Abstract
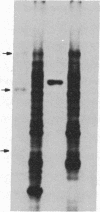
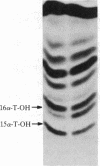
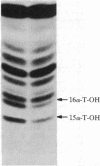
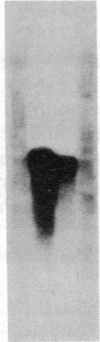
By using both double-colony hybridization and an in situ immunostaining assay for transformants, 39 cDNA clones (clone p-16 alpha) encoding mouse liver microsomal testosterone 16 alpha-hydroxylase (cytochrome P-450(16) alpha) were isolated from a cDNA library constructed in the cloning vector pUC-9 with poly(A)+ RNA immunoenriched from total liver polysomes of male 129/J mice. mRNA selected by hybridization with clone p-16 alpha translated the P-450(16) alpha apoprotein in vitro. Total cellular proteins, which were prepared from immunopositive transformant Escherichia coli cells, were conjugated with Sepharose 4B. Antibody purified with the Sepharose 4B conjugate from mixed antiserum to P-450(16) alpha and P-450(15) alpha specifically inhibited testosterone 16 alpha-hydroxylase activity in microsomes. The cDNA insert of one recombinant plasmid (clone P-16 alpha-1) was 1.75 kilobases in size and contained one or more internal restriction sites for HindIII, BamHI, Bgl I, Pst I, Alu I, HinpI, and Rsa I. 32P-labeled clone p-16 alpha-1 hybridized with a single mRNA (2000 bases) that was 10 times more concentrated in liver cells from male 129/J mice than in female mice. This result was consistent with the finding that poly(A)+ RNA from male mice translated 10 times as much P-450(16) alpha in vitro as did the poly(A)+ RNA from females. Thus, the predominant expression of testosterone 16 alpha-hydroxylase in male 129/J mice is regulated pretranslationally, presumably at the transcriptional level of the P-450(16) alpha gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Ford H. C., Lee E., Engel L. L. Circannual variation and genetic regulation of hepatic testosterone hydroxylase activities in inbred strains of mice. Endocrinology. 1979 Apr;104(4):857–861. doi: 10.1210/endo-104-4-857. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Adams J. M. Immunoprecipitation of specific polysomes using Staphylococcus aureus: purification of the immunoglobulin k chain messenger RNA from the mouse myeloma MPC11. Biochemistry. 1978 Dec 12;17(25):5560–5566. doi: 10.1021/bi00618a036. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Mode A., Norstedt G., Eneroth P., Hökfelt T. Growth hormone: a regulator of the sexually differentiated steroid metabolism in rat liver. Prog Clin Biol Res. 1983;135:37–59. [PubMed] [Google Scholar]
- Harada N., Negishi M. Mouse liver testosterone 15 alpha-hydroxylase (cytochrome P-450(15) alpha). Purification, regioselectivity, stereospecificity, and sex-dependent expression. J Biol Chem. 1984 Jan 25;259(2):1265–1271. [PubMed] [Google Scholar]
- Knopf J. L., Gallagher J. F., Held W. A. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol. 1983 Dec;3(12):2232–2240. doi: 10.1128/mcb.3.12.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Ryan D., Kuntzman R., Conney A. H. Neonatal imprinting and the turnover of microsomal cytochrome P-450 in rat liver. Mol Pharmacol. 1975 Mar;11(2):190–200. [PubMed] [Google Scholar]
- Mode A., Norstedt G., Simic B., Eneroth P., Gustafsson J. A. Continuous infusion of growth hormone feminizes hepatic steroid metabolism in the rat. Endocrinology. 1981 Jun;108(6):2103–2108. doi: 10.1210/endo-108-6-2103. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Morville A. L., Thomas P., Levin W., Reik L., Ryan D. E., Raphael C., Adesnik M. The accumulation of distinct mRNAs for the immunochemically related cytochromes P-450c and P-450d in rat liver following 3-methylcholanthrene treatment. J Biol Chem. 1983 Mar 25;258(6):3901–3906. [PubMed] [Google Scholar]
- Negishi M., Nebert D. W. Structural gene products of the Ah complex. Increases in large mRNA from mouse liver associated with cytochrome P1-450 induction by 3-methylcholanthrene. J Biol Chem. 1981 Mar 25;256(6):3085–3091. [PubMed] [Google Scholar]
- Norstedt G., Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984 Apr;36(4):805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reubi I., Griffin K. J., Raucy J. L., Johnson E. F. Three monoclonal antibodies to rabbit microsomal cytochrome P-450 1 recognize distinct epitopes that are shared to different degrees among other electrophoretic types of cytochrome P-450. J Biol Chem. 1984 May 10;259(9):5887–5892. [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B. Sexual dimorphism in the liver. Annu Rev Physiol. 1983;45:37–50. doi: 10.1146/annurev.ph.45.030183.000345. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Kurkinen M., Greaves M. Isolation of cDNA clones for the human transferrin receptor. EMBO J. 1983;2(12):2259–2263. doi: 10.1002/j.1460-2075.1983.tb01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Roop D. R., Tsai M. J., Stein J. P., Means A. R., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Regulation of the ovomucoid gene. Biochemistry. 1978 Dec 26;17(26):5773–5780. doi: 10.1021/bi00619a026. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]