Abstract
Dasatinib is a second generation tyrosine kinase inhibitor (TKI) approved for clinical use in patients with imatinib-resistant chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Large granular lymphocytes (LGLs) are medium to large cells with eccentric nuclei and abundant cytoplasm with coarse azurophilic granules. LGL lymphocytosis is caused by a proliferation of cytotoxic (CD8+) T cells and/or NK cells. In a proportion of CML and Ph+ ALL patients, there is a significant expansion of LGLs during dasatinib therapy. LGL lymphocytosis is seen in some cases with fevers, colitis, and pleural effusions (PE), suggesting an aberrant immune response mediated by these LGLs. LGLs may participate in the elimination of the residual leukemic cells, and LGL clonal expansion is associated with excellent, long-lasting therapy responses in dasatinib-treated patients. For a more comprehensive analysis, we analyzed the morphologic, phenotypic, clinical, and functional features of the LGL subsets amplified in vivo during dasatinib therapy.
Keywords: large granular lymphocyte, tyrosine kinase inhibitor, dasatinib, pleural effusion, response
Introduction
Large granular lymphocytes (LGLs) represent 10–15% of the total peripheral blood (PB) mononuclear cells in normal adults.1 They are essential for the acquired immunity against viral infection and neoplasm development. LGL lymphocytosis refers to a spectrum of distinct hematological disorders, whose common property is the abnormal expansion of LGLs. The syndrome is characterized by an abnormal antigen-driven clonal expansion of mature LGLs that would appear to persist in vivo probably owing to impaired survival signaling and/or resistance to apoptosis. LGL lymphocytosis is caused by a proliferation of cytotoxic (CD8+) T cells and/or NK cells and is associated with viral infections, autoimmune diseases, malignancies, immunodeficiencies, and immunosuppression after organ transplantation. LGL lymphocytosis can be observed in a wide spectrum of different diseases, ranging from reactive polyclonal self-limited expansion, to oligo/monoclonal asymptomatic lymphocytosis or even to overt leukemia, which is often accompanied by infiltration of the bone marrow (BM), spleen, and blood.2,3 Polyclonal expansions are usually transient, secondary to viral infections such as Epstein–Barr virus and cytomegalovirus (CMV), or they may be associated with neoplasms and autoimmune disorders; occasionally they may also occur post-splenectomy.4 Clonal LGL expansions are known to be responsible for causing cytopenias and autoimmune disorders.5 According to the current WHO classification of tumors of the hematopoietic and lymphoid tissues, clonal LGL expansions may be further classified as T-LGL leukemia, chronic lymphoproliferative disorders of NK cells (CLPD-NK, a provisional entity with similar indolent clinical presentation to T-LGL leukemia), and aggressive NK cell leukemia.6,7
In recent years, several new small molecule tyrosine kinase inhibitors (TKIs) have emerged and have shown excellent clinical activity in chronic myeloid leukemia (CML). Despite their relative target specificity, many second line TKIs also possess a wide-spectrum inhibitory profile on the kinome. Dasatinib (Sprycel, Bristol-Myers Squibb) is a second generation TKI approved for clinical use and has been highly effective in patients with imatinib-resistant CML and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).8-11 In addition to Bcr-Abl, dasatinib also targets Src and Tec kinases that are known to be key factors in the regulation of immune responses; however, Src and Tec kinases not inhibited by imatinib.12-14 The broader target profile of dasatinib not only implies its less susceptibility to the development of resistance, but also the potential for more potent modulation of immune cell subsets. This may be the therapeutic advantage, but as long-term effects on normal cells are largely unknown, significant side effects may emerge.
There is a significant expansion of LGLs during dasatinib therapy, but this phenomenon rarely happens in other TKIs. The expanded LGLs had either a CD3+CD8+ effector memory T cell or a CD3–CD16/CD56+ NK cell phenotype. The inhibited kinases responsible for LGL expansion are unknown. In this review, dasatinib-related LGL lymphocytosis are evaluated and discussed with clinical responses. Understanding the mechanism of this phenomenon is helpful to judge the therapeutic effect and prognostic significance.
The Association between LGLs and Dasatinib
Several reports demonstrated a monoclonal or oligoclonal expansion of LGLs in patients treated with dasatinib, and dasatinib has immunostimulatory effects in the form of persistent monoclonal or oligoclonal LGL lymphocytosis in a distinct proportion of patients, ranging from 27% to 73.3%, as shown in Table 1. Nagata et al.17 observed LGL lymphocytosis in 9 patients during follow-up of leukocyte counts in 20 consecutive patients treated with dasatinib. In the latest study, Tanaka et al.19 retrospectively investigated increases in LGLs in PB during dasatinib treatment in 25 CML patients. Fifteen of them (60%) showed an increase in LGLs. All 15 of these patients also showed an increase in NK cells, and 11 showed an increase in CD8+ T cells.
Table 1. Summary of articles about LGL lymphocytosis during daatinib therapy.
| Reference | No. of patients | Median age (years) | Dasatinib dose (mg/d) | Rate of LGL lymphocytosis | Lymphocyte counts (109/L) | Median interval from starting dasatinib to LGL | Median number of LGL (109/L) |
|---|---|---|---|---|---|---|---|
| Mustjoki et al.15 | 30 | 54 (25–76) | 140 | 73% (22) | 4–20 | 3 mo (range 1–15 mo) | NR |
| Kreutzman et al.16 | 20 | 51 (29–78) | NR | 50% (10) | NR | NR | NR (range 4–20) |
| Nagata et al.17 | 20 | 50 (29–81) | 100–180 | 45% (9) | NR | 1 mo (range 1–8 mo) | 6.5 (range 2.9–17.4) |
| Kim et al.18 | 18 | NR | 140 | 44% (8) | 4.6–8.8 | 4 mo (range 1.5–15 mo) | NR (range 2.2–5.6) |
| Tanaka et al.19 | 25 | 64 (20–84) | 50–140 | 60% (12) | NR | 11 wk (range 6–44 wk) | 3.0 (range 1.6–6.0) |
| Valent et al.20 | 15 | NR | 100–140 | 27% (4) | 5.0–6.9 | NR | NR (range 2.3–4.83) |
| Lee et al.21,* | 50 | 49 | 100–140 | 46% (23) | 3.7–7.9 | 4 mo (range 1–13 mo) | NR |
| Powers et al.22 | 16 | 56 (48–65) | NR | 31% (5) | NR | NR | 1.6 (range 0.6–2.7) |
Note: *In this study, the development of lymphocytosis could not be directly interpreted as an expansion of large granular lymphocytes because a PB smear was not available; NR, not reported.
The phenomenon of the increase of LGLs in the PB occurs exclusively by dasatinib intake, but not by imatinib or nilotinib intakes. Up to now, LGL lymphocytosis has rarely been reported in patients treated with imatinib or nilotinib which cannot inhibit Src family kinases. Powers et al.22 showed that 31% (5 of 16) of dasatinib-treated patients had LGL expansions, whereas the likelihood of such expansions under other TKI treatments was relatively low: 3% (1 of 29) of imatinib-treated and 14% (2 of 14) of nilotinib-treated patients.
The dosage of dasatinib is associated with the incidence of LGL lymphocytosis. In one report, 1 (14%) of 7 patients who received dasatinib 100 mg once daily developed LGL lymphocytosis, compared with two (50%) patients taking 50 mg twice daily, 5 (63%) of 8 patients taking 70 mg twice daily, and 1 (100%) of 1 patient taking 90 mg twice daily.17 The greater the dosage of dasatinib was, the higher the incidence of LGL lymphocytosis was.
The interval from starting dasatinib to LGL lymphocytosis ranges from 1 to 18 mo, as shown in Table 1. In most patients, LGL lymphocytosis was long lasting and continued throughout dasatinib therapy, albeit with marked fluctuations in absolute lymphocyte count. However, cessation of dasatinib treatments induced rapid decline of the LGL counts almost to the baseline levels.15 The LGL expansions do not persist after discontinuation of dasatinib and therefore cannot be considered LGL leukemia.
Dasatinib had a clinically significant direct effect on the immune effector cells, resulting in rapid lymphocyte mobilization, activation, and transmigration. The increase of LGLs typically developed abruptly, with a peak lymphocyte count ranging from 1.6 to 20 × 109/L.16-20 We defined patients with or without LGL lymphocytosis as LGL+ patients or LGL– patients. Both lymphocyte counts and LGL counts significantly increased 2 h after the oral dasatinib intake as compared with those before intake in all patients. LGL-increase at 2 h after the intake of dasatinib was seen even in LGL– patients as well as LGL+ patients, and that relative fold-increases of LGL were similar.19 Thus, both lymphocyte and LGL stayed at the basal levels at 2 h after the oral intakes of imatinib or nilotinib.
Immunophenotype
Dasatinib-associated lymphocytosis typically comprises monoclonal or oligoclonal expansion of cytotoxic T or NK cell. The immunophenotype of cells is CD3+CD8+ cytotoxic T cell and CD3–CD16+/56+ NK cell phenotype. CD3+CD8+ cytotoxic T cells displayed activation markers, including HLA-DR and CD57 and had TCR-ɑ/β chains on the cell surface. CD3+CD8+ T cells also showed low expression of CD62L antigen. The LGLs exhibited late differentiated (CD27–CD57+) phenotype.12 CD57 is a 110-kDa glycoprotein found on NK cells, activated effector CD8+ T cells and was a marker of replicative senescence and indicative of altered functional capacities.23
Clonality
Clonal TCR gene rearrangements were commonly observed in dasatinib-treated patients, and the incidence was higher in the LGL+ patients than in the LGL– patients. T-cell ontogenesis is associated with TCR gene rearrangement resulting in a unique molecular fingerprint for each T lymphocyte. The presence of clonal TCR gene rearrangements has been recently described in more than 80% of LGL+ patients during dasatinib therapy. High frequencies of clonal rearrangements of TCR-β, -γ, and -δ genes were observed in LGL+ patients, and at lower frequencies in LGL– patients. The LGL+ patients had more often clonal TCRδ gene rearrangements than the LGL– patients (90% vs. 10%, respectively).16 Tanaka et al.19 found clonal TCR gene rearrangements during dasatinib treatments were amazingly high, in which at least one of the three TCR genes for β, γ, and δ were rearranged in 100% of the LGL+ patients, and in 70% of the LGL– patients as well.
Constitutive activation of the signal transducer and activator of transcription 3 (STAT3) has been observed in patients with LGL leukemia. Recently, the discovery of somatic mutations in the SH2 domain of STAT3 in T-LGL leukemia and CLPD-NK, displays its main value in the diagnostic setting.24,25 STAT3 mutations are also found in bone marrow failure disorders with LGL leukemia or without a LGL expansion.26 STAT3 mutations are frequently observed suggesting that aberrant STAT3 signaling underlies the pathogenesis of clonal expansions of LGL. STAT3 mutations may be a useful tool to discriminate malignant NK/T lymphoproliferations from reactive expansions, in particular to establish clonality using STAT3 mutation as a clonal marker.25 Because of pathogenetic and clinical similarities, LGL lymphocytosis warrants screening for STAT3 mutations in NK or T lymphocytes from LGL+ patients. LGLs from 6 patients with LGL lymphocytosis during TKI therapy were studied and found to be negative for STAT3 mutations,24 but it needs further validation whether there are acquired STAT3 mutations.
Diagnosis
One of the issues currently unsolved is that “increase of LGL” has not been clearly defined during dasatinib therapy up to now. Transient or chronic, polyclonal T or NK cell lymphocytosis is occasionally seen in normal individuals with viral infections and autoimmune disorders.27 Oligoclonal and small clonal populations of LGLs have been observed in healthy elderly individuals.28 This phenomenon has been attributed to dysregulated systemic immunity in the elderly. Based on the published literature, the inclusion criteria of LGL lymphocytosis is: (1) the number of PB lymphocyte count is equal or more than 3.0 × 109/L and absolute LGL count is equal or more than 1.5 × 109/L for at least three months, (2) the predominance of LGLs in the PB smear samples, (3) this appears during the course of the dasatinib treatment, (4) the phenotypes of LGLs is either CD3–CD56+ NK cell or CD3+CD8+ T cell, (5) clonal rearrangements of the TCR genes during dasatinib therapy.
The relationship between favorable response and LGL lymphocytosis
LGL lymphocytosis during dasatinib treatment may be correlated with favorable response, as shown in Table 2. In some dasatinib-treated patients, LGL+ patients showed higher response rates than did LGL– patients. Nagata et al.17 reported 8 (89%) of 9 patients with LGL lymphocytosis achieved MR, while only 6 (55%) of 11 patients without LGL lymphocytosis eventually achieved MR. Kim et al.18 also reported 8 patients with LGL lymphocytosis achieved major molecular response (MMR). There were similarly results in the articles of Tanaka et al.19 and Lee et al.21 (Table 2). These results suggest that the LGL+ patients may achieve more favorable molecular responses, as compared with the LGL– patients.
Table 2. The relationship between favorable response and LGL lymphocytosis .
| Reference | MMR | CMR | ||||
|---|---|---|---|---|---|---|
| LGL+ | LGL− | P | LGL+ | LGL− | P | |
| Mustjoki et al.15 | 17/22 (77%) | 2/8 (25%) | 0.015 | 12/22 (55%) | 1/8 (13%) | 0.047 |
| Nagata et al.17 | 8/9 (89%) | 6/11 (55%) | 0.08 | NR | ||
| Kim et al.18 | 8/8 (100%) | 3/10 (30%) | 0.01 | 7/8 | 2/10 | 0.003 |
| Tanaka et al.19 | NR | 4/5 (80%) | 0/3 (0%) | 0.01 | ||
| Lee et al.21 | 12/23 (52%) | 5/27 (15%) | 0.05 | 10/23 (44%) | 4/27 (15%) | 0.024 |
| Mustjoki et al.29 | 7/7 (100%) | 1/30 (3%) | <0.00 | 6/7 (86%) | 1/30 (3%) | <0.00 |
MMR, major molecular response; CMR, complete molecular response; NR, not reported.
In a phase II clinical trial involving 46 patients with Ph+ ALL, survival was better in LGL+ patients than in LGL– patients.15 Median progression free survival (PFS) was 2.7 mo in LGL– patients, whereas no median PFS was reached in LGL+ patients during 24 mo follow-up time. Similarly overall survival (OS) was superior in LGL+ patients compared with LGL– patients (no median time reached vs. 5.8 mo, respectively). The prognosis of Ph+ ALL or advanced CML relapse after allogeneic stem cell transplantation is often extremely poor, but eight such heavily relapsed patients achieved complete molecular response (CMR) with dasatinib in conjunction with LGL lymphocytosis. Three of these patients were still in CMR after >18 mo of treatment.15 The median time from therapy to favorable response was shorter in LGL+ patients than that in LGL– patients (5 mo vs. 6 mo, respectively).17
The possible hypothesis to account for the favorable responses is that dasatinib-induced LGLs may have potential to exert immune-associated anti-leukemia effects in vivo. By performing NK cell cytotoxicity testing of 51Cr release assays, the NK cell cytotoxicity in LGL+ patients superior to that in LGL– patients and normal healthy donors.18 When NK cells were pretreated for 24 h and the functional assays were performed after dasatinib was washed out, an amplification of cytokine production, degranulation, and partial kill rates against different target cell lines were observed.30 Another possible hypothesis to account for the favorable responses is that dasatinib has potential to reduce the number of CD4+CD25+FOXP3+ regulatory T cells (Tregs) in both PB and BM, which are negatively regulating other immune cells, and that this reduction of Tregs were more substantially observed in LGL+ patients as compared with LGL– patients.15,31 Third, in LGL+ patients the level of IP-10, IL-6, MIG, and IL-2R were increased, and these cytokines/chemokines could contribute to improved therapeutic responses because they direct the chemotaxis of monocytes, T cells, NK cells, and dendritic cells.32 Such chemotactic effects have been shown to be relevant for leukemia control. Taken together, these changes may boost the immune system and in the long-term lead to enhanced antitumor immunity and contribute to favorable responses in dasatinib-treated patients with LGL lymphocytosis.
Adverse effects associated and laboratory parameters with LGLs
Dasatinib-related adverse effects were common. Adverse effects were temporally related to dasatinib therapy and emerged after the appearance of LGL lymphocytosis. LGL lymphocytosis are seen in some cases with fevers, colitis, and pleural effusions (PE), suggesting an aberrant immune response mediated by these cytotoxic cells. Initial attention was focused on off-target inhibition of tyrosine kinase, but TKI-induced immune deregulation has been suggested as a possible cause of adverse effects.
PE
In most recent reports, LGL lymphocytosis has been reported to be associated with PE in patients treated with dasatinib. Drug-induced PE is an uncommon occurrence that has been linked to a relatively limited number of drugs.33 Fluid retention resulting in anasarca, pleural, or pericardial effusion, or ascites has been reported rarely with imatinib.34 Recently, some studies have suggested that PE induced by dasatinib may be immune mediated.35-37 Mustjoki et al.15 reported a possible relationship between the expansion of LGLs and PE. During follow-up of leukocyte counts in 20 consecutive patients treated with dasatinib, LGL lymphocytosis was observed in 9 patients, and PE occurred in 8 patients. Five patients (25%) developed both PE and LGL lymphocytosis during dasatinib therapy.
Although the precise mechanism of dasatinib-induced PE is not completely elucidated, it may be mediated by LGLs. First, lymphocytes were observed in pleural fluid samples and the majority of lymphocytes were CD3+CD8+CD57+ T cells and no leukemic cells were detected.15 By TCR gene rearrangement analysis, an identical T cell clone was observed both in PB and in pleural fluid. Second, LGL lymphocytosis appearance proceeded to PE, which was shown in all patients who developed PE by Nagata et al.17 Approximately one month after LGL appearance all patients developed PE. During dasatinib therapy, 11 of 13 patients who developed PE had LGL lymphocytosis before. The median interval from starting dasatinib to either LGL lymphocytosis or PE was 1 mo (range 1–8 mo) and 3.5 mo (range 2–24 mo), respectively, and LGL lymphocytosis preceded PE.15 Third, PE was more commonly observed in LGL+ patients than in LGL– patients (see Table 3). Three (27%) of 11 LGL– patients developed PE, while 5 (56%) of 9 LGL+ patients developed PE.17 In another report, PE were observed in 9 of 15 LGL+ patients (60%), and 2 of 10 LGL– patients (20%).19 Lastly, the mean number of LGLs was higher in patients with PE than in those without PE. Among patients with PE, the mean peak number of LGL was 9.2 × 109/L, which was much higher than the mean peak number (4.6 × 109/L) of LGL in patients without PE.17
Table 3. The relationship between PE and LGL lymphocytosis.
| Reference | PE | |
|---|---|---|
| LGL+ | LGL− | |
| Mustjoki et al.15 | 13/22 (59%) | NR |
| Nagata et al.17 | 5/9 (56%) | 3/11 (27%) |
| Tanaka et al.19 | 9/15 (60%) | 2/10 (20%) |
| Lee et al.21 | 5/23 (22%) | 6/27 (22%) |
| Powers et al.22 | 3/5 (60%) | 2/11 (18%) |
PE, pleural effusions; NR, not reported.
Dasatinib had a clinically significant direct effect on the lymphocytes, resulting in rapid lymphocyte mobilization, activation, and transmigration. The rapid lymphocyte mobilization and activation may explain the pathogenesis of PE.29 CD8+ T cells isolated from patients with LGL expansion had increased cytotoxicity against pulmonary non-transformed endothelial cells, which may lead to the formation of PE.22 Therefore, there might be a tendency for LGL+ patients to have an influence on the development of PE.
The characteristic of LGL-related PE: (1) Pleural fluid analysis showed the majority of the cells were CD3+CD8+CD57+ T cells; (2) LGL appearance proceeded to PE; (3) PE was more commonly observed in LGL+ patients than in LGL– patients; (4) The mean number of LGLs was higher in patients with PE than in those without PE.
The management of PE includes transient dose reduction or stopping dasatinib treatment and reintroducing the treatment with caution at a lower dose after PE have been resolved. Steroid may assist in management, but treatment with diuretics is likely to be ineffective because an immune-mediated mechanism is suspected. Insertion of a chest tube is attempted to control frequent exacerbations of PE that are resistant to noninvasive treatments.
Pulmonary artery hypertension (PAH)
More recently, there have been reports of PAH developing in patients receiving long-term therapy with dasatinib.17,36 PAH is also associated with lymphoproliferative diseases, and lung biopsies performed in some of these cases revealed infiltration of lung parenchyma with LGLs.38 Dasatinib can induce LGL proliferation and LGLs have acquired the ability to directly lyse endothelial cells,39 which maybe one mechanism leading to PAH. Powers et al.22 revealed significant increases in endothelial cytotoxicity in LGL+ patients compared with results in LGL– patients during dasatinib therapy. They assayed CD8+ T-cell cytotoxicity against CRL2598 cells, a human pulmonary artery endothelial cell line in 4 dasatinib-treated patients and got this result. It has been previously demonstrated that the CRL2598 cell line is resistant to allogeneic NK cell cytotoxicity, yet susceptible to lysis by autoreactive LGL cells.39
The plasma levels of PDGF-BB, a known LGL survival signal, were particularly high in LGL+ patients.22 Tajsic and Morrell documented that the PDGF-BB homodimer potently stimulates pulmonary arterial smooth muscle cell proliferation and remodeling.40 We assumed that serum levels of PDGF-BB may play an important role in contributing to the development of PAH in LGL+ patients.
Colitis and pleuritis
Patients who developed LGL lymphocytosis had an increased risk of developing colitis and pleuritis. In most recent reports, LGLs have been reported to be associated with colitis or pleuritis in patients treated with dasatinib. Mustjoki et al.15 found colitis and pleuritis were common in LGL+ patients (18 of 22) and were preceded by LGL lymphocytosis, and none of the control patients had pleuritis or colitis during dasatinib therapy. Eleven of 22 of patients had colitis, and in 10 of 11 patients LGL lymphocytosis had been detected before the development of colitis; eight patients without LGL lymphocytosis during dasatinib treatment did not encountered colitis or pleuritis.15 Accumulation of identical cytotoxic T cells with the same phenotype was also detected in colon biopsy samples. Immunohistochemistry was performed from colonoscopy biopsies of two patients with severe colitis during dasatinib therapy and colon biopsies showed submucosal infiltration of cytotoxic CD3+CD8+ T cells.15 In another report, it was demonstrated that dasatinib-induced LGLs were cytotoxic to endothelial cells, and this may explain the association with development of pleuritis.22 LGL lymphocytosis preceded in most patients the occurrence of pleuritis and colitis implying a temporal relationship. As far as we know, no clonal LGL proliferation has been reported in the literature with colitis or pleuritis caused by other factors.
Decrease of Tregs
It has been shown that there is a decrease in Tregs in dasatinib-treated patients developing LGL lymphocytosis. LGL+ patients exhibited substantially lower Tregs level compared with LGL– patients. Compared with healthy controls (n = 15) or to LGL– patients (n = 6), the LGL+ patients (n = 7) had significantly lower percentage of Tregs in PB (median 5.1%, 4.3%, 1.9%, respectively, P < 0.001).15 LGL+ patients had the lowest Tregs’ level, suggesting that Treg-mediated regulatory mechanisms may be compromised.
The elevated cytokine/chemokine levels
Plasma IP-10 (CXCL10), IL-6, MIG (CXCL9), and IL-2R levels increased in LGL+ patients treated with dasatinib. Plasma levels of 25 cytokines were measured in healthy controls (n = 10), LGL+ patients (n = 15), and LGL– patients (n = 8) during dasatinib therapy. Significantly elevated levels of IP-10, IL-6, MIG, and IL-2R were present in the plasma of patients with LGL expansions compared with healthy controls.32 The elevated cytokine/chemokine levels in LGL+ patients could therefore indicate a higher degree of viral antigen-driven cellular immune activation enhanced by an intrinsic dysregulation of homeostasis. Consistent with these ideas, highest levels of IP-10, IL-6, MIG, and IL-2R were observed in the plasma of LGL+ patients with symptomatic CMV reactivation.32
Thrombocytopenia
Thrombocytopenia was observed more commonly in the LGL– group, as compared with the LGL+ group, and there was statistical significance (41% vs. 9%, respectively; P = 0.010).21 Thrombopenia has been related to LGL proliferation and the reason for this has been unclear.
Etiopathogenesis
What are the origins of LGLs, and how are these cells activated? Recently, several in vitro studies have indicated profound inhibitory effects of dasatinib on T- and NK-cell activation and proliferation.41-44 The putative in vivo immunomodulatory effects of dasatinib have thus far not been explored. The pathogenesis of LGL lymphocytosis, although not properly elucidated thus far, seems to be multifactorial (Fig. 1).
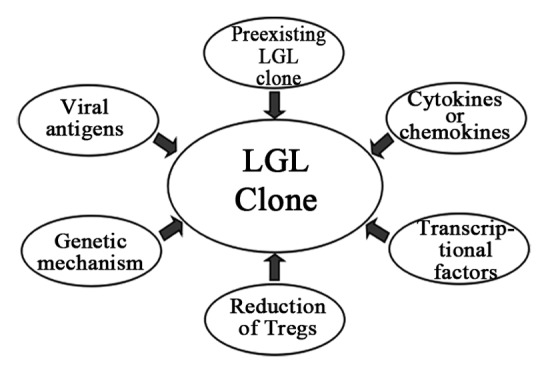
Figure 1. The pathogenesis of LGL lymphocytosis during dasatinib therapy.
Cellular mechanisms underlying the proliferation of clonal LGLs
Dasatinib and other tyrosine kinase inhibitors exert immunosuppressive effects on T cells and NK cells in vitro.41,42,45-49 It has been well demonstrated that Tregs potently suppress anti-self and anti-leukemic immune responses.50 Tregs that normally stem the expansion of autoreactive T effector cells, and decreased percentage of Tregs may enhance the expansion of the T cell clones. Dasatinib has potential to reduce the number of Tregs, and this reduction of Tregs was more substantially observed in LGL+ patients as compared with LGL– patients.22 Dasatinib have a greater impact on the number of Tregs, skewing the balance of T cell suppression toward activation and proliferation and leads to the proliferation of clonal LGLs.
Molecular mechanism underlying the proliferation of clonal LGLs
Many survival factors considered necessary for LGL survival and expansion. Recent efforts in bioinformatics and literature compilation have verified IL-15 transpresentation, PDGF-BB signaling, NFκB and T-bet activation as critical factors in the survival and expansion of leukemic LGLs.51 Although the LGL expansions do not persist after discontinuation of therapy and therefore cannot be considered LGL leukemia, similar survival factors may be transiently induced by certain TKIs. In one study, the LGL clonal expansions accompanied the increased IL-15 transpresentation and plasma levels of PDGF-BB, NFκB, and T-bet signaling.22 The plasma levels of PDGF-BB, a known LGL survival signal, were particularly high (2000–3000 pg/ml) in LGL+ patients.22 Increased plasma levels of PDGF-BB could be attributed to inhibition of kinases involved in PDGF receptor (PDGFR) α/β phosphorylation. IL-15 transpresentation was increased in LGL+ patients.22 Increased IL-15 transpresentation is responsible for eliciting oligoclonal lymphocyte expansions and this result is due to the well-known NK and T cell stimulatory activity of IL-15 signaling. NFκB and T-bet were constitutively active in CD8+ T lymphocytes from dasatinib-treated patients.22 IL-15 is a direct transcriptional target of NFκB, and IL-15 transpresentation indirectly confirm NFκB activation. Serum IL-2 has been shown to inversely correlate with T-bet activation in the context of LGL leukemia, and IL-2 levels were generally lower in patients treated with dasatinib.22 Many factors were considered necessary for clonal LGL expansion, which should be considered a starting point for future research and high-power association studies.
At present, there are only a few transcription factors that are known to play an essential role in NK cell development, such as T-box proteins, T-bet, and Eomesodermin (Eomes).52-54 Dasatinib could promote the proliferation of NK cells from cord blood under the condition of IL-2 and IL-15 stimulation through increased expression of transcription factors such as Eomes. After a 7-d culture of umbilical cord blood cells, the absolute number of NK cells had significantly increased in the culture with dasatinib compared with the culture with cytokines only. By analyzing the transcriptional factors Eomes and T-bet, NK cells showed increased expression of Eomes and T-bet compared with the expression in whole cord blood cells.55 This observation may have potentially important implication for the treatment of other diseases with dasatinib.56
Viral antigens relating with expansion of LGLs
A hypothesis was proposed that CMV reactivation was first triggered in the patients treated with dasatinib by its immunosuppressive effects, subsequently leading to the preferential expansion of LGLs.32 The expansion of LGLs is common during a primary immune response against viral antigens. Some viruses, such as CMV are relates with expansion of LGLs. CMV-infection status during dasatinib treatments was assessed by serum CMV-IgG levels, serum CMV-IgM levels, and “CMV antigen test TEIJIN C7-HRP,” which can detect CMV pp65 antigen in leukocytes.57,58 The CMV-IgG seropositivity does not necessarily represent recent CMV reactivation. The CMV-IgG seropositivity indicates that the person was infected with CMV at some time during their life. CMV-seropositive patients had a higher median lymphocyte counts at 1h compared with CMV-negative patients (4.8 vs. 2.0 × 109/L, P = 0.016).29Another report demonstrated that 15 of 16 patients with expansion of LGL were CMV-IgG seropositive, whereas only 3 of 9 patients without expansion of LGL were CMV-IgG seropositive.32 They also reported that 5 of 16 (31%) patients with expansion of LGLs, while 0 of 9 (0%) patients without expansion of LGLs, experienced symptomatic CMV reactivations by PCR-based assay to detect CMV DNA in plasma samples during dasatinib therapy.32 The use of dasatinib can results in immunosuppression, and CMV reactivation is triggered by this effects, subsequently leading to monoclonal or oligoclonal LGL expansions. The observation that LGL expansions in dasatinib-treated patients are associated with CMV reactivation provides additional insight into these seemingly contradictory observations.
A potential association was observed between positive CMV-IgG serology and clonal TCR rearrangements because more than 90% of patients with clonal TCR rearrangements were CMV-IgG seropositive, whereas only 2 of 6 patients without clonal TCR rearrangements were CMV-IgG seropositive. However, some researchers also reported that dasatinib treatments did not increase opportunistic infections including CMV by retrospective analysis of 1150 patients.59 In one report, CMV C7-HRP tests were negative in all patients and serum CMV-IgM antibodies were positive in only 2 of 25 patients without symptom of infection.19 Although the number of the patients in this study was relatively limited, the results suggest that CMV reactivation during dasatinib treatment was uncommon. These results indicate that most of the patients have clinical histories of CMV infection throughout their lives, but it is unlikely that they caught recent infection or recent reactivation of CMV. Whether CMV is related with LGL lymphocytosis, additional studies are warranted to address the role of CMV in the pathogenesis of LGL lymphocytosis.
The expansion of LGLs
Dasatinib-associated LGL lymphoproliferation is an expansion of preexisting CD8+ T or NK cells clones. These monoclonal or oligoclonal CD8+ T or NK cells already existed at the time of diagnosis, and the dasatinib-treated patients showed marked expansion of these clone during therapy whereas imatinib-treated patients did not. Thus, dasatinib does not induce these clones, but rather appears to promote their preferential expansion. Dasatinib has immunostimulatory effects in the form of LGL lymphocytosis in a proportion of patients. It has been reported that these clonal T cell and NK cells already existed in over 80% (15 of 18) of patients with CML at the time of diagnosis, and that dasatinib-treated patients showed expansion of their pre-existing clones during the therapy.16 Furthermore, during dasatinib therapy, 59% of the patients with clonal TCR γ/δ rearrangements were negative for Bcr-Abl transcripts in blood by RQ-PCR, and in five patients also high-resolution FISH analysis was performed and no Ph+ cells could be detected. Thus, it can be concluded that the detected clonal LGLs did not belong to the malignant Ph+ clone.14
Precise mechanism of the existence of these cells in CML patients at the time of diagnosis and the proliferation of these clonal cells by dasatinib treatments is still in vague. However, it is postulated that the proliferation of these cells is probably related to the off-target kinase inhibitory effects mediated by dasatinib. One hypothesis is that dasatinib could directly activate or modulate the proliferation and function of NK cells. The pre-existing clonal T or NK cells probably expand by dasatinib through inhibition of tyrosine kinases, and several subtypes of Src family kinases such as Fyn or Lck,60-63 which are known to be involved in the regulation or activation of NK cells. Up to now, the phenomenon of LGL-increase has not been reported in patients treated with imatinib or nilotinib which cannot inhibit Src family kinases. These findings imply that there is a preexisting clone for lymphocytes that might be activated by dasatinib through an activating mechanism, such as the Src kinase-mediated pathway.
Another potential mechanism for the pathogenesis of LGLs concerns dasatinib-mediated restoration of the functions or proliferative capacities of NK cells. Malignant CML cells have a capacity to produce reactive oxygen species, the main inhibitory mediator toward NK cells.64 NK cells from CML patients are profoundly defective in NK cell activity65 and NK cell numbers decrease as the disease progresses to the advanced phase.66 Nakajima et al.67 concluded that the Bcr-Abl transgene causes abnormal NK cell differentiation, and it is possible that dasatinib therapy could reduce Bcr-Abl transcription and restore NK cell numbers and/or functions.
Genetic mechanism associated with the expansion of LGLs
In speculation though, the expansion of LGLs during dasatinib therapy might also be associated with HLA-A*02. This might explain the existence of some patients who did not show LGL-increase during dasatinib therapy.32 Whether patients with HLA-A*02 are actually associated with LGL lymphocytosis or not, this could be one of the important issues to be clarified, and racial differences may exist in terms of LGL lymphocytosis and clinical effectiveness by dasatinib treatments.
It is tempting to speculate that crossreactive CD8+ T cells21 driven by viral recrudescence and fortuitously targeting leukemia-associated antigens, might mediate these beneficial effects. In this light, it is notable that the majority of LGL patients (11/16) in this study were HLA-A*02, which might restrict both virus-specific and leukemia-associated antigen-specific epitopes in this setting. Previous descriptions of improved survival associated with HLA-A*02 and CMV IgG seropositivity in the allogeneic transplantation setting are consistent with this notion.22
However, the current study showed the somewhat paradoxical finding that blockade of Src kinases by dasatinib could deregulate or modulate NK or NK/T-cell activation, and that it enhances NK or NK/T-cell proliferation and/or activation, thus modulating LGLs to attack CML stem cells. It still needs to be fully clarified whether the effect of dasatinib on NK or NK/T-cell proliferation or activation is mediated via Src kinase or other unknown potential pathways involved in the proliferation and differentiation of NK or NK/T cells.
Conclusions
The effects of dasatinib are important contents to research, and LGL lymphocytosis is a common phenomenon during dasatinib therapy, but the progress in molecular, cellular biology and clinical investigation is limited. Although the mechanisms currently are unresolved, the phenomenon is clinically relevant and may have both beneficial (anti-leukemic response) and harmful (anti-host reactivity) sequelae. This warrants further laboratory and clinical studies in larger patient populations and may result in novel applications for TKIs.
In conclusion, LGLs markedly expanded during dasatinib therapy, and LGLs were not only confined to CD3+CD8+ cytotoxic T cells but also resided in CD3–CD56+ NK cells. This knowledge allows patients with localized disease to avoid over-treatment and patients with advanced cancer to avoid under-treatment. These unique activities of dasatinib also implicate the possibilities of the usage of this drug for other malignancies. There are still a lot of issues to be clarified in the future. If all these findings are confirmed in larger studies, clinical implication for the treatment of patients would be significant. We believe that elucidation of this pathogenesis help to further accelerate progress in the laboratory and clinical research, and the participation of clinicians and patients in experimental and clinical studies is invaluable.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This study was supported by National Natural Science Foundation of China (30871104, 30971296, 81170485, 81170488, 81370657), Natural Science Foundation of Jiangsu Province (BK2010584), Key Projects of Health Department of Jiangsu Province (K201108), Jiangsu Province’s Medical Elite Program (RC2011169), National Public Health Grand Research Foundation (201202017), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (JX10231801), Program for Development of Innovative Research Teams in the First Affiliated Hospital of Nanjing Medical University, and Project of National Key Clinical Specialty.
Authors’ Contributions
All authors contributed to initial drafts, edited versions, and the final version. All authors read and approved the final manuscript.
Glossary
Abbreviations:
- LGLs
large granular lymphocytes
- PB
peripheral blood
- BM
bone marrow
- CMV
cytomegalovirus
- CLPD-NK
chronic lymphoproliferative disorders of NK cells
- TKIs
tyrosine kinase inhibitors
- CML
chronic myeloid leukemia
- Ph+ ALL
Philadelphia chromosome-positive acute lymphoblastic leukemia
- STAT3
signal transducer and activator of transcription 3
- MMR
major molecular response
- PFS
progression free survival, OS, overall survival
- CMR
complete molecular response
- Tregs
regulatory T cells
- PE
pleural effusions
- PAH
pulmonary artery hypertension
- Eomes
Eomesodermin
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27310
References
- 1.Lamy T, Loughran TP. Large granular lymphocyte leukemia. Cancer Control. 1998;5:25–33. doi: 10.1177/107327489800500103. [DOI] [PubMed] [Google Scholar]
- 2.Loughran TP., Jr. Clonal diseases of large granular lymphocytes. Blood. 1993;82:1–14. [PubMed] [Google Scholar]
- 3.Sokol L, Loughran TP., Jr. Large granular lymphocyte leukemia. Oncologist. 2006;11:263–73. doi: 10.1634/theoncologist.11-3-263. [DOI] [PubMed] [Google Scholar]
- 4.Zambello R, Semenzato G. Large granular lymphocyte disorders: new etiopathogenetic clues as a rationale for innovative therapeutic approaches. Haematologica. 2009;94:1341–5. doi: 10.3324/haematol.2009.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamy T, Bauer FA, Liu JH, Li YX, Pillemer E, Shahidi H, Gregory SA, Zambello R, Marcolongo R, Semenzato G, et al. Clinicopathological features of aggressive large granular lymphocyte leukaemia resemble Fas ligand transgenic mice. Br J Haematol. 2000;108:717–23. doi: 10.1046/j.1365-2141.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan WC, Foucar KM, Morice WG, Catovsky D. T-cell large granular lymphocyte leukaemia. In: WHO Classiication of Tumours of the Hematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL et al. (Eds). IARC Press, Lyon, France, 272–273 (2008). [Google Scholar]
- 7.Villamor N, Morice WG, Chan WC, Foucar K. Chronic lyphoproliferative disorders of NK cells. In: WHO Classiication of Tumours of Hematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL et al. (Eds). IARC Press, Lyon, France, 274–275 (2008). [Google Scholar]
- 8.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–50. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, Hochhaus A, Guilhot F, Saglio G, Apperley J, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–13. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 10.Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, Facon T, Goldberg SL, Cervantes F, Niederwieser D, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–9. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 11.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345–56. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 12.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–44. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 13.Hantschel O, Rix U, Schmidt U, Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL, Ellmeier W, Valent P, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104:13283–8. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–63. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 15.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M, Kovanen P, Laurinolli T, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]
- 16.Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R, Porkka K, Mustjoki S. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116:772–82. doi: 10.1182/blood-2009-12-256800. [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y, Ohashi K, Fukuda S, Kamata N, Akiyama H, Sakamaki H. Clinical features of dasatinib-induced large granular lymphocytosis and pleural effusion. Int J Hematol. 2010;91:799–807. doi: 10.1007/s12185-010-0565-1. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim HJ, Lee JJ, Lipton JH. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica. 2009;94:135–9. doi: 10.3324/haematol.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka H, Nakashima S, Usuda M. Rapid and sustained increase of large granular lymphocytes and rare cytomegalovirus reactivation during dasatinib treatment in chronic myelogenous leukemia patients. Int J Hematol. 2012;96:308–19. doi: 10.1007/s12185-012-1132-8. [DOI] [PubMed] [Google Scholar]
- 20.Valent JN, Schiffer CA. Prevalence of large granular lymphocytosis in patients with chronic myelogenous leukemia (CML) treated with dasatinib. Leuk Res. 2011;35:e1–3. doi: 10.1016/j.leukres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Jung CW, Kim DY, Lee KH, Sohn SK, Kwak JY, Kim HJ, Kim IH, Park S, Kim DH. Retrospective multicenter study on the development of peripheral lymphocytosis following second-line dasatinib therapy for chronic myeloid leukemia. Am J Hematol. 2011;86:346–50. doi: 10.1002/ajh.21980. [DOI] [PubMed] [Google Scholar]
- 22.Powers JJ, Dubovsky JA, Epling-Burnette PK, Moscinski L, Zhang L, Mustjoki S, Sotomayor EM, Pinilla-Ibarz JA. A molecular and functional analysis of large granular lymphocyte expansions in patients with chronic myelogenous leukemia treated with tyrosine kinase inhibitors. Leuk Lymphoma. 2011;52:668–79. doi: 10.3109/10428194.2010.550074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 24.Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ, Olson T, Jalkanen SE, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–13. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerez A, Clemente MJ, Makishima H, Koskela H, Leblanc F, Peng Ng K, Olson T, Przychodzen B, Afable M, Gomez-Segui I, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–57. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerez A, Clemente MJ, Makishima H, Rajala H, Gómez-Seguí I, Olson T, McGraw K, Przychodzen B, Kulasekararaj A, Afable M, et al. STAT3 mutations indicate the presence of subclinical T-cell clones in a subset of aplastic anemia and myelodysplastic syndrome patients. Blood. 2013;122:2453–9. doi: 10.1182/blood-2013-04-494930. [Prepublished online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith PR, Cavenagh JD, Milne T, Howe D, Wilkes SJ, Sinnott P, Forster GE, Helbert M. Benign monoclonal expansion of CD8+ lymphocytes in HIV infection. J Clin Pathol. 2000;53:177–81. doi: 10.1136/jcp.53.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, Kourilsky P, Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–9. [PubMed] [Google Scholar]
- 29.Mustjoki S, Auvinen K, Kreutzman A, Rousselot P, Hernesniemi S, Melo T, Lahesmaa-Korpinen AM, Hautaniemi S, Bouchet S, Molimard M, et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia. 2013;27:914–24. doi: 10.1038/leu.2012.348. [DOI] [PubMed] [Google Scholar]
- 30.Hassold N, Seystahl K, Kempf K, Urlaub D, Zekl M, Einsele H, Watzl C, Wischhusen J, Seggewiss-Bernhardt R. Enhancement of natural killer cell effector functions against selected lymphoma and leukemia cell lines by dasatinib. Int J Cancer. 2012;131:E916–27. doi: 10.1002/ijc.27537. [DOI] [PubMed] [Google Scholar]
- 31.Rohon P, Porkka K, Mustjoki S. Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur J Haematol. 2010;85:387–98. doi: 10.1111/j.1600-0609.2010.01501.x. [DOI] [PubMed] [Google Scholar]
- 32.Kreutzman A, Ladell K, Koechel C, Gostick E, Ekblom M, Stenke L, Melo T, Einsele H, Porkka K, Price DA, et al. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia. 2011;25:1587–97. doi: 10.1038/leu.2011.135. [DOI] [PubMed] [Google Scholar]
- 33.Morelock SY, Sahn SA. Drugs and the pleura. Chest. 1999;116:212–21. doi: 10.1378/chest.116.1.212. [DOI] [PubMed] [Google Scholar]
- 34.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–9. doi: 10.1182/blood.V99.10.3530. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron A, Réa D, Levy V, Picard C, Meignin V, Tamburini J, Bruzzoni-Giovanelli H, Calvo F, Tazi A, Rousselot P. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med. 2007;176:814–8. doi: 10.1164/rccm.200705-715CR. [DOI] [PubMed] [Google Scholar]
- 36.Quintás-Cardama A, Kantarjian H, O’brien S, Borthakur G, Bruzzi J, Munden R, Cortes J. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–14. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 37.de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, Chaidos A, Olavarria E, Goldman JM, Apperley JF, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008;141:745–7. doi: 10.1111/j.1365-2141.2008.07108.x. [DOI] [PubMed] [Google Scholar]
- 38.Rossoff LJ, Genovese J, Coleman M, Dantzker DR. Primary pulmonary hypertension in a patient with CD8/T-cell large granulocyte leukemia: amelioration by cladribine therapy. Chest. 1997;112:551–3. doi: 10.1378/chest.112.2.551. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Bai F, Sokol L, Zhou J, Ren A, Painter JS, Liu J, Sallman DA, Chen YA, Yoder JA, et al. A critical role for DAP10 and DAP12 in CD8+ T cell-mediated tissue damage in large granular lymphocyte leukemia. Blood. 2009;113:3226–34. doi: 10.1182/blood-2008-07-168245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tajsic T, Morrell NW. Cellular and molecular mechanisms of pulmonary vascular smooth muscle cell proliferation In: Yuan JX-J, Garcia JGN, West JB, Hales CA, Rich S, Archer SL, editors. Textbook of pulmonary vascular disease. New York:Springer 2011.pp 323–334. [Google Scholar]
- 41.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–77. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK, Zezula J, Greiner E, Gostick E, Price DA, et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res. 2008;14:2484–91. doi: 10.1158/1078-0432.CCR-07-4393. [DOI] [PubMed] [Google Scholar]
- 43.Blake SJ, Bruce Lyons A, Fraser CK, Hayball JD, Hughes TP. Dasatinib suppresses in vitro natural killer cell cytotoxicity. Blood. 2008;111:4415–6. doi: 10.1182/blood-2008-02-138701. [DOI] [PubMed] [Google Scholar]
- 44.Blake S, Hughes TP, Mayrhofer G, Lyons AB. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol. 2008;127:330–9. doi: 10.1016/j.clim.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, Götz M, Guillaume P, Döhner H, Bunjes D, et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol. 2008;36:1297–308. doi: 10.1016/j.exphem.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Fraser CK, Blake SJ, Diener KR, Lyons AB, Brown MP, Hughes TP, Hayball JD. Dasatinib inhibits recombinant viral antigen-specific murine CD4+ and CD8+ T-cell responses and NK-cell cytolytic activity in vitro and in vivo. Exp Hematol. 2009;37:256–65. doi: 10.1016/j.exphem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Seggewiss R, Loré K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–9. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Schmitt A, Chen B, Rojewski M, Rübeler V, Fei F, Yu Y, Yu X, Ringhoffer M, von Harsdorf S, et al. Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling. J Cell Mol Med. 2008;12(5B):2107–18. doi: 10.1111/j.1582-4934.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blake SJ, Lyons AB, Hughes TP. Nilotinib inhibits the Src-family kinase LCK and T-cell function in vitro. J Cell Mol Med. 2009;13:599–601. doi: 10.1111/j.1582-4934.2009.00500_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley TW, Parker CJ. CD4 (+)CD25 (+)Foxp3 (+) regulatory T cells and hematologic malignancies. Front Biosci (Schol Ed) 2010;2:980–92. doi: 10.2741/S114. [Schol Ed] [DOI] [PubMed] [Google Scholar]
- 51.Zhang R, Shah MV, Yang J, Nyland SB, Liu X, Yun JK, Albert R, Loughran TP., Jr. Network model of survival signaling in large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2008;105:16308–13. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tayade C, Fang Y, Black GP, v A P, Jr., Erlebacher A, Croy BA. Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J Leukoc Biol. 2005;78:1347–55. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- 53.Ramirez K, Kee BL. Multiple hats for natural killers. Curr Opin Immunol. 2010;22:193–8. doi: 10.1016/j.coi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka J, Sugita J, Shiratori S, Shigematsu A, Imamura M. Dasatinib enhances the expansion of CD56+CD3- NK cells from cord blood. Blood. 2012;119:6175–6. doi: 10.1182/blood-2012-03-416800. [DOI] [PubMed] [Google Scholar]
- 56.Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17:5546–52. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- 57.Eizuru Y, Minematsu T, Minamishima Y, Ebihara K, Takahashi K, Tamura K, Hosoda K, Masuho Y. Rapid diagnosis of cytomegalovirus infections by direct immunoperoxidase staining with human monoclonal antibody against an immediate-early antigen. Microbiol Immunol. 1991;35:1015–22. doi: 10.1111/j.1348-0421.1991.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 58.Gondo H, Minematsu T, Harada M, Akashi K, Hayashi S, Taniguchi S, Yamasaki K, Shibuya T, Takamatsu Y, Teshima T, et al. Cytomegalovirus (CMV) antigenaemia for rapid diagnosis and monitoring of CMV-associated disease after bone marrow transplantation. Br J Haematol. 1994;86:130–7. doi: 10.1111/j.1365-2141.1994.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 59.Al-Ameri A, Kantarjian H, Borthakur G, Bahceci E, Szatrowski T, Damokosh A, et al. Opportunistic infection are uncommon with dasatinib in patients with chronic myeloid leukemia in chronic phase (CP-CML). The 51th ASH Annual Meeting and Exposition 2009. [Google Scholar]
- 60.Lowin-Kropf B, Kunz B, Schneider P, Held W. A role for the src family kinase Fyn in NK cell activation and the formation of the repertoire of Ly49 receptors. Eur J Immunol. 2002;32:773–82. doi: 10.1002/1521-4141(200203)32:3<773::AID-IMMU773>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 61.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–96. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pignata C, Prasad KV, Hallek M, Druker B, Rudd CE, Robertson MJ, Ritz J. Phosphorylation of src family lck tyrosine kinase following interleukin-12 activation of human natural killer cells. Cell Immunol. 1995;165:211–6. doi: 10.1006/cimm.1995.1207. [DOI] [PubMed] [Google Scholar]
- 63.Bloch-Queyrat C, Fondanèche MC, Chen R, Yin L, Relouzat F, Veillette A, Fischer A, Latour S. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J Exp Med. 2005;202:181–92. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96:1961–8. [PubMed] [Google Scholar]
- 65.Chang WC, Hsiao MH, Pattengale PK. Natural killer cell immunodeficiency in patients with chronic myelogenous leukemia. IV. Interleukin-1 deficiency, γ-interferon deficiency and the restorative effects of short-term culture in the presence of interleukin-2 on natural killer cytotoxicity, natural killer-target binding and production of natural killer cytotoxic factor. Nat Immun Cell Growth Regul. 1991;10:57–70. [PubMed] [Google Scholar]
- 66.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88:2279–87. [PubMed] [Google Scholar]
- 67.Nakajima H, Zhao R, Lund TC, Ward J, Dolan M, Hirsch B, Miller JS. The BCR/ABL transgene causes abnormal NK cell differentiation and can be found in circulating NK cells of advanced phase chronic myelogenous leukemia patients. J Immunol. 2002;168:643–50. doi: 10.4049/jimmunol.168.2.643. [DOI] [PubMed] [Google Scholar]


