Abstract
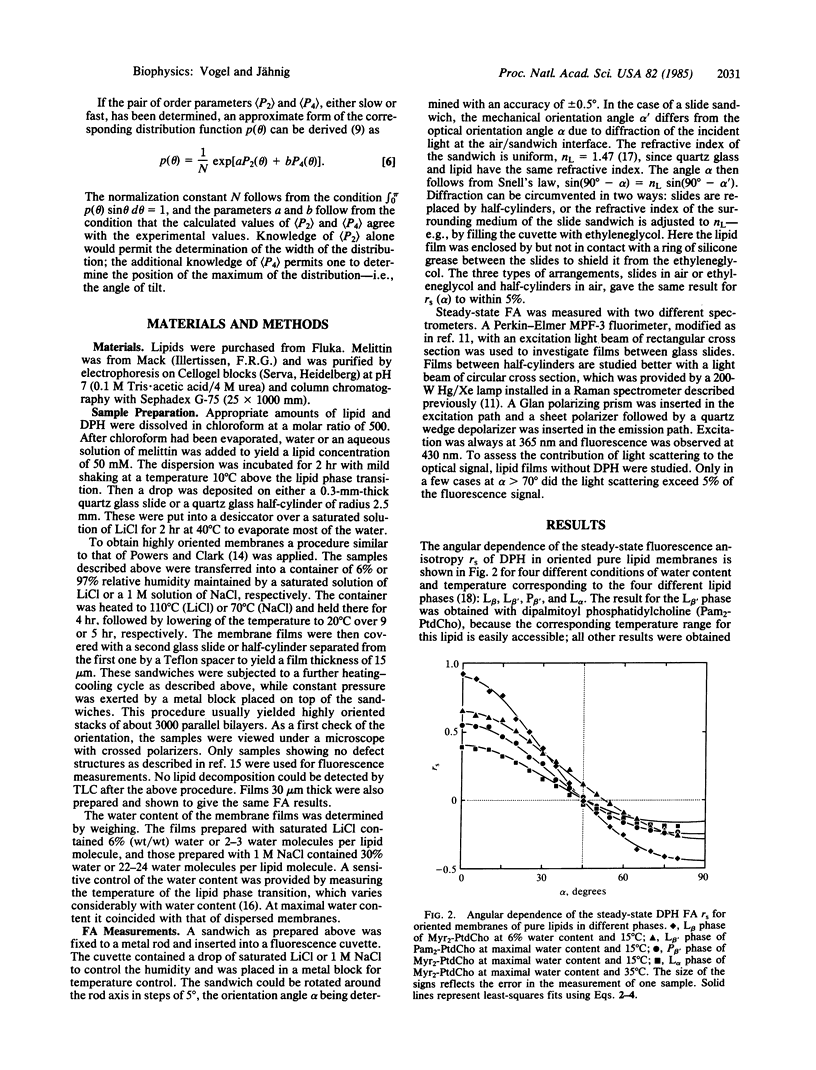
Fluorescence anisotropy measurements on isotropically distributed membranes yield the well-known orientational order parameter as a measure of orientational fluctuations of the fluorophore that are fast compared to the fluorescence lifetime. Measurements on oriented membranes provide four order parameters, two for fast orientational fluctuations and two for slow, from which their approximate angular distributions can be derived. This is exemplified for the fluctuations of lipids in membranes with and without protein. Steady-state fluorescence anisotropy experiments on oriented membranes are carried out, using diphenylhexatriene as a fluorescence probe for lipid fluctuations and melittin as a membrane protein. Protein molecules in a fluid lipid membrane restrict the fast lipid fluctuations and induce slow lipid fluctuations. The angular distribution of the slow fluctuations indicates that lipid molecules at the protein surface are tilted with respect to the membrane normal. The spatial coherence length of these fluctuations is of the order of 15 Å.
Keywords: fluorescence anisotropy, oriented membranes, order parameters, lipid fluctuations, lipid-protein interaction
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrich M. P., Vanderkooi J. M. Temperature dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phophoslipid artificial membranes. Biochemistry. 1976 Mar 23;15(6):1257–1261. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- Asher S. A., Pershan P. S. Alignment and defect structures in oriented phosphatidylcholine multilayers. Biophys J. 1979 Sep;27(3):393–421. doi: 10.1016/S0006-3495(79)85225-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig J., Zaccai G. Neutron diffraction studies on phosphatidylcholine model membranes. I. Head group conformation. J Mol Biol. 1979 Nov 15;134(4):673–691. doi: 10.1016/0022-2836(79)90479-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. H., Eanes E. D. Coexistence of rigid crystalline and liquid crystalline phases in lecithin-water mixtures. Biophys J. 1974 May;14(5):335–342. doi: 10.1016/S0006-3495(74)85920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F., Vogel H., Best L. Unifying description of the effect of membrane proteins on lipid order. Verification for the melittin/dimyristoylphosphatidylcholine system. Biochemistry. 1982 Dec 21;21(26):6790–6798. doi: 10.1021/bi00269a027. [DOI] [PubMed] [Google Scholar]
- Meier P., Blume A., Ohmes E., Neugebauer F. A., Kothe G. Structure and dynamics of phospholipid membranes: an electron spin resonance study employing biradical probes. Biochemistry. 1982 Feb 2;21(3):526–534. doi: 10.1021/bi00532a018. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Gilmore R., Glaser M., Gutowsky H. S., Hshung J. C., Kang S. Y., King T. E., Meadows M., Rice D. Deuterium nuclear magnetic resonance investigation of the effects of proteins and polypeptides on hydrocarbon chain order in model membrane systems. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4657–4660. doi: 10.1073/pnas.75.10.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers L., Clark N. A. Preparation of large monodomain phospholipid bilayer smectic liquid crystals. Proc Natl Acad Sci U S A. 1975 Mar;72(3):840–843. doi: 10.1073/pnas.72.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Stamatoff J., Feuer B., Guggenheim H. J., Tellez G., Yamane T. Amplitude of rippling in the P beta phase of dipalmitoylphosphatidylcholine bilayers. Biophys J. 1982 Jun;38(3):217–226. doi: 10.1016/S0006-3495(82)84551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Wittebort R. J., Blume A., Huang T. H., Das Gupta S. K., Griffin R. G. Carbon-13 nuclear magnetic resonance investigations of phase transitions and phase equilibria in pure and mixed phospholipid bilayers. Biochemistry. 1982 Jul 6;21(14):3487–3502. doi: 10.1021/bi00257a036. [DOI] [PubMed] [Google Scholar]



