in living cells. Science. 2006;311:1293–7. doi: 10.1126/science.1122224. [DOI] [PubMed] [Google Scholar]
- 78.Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol. 2010;28:743–7. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karginov AV, Hahn KM. Allosteric activation of kinases: design and application of RapR kinases. Curr Protoc Cell Biol 2011; Chapter 14:Unit 14 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karginov AV, Zou Y, Shirvanyants D, Kota P, Dokholyan NV, Young DD, Hahn KM, Deiters A. Light regulation of protein dimerization and kinase activity in living cells using photocaged rapamycin and engineered FKBP. J Am Chem Soc. 2011;133:420–3. doi: 10.1021/ja109630v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guilluy C, Rolli-Derkinderen M, Loufrani L, Bourgé A, Henrion D, Sabourin L, Loirand G, Pacaud P. Ste20-related kinase SLK phosphorylates Ser188 of RhoA to induce vasodilation in response to angiotensin II Type 2 receptor activation. Circ Res. 2008;102:1265–74. doi: 10.1161/CIRCRESAHA.107.164764. [DOI] [PubMed] [Google Scholar]
- 82.Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, et al. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- 83.Jones SE, Palmer TM. Protein kinase A-mediated phosphorylation of RhoA on serine 188 triggers the rapid induction of a neuroendocrine-like phenotype in prostate cancer epithelial cells. Cell Signal. 2012;24:1504–14. doi: 10.1016/j.cellsig.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem. 2003;278:19023–31. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]