Abstract
Cell migration and invasion involve the formation of cell adhesion structures as well as the dynamic and spatial regulation of the cytoskeleton. The adhesive structures known as podosomes and invadopodia share a common role in cell motility, adhesion, and invasion, and form when the plasma membrane of motile cells undergoes highly regulated protrusions. Palladin, a molecular scaffold, co-localizes with actin-rich structures where it plays a role in their assembly and maintenance in a wide variety of cell lines. Palladin regulates actin cytoskeleton organization as well as cell adhesion formation. Moreover, palladin contributes to the invasive nature of cancer metastatic cells by regulating invadopodia formation. Palladin seems to regulate podosome and invodopodia formation through Rho GTPases, which are known as key players in coordinating the cellular responses required for cell migration and metastasis.
Keywords: Palladin, actin, invasion, invadopodia, podosome
Cell Motility and Metastasis
Cell migration is necessary in the normal development of multicellular organisms, and is vital for physiological processes such as embryogenesis, immune surveillance, tissue damage, and regeneration.1 However, abnormal regulation of cell migration leads to the progression of several chronic human diseases, such as cancer invasion and metastasis. Therefore, understanding the fundamental mechanisms of cell migration is central for limiting the progression of the disease.2
Cell migration is a multistep process that requires the dynamics of disassembly, relocation, and reassembly of cytoskeletal structures within the cell, as well as the rearrangement of cell–substrate adhesions.3 Metastasis is the spread of cancer cells to distant sites in the body leading to the formation of secondary tumors, which are the major cause of death in cancer patients, rather the primary tumors themselves. Metastasis begins when tumor cells detach from their primary lesion or neighboring cells, acquire a motile phenotype, gain the ability to invade to permit intravasation, enter the blood/lymphatic circulation, and finally, extravasate and proliferate at a distant site.4-7 The ability of metastatic cells to migrate and invade through the surrounding extracellular matrix depends on the formation of protrusive structures that differ by their morphological, structural, and functional characteristics. These protrusive structures are known as filopodia, lamellipodia, and invadopodia/podosomes.1,8 Cell migration and invasion is usually initiated in response to extracellular cues or chemoattractants, which vary between diffusible factors or signals from neighboring cells and extracellular matrix. Upon binding to surface receptors, these cues stimulate intracellular signaling pathways that regulate the dynamic organization of specific types of actin arrays. Different regulators of the actin cytoskeleton have been involved in the process of cell migration, invasion, and metastasis, including Rho GTPases such as Rac, Rho and Cdc42, Wiskott–Aldrich syndrome protein (WASP) family proteins/Arp2/3 complex, cortactin, LASP-1, Mena, Cofilin, AFAP-110, and profilin.1,2,9
Palladin Isoforms and Relatives
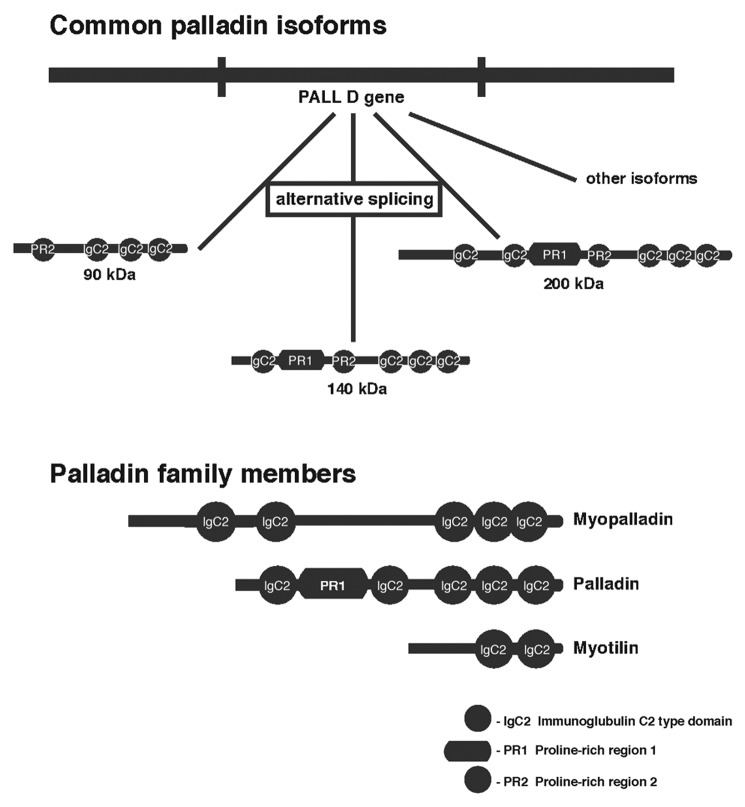
Palladin, a recently discovered actin-associated protein, is known to have an essential role in co-localizing with actin-rich structures for their assembly and maintenance in a wide variety of cell lines.10,11 A 90-kDa palladin was found to be highly expressed in vertebrates, and is the most abundant and ubiquitous in both embryonic and adult mouse tissues.11,12 The 90-kDa palladin has three Ig-domains (Fig. 1), and therefore is considered as a new member of a family of cytoplasmic proteins with these structural modules.10 The Ig-domains provide rigidity for the proteins, function as a ruler separating structural components at proper distance,13 and serve as sites for intermolecular interactions.10 Other isoforms of palladin were discovered, all resulting from alternative splicing. In mice, the palladin gene expressed three major isoforms, which are the 90, 140, and 200 kDa; however, several other isoforms could exist in specific tissue cells via alternative splicing.12,14 Their domain structures are illustrated in Figure 1. The 90 and 140 kDa isoforms, along with other isoforms, appeared to be highly expressed in embryonic developing mouse tissues and organs, whereas the 200-kDa isoform was limited to only heart and bone.12 Expression levels were greatly reduced in a number of adult tissues such as the heart, liver, skeletal muscles, and kidney, supporting the idea that palladin isoforms might be required in establishing the cytoskeletal organization of cells as they differentiate, thus contributing to cell morphology in unique ways.11,12
Figure 1. Schematic representation of major palladin isoforms, palladin family members, and their domain organization.
There are two close relatives of palladin, known as myopalladin and myotilin, all containing multiple copies of a distinctive Ig-like domain (immunoglobulin-like domain) (Fig. 1).15,16 Myopalladin, expressed only in heart and skeletal muscle, got its name due to its surprising homology with the protein palladin. Myopalladin has two distinct regions within its COOH-terminal 90-kD domain, known as the nebulin/nebulette and the α-actinin-binding sites. These sites are highly homologous with those found in palladin, suggesting that both palladin and myopalladin might be members of the same gene family.15 Since palladin is known to co-localize with α-actinin in focal adhesions, stress fibers, cell–cell junctions, and Z-lines,11 this raised the possibility that myopalladin and palladin have common regulatory roles in stress fiber and Z-line assembly.15 Myotilin, on the other hand, is limited only in skeletal and cardiac muscle serves as a cytoskeleton-organizing protein where its expression in adult tissues in restricted.10,15,16 Comparison indicated that the homology between palladin and myotilin is not only restricted to the Ig-domains, but extended to the N-terminal sequence.10 Myotilin, due to its Ig domains, functions mainly in regulating actin organization and generating actin-based arrays by serving as an F-actin-binding protein required for crosslinking F-actin into bundles.17 This suggested that palladin might also function as an F-actin-binding protein, and indeed, the full-length 90-kDa palladin was shown to bind to F-actin and crosslinked actin filaments into bundles.14,18
There is more to palladin than serving as an actin-crosslinking protein, as it also plays an important role as a cytoskeleton scaffolding molecule by interacting with different actin binding proteins required for regulation of cytoskeleton organization (Table 1).19 Palladin isoforms contain one or two polyproline stretches that act as binding sites for other regulatory proteins important for the organization of actin filament arrays. For instance, palladin binds to profilin, a key regulator of actin dynamics, via its proline-rich region (PR2). Profilin, in turn, has a large number of binding protein complexes, such as the actin-related protein (ARP2/3 complex), WASP/WAVE/Scar, and VASP, all crucial in modulating its localization and its role maintenance and organization of arrays of actin filaments.20,21 Other than profilin, palladin also binds directly to VASP and α-actinin (known as actin-crosslinking proteins), and ArgBP2 (Abl/Arg kinase adaptor protein), and it was shown to co-localize with those proteins to stress fibers and focal adhesions.11,21,22 Palladin and filament A, known as actin-associated proteins, also interact with FHL2 and ανβ5, where they are involved in linking ανβ5 at focal adhesion sites in osteoblasts enhancing their adhesion and migration.23 Palladin also binds to Eps8, and together these two proteins function in the rapid and transient remodeling of the actin cytoskeleton, promoting the formation of dorsal ruffles and podosomes upon PDGF and phorbol ester treatment.24 Lasp-1, a protein known to be associated with sites of cytoskeletal organization, was also identified to bind to palladin. The co-localization of these two proteins influenced the actin polymerization and the bundling of actin filaments.12 Moreover, palladin binds to the active form of ezrin through Ig-domains 2-3. The existence of ezrin and palladin in an ezrin–palladin complex could unify two crucial signaling pathways, which are the Rho-pathway and the VASP-mediated control of the acto-myosin system.10 Ezrin has been stated to play a significant role in the metastatic capabilities of various types of human tumors, such as pancreatic tumors, suggesting that palladin might also be involved in that process.25 In addition to the diversity of palladin binding proteins, palladin binds to Src and, through this interaction, it regulates cytoskeletal actin rearrangements for the formation of podosomes.26
Table 1. Representing palladin partners.
| Palladin | Palladin-binding partners | Functions in |
| Profilin | Maintenance and organization of arrays of actin filaments | |
| α-actinin | Co-localize to stress fibers and focal adhesion. Involved in cell motility and cell-cell adhesion | |
| Vasp | ||
| ArgBP2 | ||
| FHL2 and β5 | Co-localize at focal adhesion sites in osteoblasts. Increase cell adhesion to and migration on matrix proteins, as well as enhance osteoblast differentiation | |
| Eps8 | Dorsal ruffles and podosome formation. Also enhances the progression and metastatic invasion of cancer cells | |
| Lasp1 | Actin polymerization and bundling of actin filaments | |
| Ezrin | Control the acto-myosin system. Also crucial for the metastatic capabilities of various types of tumors | |
| Src | Actin-cytoskeleton rearrangements, and podosome formation |
Role of Palladin in Cell Motility
Previous experiments indicated that palladin has a specialized function in the organization of actin cytoskeleton needed for cell migration and cellular contractility.11,12 Since cell motility depends on the regulation of actin cytoskeleton, palladin appeared to regulate cell motility by the formation of cell adhesions and maintenance of cell structure in trophoblast and fibroblast cells. This was observed as upon knockdown of palladin in fibroblasts, it resulted in the loss of stress fibers and focal adhesions and in cell rounding.11 On the contrary, when palladin expression was upregulated in COS-7 cells, it led to actin bundling, and the overexpression of the 90-kDa and 140-kDa isoforms resulted in the formation of robust actin cables and the assembly of star-like F-actin arrays, respectively.12 Previous studies suggested that palladin has a crucial role in the normal function and development of the nervous system. Palladin was found to be expressed in the excitatory terminals of a rat’s central nervous system, where in adult rodents palladin was expressed in the brain and spinal cord, especially in the olfactory bulb, cerebral and cerebellar cortex, hippocampus, amygdala, superior colliculus, and superficial laminae of the spinal dorsal horn.27 Also, palladin appeared to be targeted to the developing axon but not the dendrites, where it was strongly expressed in the axonal growth cone. It contributed to neurite outgrowth in cortical neurons, as upon its knockdown in both B35 neuroblastoma cell line and cultured primary cortical neurons, cells failed to extend neuritis.28 Moreover, palladin seemed to have a crucial role in embryogenesis, where its knockdown led to neural tube closure defects and embryonic lethality. Loss of palladin in murine embryonic fibroblast cells (MEFs) disorganized actin cytoskeleton architecture, impaired the formation of stress fibers, decreased b1-integrin protein expression, decreased adhesion to fibronectin, and weakened cell-ECM interaction.29,30 Also, a decrease in cell motility was observed in paladin-null MEFs, which could be the result of damaged actin cytoskeleton.30 Moreover, as previously said, palladin contributed to osteoblast migration and adhesion where palladin was observed to interact with FHL2 and co-localize with αvβ5 at focal adhesion sites in osteoblasts.23
Role of Podosomes and Invadopodia in Cell Motility
As previously said, actin cytoskeleton organization is important for cell motility and adhesion. Cell motility and invasion depend on the ability of the cell to form adhesion structures to its substrate, which explains the reason why cells with reduced cell adhesions migrate at a slower rate or do not move at all.29,30 These surface structures are known as podosomes and invadopodia, which form when the plasma membrane of motile cells undergoes highly regulated protrusions and invaginations. The concept of podosomes and invadopodia is somehow similar since they are similar in appearance, and have common architectural features and functions. However, these transient surface membrane distortions are distinct, in that they differ in their way of regulation, molecular components, and depend on the cell types.31
Podosomes are highly dynamic contact sites that were initially observed in cells that were transformed by the Rous sarcoma virus, and in monocyte-derived cells such as macrophages and osteoclasts.32-34 These initially named rosettes consist of dot-shaped F-actin-rich close contacts formed of a central core of actin filaments surrounded by a juxtamembrane ring that is enriched in vinculin, talin, α-actinin, Src, and tyrosine-phosphorylated proteins.31,32,35,36 They are accompanied by actin polymerization regulatory proteins such as gelsolin, cortactin, dynamin, WASP/NWASP, and Arp2/3.31,37 A little is known about their function; however, they act as focal adhesions and they contain metalloproteases, which might contribute to matrix degradation at their formation sites.38 The exact regulation of podosome initiation and dynamics in response to the cell environmental factors has always remained unclear. Soluble factors such as chemotactic or growth factors acting through serpentine and receptor tyrosine kinases were required for cell polarization and podosome initiation in myeloid cells. Also, integrin ligands endorse the recruitment and accumulation of podosomal components.39 Recent studies showed that WASP and WIP are required for actin polymerization and protrusion formation at the cell margin, arrangement of podosome cores, and configuration and dynamics of integrins and integrin-associated proteins.40-42 Moreover, WASPs not only appear to regulate podosome formation, but also an open conformation on WASP leads to podosome disassembly.23,39,43 In brief, podosomes are highly dynamic structures that not only are involved in the adhesion of cells, but also contribute in tissue invasion and matrix remodeling. Yet, molecular mechanisms and coordination of signaling pathways triggered by environmental factors leading to cell polarization and podosome formation has yet to be determined.
Invadopodia are characterized by convolutions and extensions at the ventral or lower surface of plasma membrane of invasive cancer cells that appear morphologically similar to podosomes. They were first observed and described as rosettes, much similar to podosomes, formed by Rous sarcoma virus-transformed chicken embryonic fibroblasts.44 Much like podosomes, invadopodia formation is regulated by actin cytoskeleton regulatory proteins, such as cortactin, cofilin, Mena, fascin, formin, Neural Wiskott-Aldrich syndrome protein (N-WASP), and Arp2/3 complex.45-50 The ability of cancer cells to form invadopodia is related to their metastatic abilities, where they serve as cellular digestion of underlying matrix promoting 3D invasion through ECM. Membrane-Type-1 MMP was found to co-localize with invadopodia and osteoclast podosomes where they play a crucial role in cell invasion and cell migration.31 Various extracellular and intracellular signals such as growth factor stimulation and integrin activation regulate invadopodia formation.51,52 Moreover, oncogenic growth factor receptors and signaling proteins, such as EGF receptor, PDGF receptor, Met, Abl, Src, and phosphoinositide 3-kinase p110alpha are involved in invadopodia formation.50,53-57 So now, even though podosomes and invadopodia are similar in appearance, localization, and composition, one should note that a major difference between invadopodia and podosomes is their formation from different cell types. Unlike invadopodia, which are formed by cancer invasive cells and in transformed fibroblasts, podosomes are found in non-cancerous cells such as immune-related cells (macrophages, monocytes), dendritic cells, osteoclasts, endothelial cells, and smooth muscle.49,58
Role of Palladin in Invasion Through Podosomes and Invadopodia Formation
Since palladin plays a crucial role in regulating actin cytoskeleton organization, cell adhesion, and cell motility, this suggested the possibility that palladin might also be contributing in the invasive nature of cancer metastatic cells. Ryu et al. analyzed global patterns of gene expression using SAGE in pancreatic cancer due to its high invasive capabilities and other tumor types looking for components of invasion and insights about its process. Not shockingly, palladin appeared to be found in a cluster of invasion-specific genes in pancreatic and colorectal cancers.59 Few years after, cDNA microarray analysis was done in Condeelis lab on a population of collected cells in order to identify the gene expression profile of invasive carcinoma cells in primary mammary tumors. Specific genes were identified to be upregulated in a population of aggressively motile cells when compared with non-motile cells, one of which was the human palladin gene, which was upregulated by 3-folds.9 For instance, palladin expression levels were found higher in malignant tissues than in normal breast samples in breast cancer patients. It appeared that highly invasive breast cell lines such as BT549, Hs 578, MDA-MB-231, and SUM159 express much higher levels of palladin than in non-invasive T49D, BT474, ZR75.1, and MCF7 breast cancer cell lines.19 Previous microarrays showed that the invasive motility seen in breast cancer cell lines implanted in a mouse host is due to the overexpression of palladin, suggesting that palladin may be responsible for the migratory and invasiveness of breast cancer cells.9 Palladin appeared also to enhance podosome formation and localize to podosomes in invasive cancer cell lines, signifying the importance of palladin in their invasiveness ability. In addition to that, to determine the role of palladin in podosome formation, palladin knockdown led to a significant decrease in podosome formation in invasive breast cancer cell lines treated with PDBu.19 Performing gene expression analysis, palladin-binding protein Eps8 also plays a role in breast and thyroid cancer progression and metastatic invasion, as it appeared to be overexpressed in breast and thyroid tumors.60,61 Furthermore, palladin has been implicated in other highly invasive cancers, such as pancreatic cancer. Pancreatic cancer is one of the most aggressive malignancies found in humans and is lethal in 95–99% of the cases due to the aggressive invasive motility of pancreatic cells.62 Thus, possibly, the strongest support for the role of palladin in invasive cancer came from a study of genes involved in pancreatic cancer, where the human palladin gene was point mutated in a form of pancreatic cancer and overexpressed in sporadic pancreatic tumors shown by microarray analysis and immunohistochemical staining of tumor sections.14,63,64 Eps8 palladin-binding-protein was found to be upregulated in pancreatic tumors and metastatic tumor-derived cell lines.65
Cancer-associated fibroblasts, or myofibroblasts, are found in the stromal compartment surrounding solid tumors. These activated fibroblasts have contractile properties and an α-smooth muscle actin (α-SMA) marker as a hallmark of these cells.66,67 Palladin was found to be overexpressed in cancer-associated fibroblasts of different types of tumors such as pancreas, breast, lung, kidney, and ovary, promoting their invasion and metastasis. Moreover, paladin-expressing fibroblasts were found next to cancer cells in lymph node and liver metastases.14,68-70 In the early stages of neoplastic progression in pancreatic tumorigenesis, palladin expression levels in stromal fibroblasts increased and co-localized with α-SMA. The initiating event in pancreatic ductal adenocarcinoma followed by increased palladin expression levels in fibroblasts and their transformation into myofibroblasts, appeared also to be triggered by co-culturing normal fibroblasts with k-ras-expressing epithelial cells through k-ras mutation. In fact, palladin was behind the upregulation of α-SMA and transformation of fibroblasts into myofibroblasts. This overexpression enhanced the cellular migration and invasion of fibroblasts through the extracellular matrix creating tunnels through which cancer cells can easily follow and metastasize. This was done through invadopodia filled with matrix-destroying enzymes.66 Recent studies showed that the exposure of cells to wounding media enhances their migration, which was the case also in palladin-expressing fibroblasts.66,71 Protein Kinase C (PKC) appeared to regulate assembly of invadopodia in palladin-expressing CAFs, as it was known to stimulate podosomes and invadopodia formation in several other cell lines.5,72-74 Moreover, phorbol ester stimulation enhanced PMA-induced invadopodia formation and its co-localization with palladin in carcinoma associated fibroblasts (CAFs), leading to matrix degradation and enhancing in vitro and in vivo invasion of pancreatic cancer cells.5 These results show that not only palladin plays a crucial role in regulating cell adhesion and cell motility, but also contributes in invasive motility of invasion cancer cells.
Palladin and Rho GTPases
Rho GTPases are known to be involved in cytoskeletal remodeling during cell migration and cell adhesion.2 Few studies demonstrated that palladin plays a crucial role in their activation, suggesting that this link further contributes in podosome and invadopodia formation. To start with, a previous study showed that palladin binds to Eps8-enhancing podosome formation in vascular smooth muscle cells. Knowing that Eps8 has been reported to play a crucial role in regulating the activity of Rac, it was tested whether palladin was involved in this pathway too. As a result, paladin, through its interaction with Eps8, appeared to play a critical role in Rac activation, as the levels of active Rac decreased upon palladin knockdown in HeLa cells.24 A recent study examined the role of palladin overexpression in the invasive ability of activated fibrobalasts. Palladin-expressing HDF-WT or HDF-FX cells resulted in the destruction and degradation of the gelatin surface far more than what would be normally expected from invadopodia alone. Thus, wondering whether palladin-induced myofibroblasts had enhanced contractility causing this high tear of the matrix, RhoA levels were investigated in palladin-expressing myofibroblasts. Results showed that RhoA levels were increased by 2-folds compared with normal human dermal fibroblasts (HDFs).66 Furthermore, another more recent study speculated whether Cdc42 is involved in PMA-induced invadopodia assembly and whether palladin regulates the activity of Cdc42 in cancer-associated fibroblasts (CAFs). Knowing that Cdc42 was previously found in invadopodia in smooth muscle cells, metastatic rat carcinoma mammary cells, and human melanoma cells, Cdc42 was tested for its role in invadopodia formation in CAFs. Results showed that Cdc42-knockdown cells treated with PMA were unable to form invadopodia, proving that Cdc42 activation is crucial for invadopodia formation and increase invasion. Also, checking whether palladin modulates the activity of Cdc42, palladin expression was knocked down. As a result, the activity of Cdc42 dropped significantly in the absence of palladin expression. Thus, this concludes that palladin is required for Cdc42 activation, which in turn, is necessary for invadopodia assembly and increase invasion.5
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/28024
References
- 1.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 3.Lambrechts A, Van Troys M, Ampe C. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol. 2004;36:1890–909. doi: 10.1016/j.biocel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad A, Hart IR. Mechanisms of metastasis. Crit Rev Oncol Hematol. 1997;26:163–73. doi: 10.1016/S1040-8428(97)10002-6. [DOI] [PubMed] [Google Scholar]
- 5.Goicoechea SM, García-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG, Hwang RF, Urrutia R, Yeh JJ, Kim HJ, et al. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2013 doi: 10.1038/onc.2013.68. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker B, Sukumar S. Distant metastasis in breast cancer: molecular mechanisms and therapeutic targets. Cancer Biol Ther. 2003;2:14–21. doi: 10.4161/cbt.188. [DOI] [PubMed] [Google Scholar]
- 7.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(Suppl):1529–37. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1529::AID-CNCR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 10.Mykkänen O-M, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, Carpén O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–73. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–56. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachlin AS, Otey CA. Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- 13.Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Curr Opin Cell Biol. 1998;10:23–34. doi: 10.1016/S0955-0674(98)80083-5. [DOI] [PubMed] [Google Scholar]
- 14.Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–25. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang M-L, Mudry RE, McElhinny AS, Trombitás K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–27. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmikangas P, Mykkänen O-M, Grönholm M, Heiska L, Kere J, Carpén O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–36. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- 17.Salmikangas P, van der Ven PF, Lalowski M, Taivainen A, Zhao F, Suila H, Schröder R, Lappalainen P, Fürst DO, Carpén O. Myotilin, the limb-girdle muscular dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly. Hum Mol Genet. 2003;12:189–203. doi: 10.1093/hmg/ddg020. [DOI] [PubMed] [Google Scholar]
- 18.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, Otey CA. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–31. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- 19.Goicoechea SM, Bednarski B, García-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–98. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boukhelifa M, Moza M, Johansson T, Rachlin A, Parast M, Huttelmaier S, Roy P, Jockusch BM, Carpen O, Karlsson R, et al. The proline-rich protein palladin is a binding partner for profilin. FEBS J. 2006;273:26–33. doi: 10.1111/j.1742-4658.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- 21.Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- 22.Rönty M, Taivainen A, Moza M, Kruh GD, Ehler E, Carpen O. Involvement of palladin and α-actinin in targeting of the Abl/Arg kinase adaptor ArgBP2 to the actin cytoskeleton. Exp Cell Res. 2005;310:88–98. doi: 10.1016/j.yexcr.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Lai CF, Bai S, Uthgenannt BA, Halstead LR, McLoughlin P, Schafer BW, Chu PH, Chen J, Otey CA, Cao X, et al. Four and half lim protein 2 (FHL2) stimulates osteoblast differentiation. J Bone Miner Res. 2006;21:17–28. doi: 10.1359/JBMR.050915. [DOI] [PubMed] [Google Scholar]
- 24.Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–24. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- 25.Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA. High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun. 1999;258:395–400. doi: 10.1006/bbrc.1999.0653. [DOI] [PubMed] [Google Scholar]
- 26.Rönty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, Carpen O. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–85. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SJ, Pagliardini S, Boukhelifa M, Parast MM, Otey CA, Rustioni A, Valtschanoff JG. Palladin is expressed preferentially in excitatory terminals in the rat central nervous system. J Comp Neurol. 2001;436:211–24. doi: 10.1002/cne.1062. [DOI] [PubMed] [Google Scholar]
- 28.Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell. 2001;12:2721–9. doi: 10.1091/mbc.12.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, et al. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing beta1-integrin. J Cell Biochem. 2007;100:1288–300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- 30.Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, Xu G, Sun X, Kong H, Gu M, et al. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–15. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 32.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–91. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–14. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 35.Marchisio PC, Bergui L, Corbascio GC, Cremona O, D’Urso N, Schena M, Tesio L, Caligaris-Cappio F. Vinculin, talin, and integrins are localized at specific adhesion sites of malignant B lymphocytes. Blood. 1988;72:830–3. [PubMed] [Google Scholar]
- 36.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–57. doi: 10.1016/S0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 37.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–85. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, del Carmen Ovejero M, Hou P, Heegaard A-M, Kumegawa M, Foged NT, Delaissé JM. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J Cell Sci. 1997;110:589–96. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- 39.Monypenny J, Chou H-C, Bañón-Rodríguez I, Thrasher AJ, Antón IM, Jones GE, Calle Y. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur J Cell Biol. 2011;90:198–204. doi: 10.1016/j.ejcb.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calle Y, Antón IM, Thrasher AJ, Jones GE. WASP and WIP regulate podosomes in migrating leukocytes. J Microsc. 2008;231:494–505. doi: 10.1111/j.1365-2818.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 41.Chou H-C, Antón IM, Holt MR, Curcio C, Lanzardo S, Worth A, Burns S, Thrasher AJ, Jones GE, Calle Y. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–44. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dovas A, Gevrey J-C, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci. 2009;122:3873–82. doi: 10.1242/jcs.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, Kempski H, Burns S, Jones GE, Sinclair J, Kinnon C, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108:2182–9. doi: 10.1182/blood-2006-01-010249. [DOI] [PubMed] [Google Scholar]
- 44.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–85. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 45.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 46.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lizárraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 48.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim H-D, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91:902–7. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, Mueller SC. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006;312:1240–53. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajadurai CV, Havrylov S, Zaoui K, Vaillancourt R, Stuible M, Naujokas M, Zuo D, Tremblay ML, Park M. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. J Cell Sci. 2012;125:2940–53. doi: 10.1242/jcs.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 2010;285:40201–11. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphoinositide 3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J Cell Biol. 2011;193:1275–88. doi: 10.1083/jcb.201009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García E, Jones GE, Machesky LM, Antón IM. WIP: WASP-interacting proteins at invadopodia and podosomes. Eur J Cell Biol. 2012;91:869–77. doi: 10.1016/j.ejcb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res. 2001;61:1833–8. [PubMed] [Google Scholar]
- 60.Griffith OL, Melck A, Jones SJ, Wiseman SM. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–51. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 61.Yao J, Weremowicz S, Feng B, Gentleman RC, Marks JR, Gelman R, Brennan C, Polyak K. Combined cDNA array comparative genomic hybridization and serial analysis of gene expression analysis of breast tumor progression. Cancer Res. 2006;66:4065–78. doi: 10.1158/0008-5472.CAN-05-4083. [DOI] [PubMed] [Google Scholar]
- 62.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 63.Pogue-Geile KL, Chen R, Bronner MP, Crnogorac-Jurcevic T, Moyes KW, Dowen S, Otey CA, Crispin DA, George RD, Whitcomb DC, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salaria SN, Illei P, Sharma R, Walter KM, Klein AP, Eshleman JR, Maitra A, Schulick R, Winter J, Ouellette MM, et al. Palladin is overexpressed in the non-neoplastic stroma of infiltrating ductal adenocarcinomas of the pancreas, but is only rarely overexpressed in neoplastic cells. Cancer Biol Ther. 2007;6:324–8. doi: 10.4161/cbt.6.3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welsch T, Endlich K, Giese T, Büchler MW, Schmidt J. Eps8 is increased in pancreatic cancer and required for dynamic actin-based cell protrusions and intercellular cytoskeletal organization. Cancer Lett. 2007;255:205–18. doi: 10.1016/j.canlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One. 2012;7:e30219. doi: 10.1371/journal.pone.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 68.Goicoechea SM, Bednarski B, Stack C, Cowan DW, Volmar K, Thorne L, Cukierman E, Rustgi AK, Brentnall T, Hwang RF, et al. Isoform-specific upregulation of palladin in human and murine pancreas tumors. PLoS One. 2010;5:e10347. doi: 10.1371/journal.pone.0010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta V, Bassi DE, Simons JD, Devarajan K, Al-Saleem T, Uzzo RG, Cukierman E. Elevated expression of stromal palladin predicts poor clinical outcome in renal cell carcinoma. PLoS One. 2011;6:e21494. doi: 10.1371/journal.pone.0021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rönty MJ, Leivonen S-K, Hinz B, Rachlin A, Otey CA, Kähäri V-M, Carpén OM. Isoform-specific regulation of the actin-organizing protein palladin during TGF-β1-induced myofibroblast differentiation. J Invest Dermatol. 2006;126:2387–96. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- 71.Weinger I, Klepeis VE, Trinkaus-Randall V. Tri-nucleotide receptors play a critical role in epithelial cell wound repair. Purinergic Signal. 2005;1:281–92. doi: 10.1007/s11302-005-8132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hai C-M, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- 73.Tatin F, Varon C, Génot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–81. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 74.Xiao H, Bai X-H, Kapus A, Lu W-Y, Mak AS, Liu M. The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol. 2010;30:5545–61. doi: 10.1128/MCB.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]