Abstract
The failure of EGFR inhibitors in colorectal tumors with KRAS mutations requires the development of alternative treatment strategies for this patient subgroup. Among the hallmarks of cancer the disturbed immunosurveillance and cancer immune evasion have become emerging targets for cancer therapy. Due to their pleiotropic functions immunomodulatory drugs (IMiDs) are interesting agents for combination therapies in solid tumors. However, their possible contribution and a way of monitoring their biological effects have yet to be revealed. In a heavily pretreated patient with advanced colorectal cancer carrying mutations in APC and KRAS genes, we show an early metabolic response and enhanced NK cell activity to monotherapy with lenalidomide. After subsequent lenalidomide/cetuximab combination treatment, the patient had progressive disease. At the same time a reduced performance status, complicated by febrile neutropenia, occurred, as well as a slight increase in metabolic activity. Concordantly NK cell activity dropped back to baseline. Thus, laboratory measurements and metabolic response assessment correlated with clinical conditions. This case report describes the feasibility and potential of a functional assessment of patient derived immune competent cells in combination with functional imaging for the detection of a biological response.
Keywords: advanced colorectal cancer, immune therapy, antibody-dependent cellular cytotoxicity, functional imaging, biologic response
Case Report
A 37-y-old woman with familiar adenomatous polyposis and advanced colorectal cancer (APC and KRAS mutated primary tumor) was referred to our department for further treatment. At time of initial diagnosis she underwent subtotal colectomy for obstructing carcinoma of the left colon and resection of liver metastases (08/2006; pT3N1M1LOVORO). To treat three primary unresectable liver metastases neoadjuvant chemotherapy with FOLFOX/bevacizumab and subsequent radiofrequency ablation and a further atypical liver resection combined with ileostomy reoperation were performed (12/2006). This procedure was complicated by a perforation of the duodenum, peritonitis, and multiple organ dysfunction syndrome. After recovery the patient received four cycles of adjuvant FOLFOX chemotherapy. One year later, she suffered a relapse with liver, pulmonary, and pelvic metastases (08/2008) and underwent reinduction with FOLFOX/bevacizumab with the outcome of a progressive disease after 6 cycles. Unfortunately, the patient did not respond to three subsequent cycles with FOLFIRI/bevacizumab. Due to extensive pelvic progression the patient received palliative radiotherapy (12/2009), bilateral nephrostomy, and a reinduction with FOLFIRI until disease progression (07/2010). At this time the patient agreed to take part in a clinical investigation trial of lenalidomide and cetuximab in patients with advanced solid tumors (NCT01166035). Treatment started with a monophase of 15 mg lenalidomide orally once daily for 21 d. Afterwards the patient received one 28 d-cycle of 15 mg lenalidomide once daily and infusions of cetuximab on the days 1, 8, 15, and 22 (400 mg/m2 at the first infusion, then subsequently 250 mg/m2).
During the first 6 wk of treatment, the WHO-five well-being index increased from 40% to 60% and the patient described a reduction of pelvic pain. Along with these clinical findings, imaging with [F-18]2-deoxy-2-fluoro-d-glucose positron emission tomography (F-18-FDG PET/CT) revealed an early metabolic response. F-18-FDG PET/CT upon inclusion proved high accumulation of FDG within the target lesion in the liver (Fig. 1).
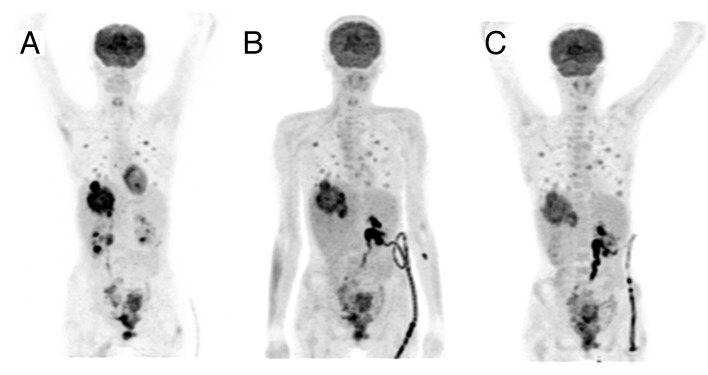
Figure 1. Functional imaging at different treatment time points. [F-18]2-deoxy-2-fluoro-d-glucose positron emission tomography (F-18-FDG PET/CT) maximum intensity projection images (antero-posterior view) demonstrating the FDG uptake during the treatment course of the patient. Upon inclusion (A) high accumulation of FDG was shown within the target lesion in the liver. Quantitative evaluation of FDG uptake of the first follow-up scan after three weeks of lenalidomide monotherapy (B) revealed a reduction of the maximum standard uptake value (SUVmax) within the target lesion by 45%, from initially 12.1 to 6.7. Restaging after three weeks of combined lenalidomide and cetuximab treatment showed a slight increase of the SUVmax to 7.6.
In the first follow-up scan after three weeks of lenalidomide monotherapy, quantitative evaluation of FDG uptake revealed a reduction of the maximum standard uptake value (SUVmax) within the target lesion by 45%, from initially 12.1 to 6.7. Restaging after three weeks of combined lenalidomide and cetuximab treatment showed a slight increase of the SUVmax to 7.6 (+13.4%), still representing a SUV reduction of 37.2% compared with the baseline value.
Therapy had been complicated by a bleeding episode after the liver biopsy before treatment start requiring angiographic embolization of the right 12th intercostal artery. At the end of the first combination cycle, therapy was terminated, because of progressive disease according to the CT scan and febrile neutropenia in combination with a high risk for urosepsis due to bilateral nephrostomy tubes. Together with the patient, who had to travel a long way to the study center, it was decided to discontinue the treatment.
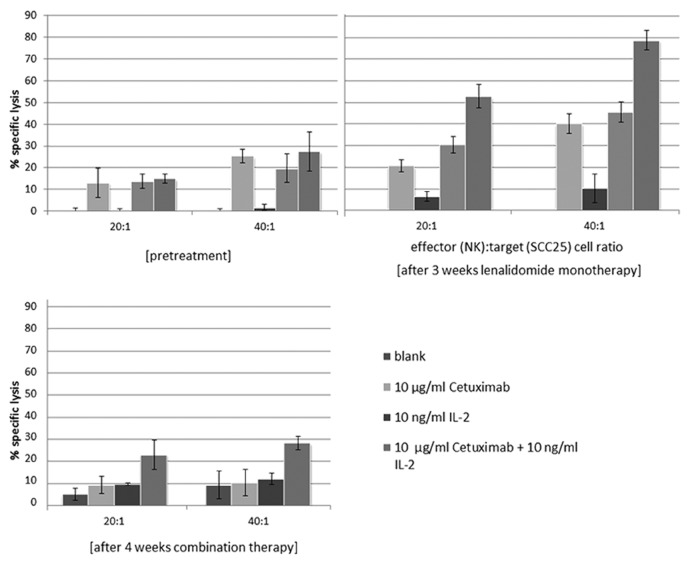
The translational research program included a functional testing of treatment response at the immune cellular level by measurement of antibody dependent cellular cytotoxicity (ADCC). Therefore, 9 ml of EDTA whole blood were collected before treatment and in parallel to the imaging procedures. The blood was processed immediately by density gradient centrifugation using LymphoprepTM (Fresenius KabiNorge AS for Axis-Shield Poc AS) according to the manufacturer’s protocol to gain peripheral blood mononuclear cells (PBMCs). Subsequently, natural killer (NK) cells were isolated from the PBMCs by negative depletion with magnetic cell sorting (MACS), using the NK isolation kit (Miltenyi Biotech). Afterwards, the purified and isolated NK cells were seeded in 96 well plates at two different cell numbers (20 000 and 40 000 cells/well) and cultured for 24 h in RPMI, completed with 10% FCS, 2 nM l-glutamine and 1% penicillin/streptomycin (PAA) either with or without Interleukin 2 (IL-2) (10 ng) (Sigma-Aldrich). NK purity had been assessed in preceding projects by flow cytometry analyses of CD3−CD56+ (BD Bioscience) stained cell population, revealing a purity of at least 90% (data not shown). ADCC measurements were performed in triplicates using a standard chrome release assay.1 Shortly, radioactive chromium 51Cr labeled SCC25 (oral squamous carcinoma cell line) cells (100 µCi) were added (1000 cells/well) with or without cetuximab (110 µg/well) to the pre-seeded NK cells. After 4 h of co-incubation, the amount of radioactive chromium release was measured in the cell culture supernatants with a gamma counter. Percentage of specific lysis was calculated according to the formula reported by Strohlein et al.2: % specific lysis = 100 × (mean experimental release – mean spontaneous release)/(mean maximal release – mean spontaneous release). In accordance with the clinical benefit described above and the findings of the metabolic imaging, the ADCC measurements of patient-derived NK cells showed the highest increase in antibody-dependent cellular cytotoxicity after lenalidomide monotherapy (Fig. 2).
Figure 2. Chromium-release measurement of ADCC of patient blood samples at different treatment time points. Time- and treatment-dependent enhancement of specific tumor cell lysis mediated by patient-derived NK cells toward naive or cetuximab-coated SCC25 cells (ADCC) (effector [NK]: target [SCC25] cell ratio 20:1 or 40:1). Specific lysis was calculated from radioactive chrome release assay. Data represent mean percent of lysis ± SD of triplicates.
Incubation of patient-derived NK cells after 3 weeks of lenalidomide monotherapy with cetuximab-labeled SCC25 cells revealed an increase of specific tumor cell lysis of 10% for both effector:target ratios. The additional combination with the cytokine IL-2 even displayed an increase of up to 50% compared with baseline (prior therapy). Concurrently to the clinical progression, at the third timepoint of investigation the ADCC decreased to baseline levels, but the cellular cytotoxicity (NK cells only) yet remained 5% and 10%, respectively above baseline.
Discussion
Standard therapy of metastatic colorectal cancer comprises of chemotherapy regimen partially in combination with monoclonal antibodies blocking the vascular endothelial growth factor (VEGF) or the epidermal growth factor receptor (EGFR). As KRAS mutant tumors do not respond to antibodies that block EGFR, these patients have less therapeutic options than those with wild-type tumors.
Among the hallmarks of cancer and mechanism-based target-specific treatments, the therapeutic manipulation of immunoevasion of tumors is an emerging concept.3 Especially the IgG1 subclass of therapeutic monoclonal antibodies (mAb) appear to trigger various immunological responses that may be responsible for their clinical activity apart from targeting the signaling pathway itself. This cascade may be triggered by mAb-mediated antibody-dependent cellular cytotoxicity (ADCC), leading to the generation of a large amount of released tumor antigen (TA), which can be processed and presented by dendritic cells to induce a tumor antigen-specific T lymphocytic response. Cetuximab, an IgG1 EGFR blocking antibody approved for use in metastatic colorectal cancer has shown an increase of ADCC independent of KRAS status in preclinical colorectal models in combination with immunomodulatory drugs.1
Additional combination with immune activating agents might enable relevant effects in patients with otherwise cancer-mediated immunosuppression.4 Especially thalidomide analogs, the so called immunomodulatory drugs (IMiDs), are potential combination partners with pleiotropic properties including immunomodulation, antiangiogenic, anti-inflammatory, and some antiproliferative effects.5,6 Lenalidomide (Revlimid®) is a second generation thalidomide derivative and is able to function as co-stimulatory molecule for mature T cells, to mitigate the negative effects of CTLA-4 signaling, to induce phosphorylation of CD28, and to inhibit regulatory T cells.7 Beside its actions on the adaptive immune system, lenalidomide also targets the innate immune response by activation of natural killer cells (NK) as well as natural killer T cells. This drug has already demonstrated efficacy in the treatment of a number of hematological malignancies, most notably multiple myeloma. It also appears to be highly active in combination with the anti-CD20 antibody rituximab, most likely due to an enhancement of ADCC.8 In the solid tumor setting, lenalidomide combinations with selected cytotoxic agents are currently being assessed.9-11 In vitro, lenalidomide enhanced NK cell-mediated ADCC of cetuximab-coated tumor cells.1 Furthermore, lenalidomide has also been shown to enhance the ability of immune cells to kill prostate cancer cells in vitro and to enhance markers of immune activation in patients with advanced solid tumors.9,12,13 Despite the diverse effects of IMiDs in vitro, the relative contribution of each of these to their ultimate antitumor activity remains unclear. Although the co-stimulatory effects on NK cells have been heralded as an important feature, these in vitro effects have yet to be firmly corroborated in vivo. In addition, it is presently not known how the biologic responses of individual patients, especially of heavily pretreated patients can be monitored.
In this case report a woman with APC and KRAS mutation positive advanced colorectal cancer took part in a phase I/II study with a lenalidomide prephase monotherapy followed by a lenalidomide/cetuximab combination therapy. Study endpoints included evaluation of dose-limiting toxicity and early biologic response. Despite multiple lines of pretreatment, we detected reduced tumor metabolism assessed by FDG-PET imaging after administration of lenalidomide monotherapy for 3 weeks. In parallel, patient derived NK cells exerted potentiated ex vivo ADCC activity compared with the pretreatment assays and the WHO-five well-being index increased. Since direct antiproliferative effects of lenalidomide are unlikely at the used dose level,14 our findings support the hypothesis that the metabolic response in this patient at this time point was caused by enhanced immune response.
As described above, the patient was taken off protocol after the following four weeks of combination treatment with lenalidomide and cetuximab due to progressive disease according to RECIST and along with a reduced performance status, complicated by febrile neutropenia. As recently demonstrated for other immunomodulatory drugs immunotherapeutic agents may result in response even after initial progressive disease.15,16 According to immune-related response criteria16 continuation of medication could be discussed. In addition, at that time point the functional imaging of the liver target lesion still showed a SUV reduction (37.2%) compared with the baseline value, even though SUV increased slightly compared with the initial 45% reduction. In a recent publication a SUV reduction of 36% or greater was shown to predict a histopathological response,17 but no clear cut off to define tumor progression is available. Furthermore, metabolic imaging cannot distinguish between tumor growth and inflammation, thus there are limitations to metabolic imaging, especially regarding immunostimulatory agents.
The lack of immunological response to the combination therapy in our patient remains elusive. Wu et al.1 reported about the complex interactions between lenalidomide-pretreated NK effector cells and solid tumor cells and described the requirement of specific NK cell-activating receptors to trigger ADCC against tumor cells in vitro. Therefore, cytokine release in febrile neutropenia and escape mechanisms modulating the surface receptors of NK cells or the change in target expression on tumor cells could have influenced the response and ADCC measurements. In addition, despite primarily suggested ADCC effects in k-ras mutant tumors,18 recent data discuss this controversially.19
In summary, our case report demonstrates for the first time the feasibility of functional immunoassays with patient-derived NK cells and early changes in metabolic imaging in a therapeutic setting with lenalidomide and cetuximab. In addition to the already widely used imaging techniques to assess early response, functional immunoassays might improve the development of new cancer drugs.
Future studies including earlier tumor stages will show, whether NK cell activity correlates with clinical outcome and may serve as a biomarker to monitor early response. The combination of IMiDs with antibodies capable of directing antibody-dependent cell-mediated cytotoxicity represents an attractive approach, since this strategy may bypass the intrinsic genetic defects in the cellular proliferative machinery of cancer.
Disclosure of Potential Conflicts of Interest
The clinical trial NCT01166035 was academia-initiated, for which Celgene provided the study drug and a scientific grant.
Acknowledgments
Special thanks goes to the members of the TEXO (tyrolean experimental oncology group) for their help and advice on study design and their financial support of the scientific program. A complete list of members of the TEXO appears on the website http://www.texo.at/.
Glossary
Abbreviations:
- IMiDs
immunomodulatory drugs
- NK cells
natural killer cells
- FDG-PET
[F-18]2-deoxy-2-fluoro-d-glucose positron emission tomography
- ADCC
antibody-dependent cellular cytotoxicity
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27327
References
- 1.Wu L, Parton A, Lu L, Adams M, Schafer P, Bartlett JB. Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers. Cancer Immunol Immunother. 2011;60:61–73. doi: 10.1007/s00262-010-0919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ströhlein MA, Grützner KU, Schildberg FW, Heiss MM. Induction of cytotoxicity against autologous tumour cells by interleukin-12: evidence for intrinsic anti-tumor immune capacity in curatively resected gastrointestinal tumour patients. Cancer Immunol Immunother. 2002;51:505–12. doi: 10.1007/s00262-002-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin Cancer Res. 2007;13:727s–32s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 5.Kalmadi S, Baz R, Mahindra A. Lenalidomide: the emerging role of a novel targeted agent in malignancies. Drugs Today (Barc) 2007;43:85–95. doi: 10.1358/dot.2007.43.2.1037480. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JB, Tozer A, Stirling D, Zeldis JB. Recent clinical studies of the immunomodulatory drug (IMiD) lenalidomide. Br J Cancer. 2005;93:613–9. doi: 10.1038/sj.bjc.6602774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Qian Z, Cai Z, Sun L, Wang H, Bartlett JB, Yi Q, Wang M. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–9. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 9.Henry JY, Lu L, Adams M, Meyer B, Bartlett JB, Dalgleish AG, Galustian C. Lenalidomide enhances the anti-prostate cancer activity of docetaxel in vitro and in vivo. Prostate. 2012;72:856–67. doi: 10.1002/pros.21488. [DOI] [PubMed] [Google Scholar]
- 10.Sanborn SL, Gibbons J, Krishnamurthi S, Brell JM, Dowlati A, Bokar JA, Nock C, Horvath N, Bako J, Remick SC, et al. Phase I trial of docetaxel given every 3 weeks and daily lenalidomide in patients with advanced solid tumors. Invest New Drugs. 2009;27:453–60. doi: 10.1007/s10637-008-9200-x. [DOI] [PubMed] [Google Scholar]
- 11.Kalmadi S, Davis M, Dowlati A, O’Keefe S, Cline-Burkhardt M, Pelley RJ, Borden E, Dreicer R, Bukowski R, Mekhail T. Phase I trial of three-weekly docetaxel, carboplatin and oral lenalidomide (Revlimid) in patients with advanced solid tumors. Invest New Drugs. 2007;25:211–6. doi: 10.1007/s10637-006-9025-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–59. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, Polychronis A, Pandha H, Muller GW, Stirling DI, et al. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer. 2004;90:955–61. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds CP, Kang MH, Keir ST, Gorlick R, Kolb EA, Lock R, Maris JM, Carol H, Morton CL, Billups CA, et al. Initial testing of lenalidomide by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;57:606–11. doi: 10.1002/pbc.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24:2174–80. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 17.Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, Hünerbein M, Felix R, Wust P, Amthauer H. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658–66. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- 18.Overdijk MB, Verploegen S, van den Brakel JH, Lammerts van Bueren JJ, Vink T, van de Winkel JG, Parren PW, Bleeker WK. Epidermal growth factor receptor (EGFR) antibody-induced antibody-dependent cellular cytotoxicity plays a prominent role in inhibiting tumorigenesis, even of tumor cells insensitive to EGFR signaling inhibition. J Immunol. 2011;187:3383–90. doi: 10.4049/jimmunol.1003926. [DOI] [PubMed] [Google Scholar]
- 19.Kasper S, Breitenbuecher F, Reis H, Brandau S, Worm K, Köhler J, Paul A, Trarbach T, Schmid KW, Schuler M. Oncogenic RAS simultaneously protects against anti-EGFR antibody-dependent cellular cytotoxicity and EGFR signaling blockade. Oncogene. 2013;32:2873–81. doi: 10.1038/onc.2012.302. [DOI] [PubMed] [Google Scholar]