SUMMARY
The mismatch repair (MMR) initiation protein MutS forms at least two types of sliding clamps on DNA: a transient mismatch searching clamp (~1 s) and an unusually stable (~600 s) ATP-bound clamp that recruits downstream MMR components. Remarkably, direct visualization of single MutS particles on mismatched DNA has not been reported. We have combined real-time particle tracking with fluorescence resonance energy transfer (FRET) to image MutS diffusion dynamics on DNA containing a single mismatch. We show searching MutS rotates during diffusion independent of ionic strength or flow rate, suggesting continuous contact with the DNA backbone. In contrast, ATP-bound MutS clamps that are visually and successively released from the mismatch spin freely around the DNA, and their diffusion is affected by ionic strength and flow rate. These observations show that ATP binding alters the MutS diffusion mechanics on DNA, which has a number of implications for the mechanism of MMR.
INTRODUCTION
Proteins that bind DNA often exploit one-dimensional (1D) thermal fluctuation-driven translocation (diffusion) to search for specific target sites (Berg et al., 1981). The application of 1D diffusion may reduce the time required to locate a DNA target site by several orders-of-magnitude compared to random three-dimensional (3D) collision-dependent diffusion. There are at least two types of diffusion-driven DNA searching mechanisms (Berg et al., 1981). Proteins that couple 1D diffusion with a large number of microscopic DNA dissociation-association events exhibit a “hopping” mechanism (Berg et al., 1981; Gowers and Halford, 2003). In its extreme case, a hopping protein completely dissociates from the DNA at every step and may only sample DNA sequences by 3D diffusion (Berg et al., 1981; Gowers and Halford, 2003). Proteins that maintain continuous but nonspecific contact with the DNA during 1D diffusion exhibit a “sliding” mechanism (Berg et al., 1981). If a sliding protein retains continuous contact with the phosphate backbone it will rotate 360° about the DNA approximately every 10.5 bp. Coupling with rotation decreases the translational diffusion rate largely because of the hydrodynamic resistance to rotation (Schurr, 1979).
Distinguishing between the hopping and sliding diffusion mechanisms may be indirectly determined by examining the diffusion response to ionic strength (Berg et al., 1981; Blainey et al., 2006; Halford and Szczelkun, 2002). A hopping protein may be expected to display faster diffusion at higher ionic strength because of an increase in the microscopic DNA association-dissociation events that results from electrostatic screening between the phosphate backbone and the DNA binding residues of the protein (Komazin-Meredith et al., 2008a). In contrast, ionic strength will not affect the diffusion of a sliding protein because microscopic ionic interactions between the protein and DNA are continually preserved and shielded during movement (Gorman et al., 2010; Tafvizi et al., 2011).
MutS homologs (MSH) recognize mismatched nucleotides to initiate mismatch repair (MMR) (Fukui, 2010). Mismatch binding provokes ADP→ATP exchange by MSHs, which results in the formation of an ATP-bound sliding clamp that dissociates from the mismatch and appears capable of hydrolysis-independent diffusion along the DNA (Acharya et al., 2003; Gradia et al., 1997, 1999). The ATP-bound MSH sliding clamps have been proposed to target downstream components and communicate the mismatch binding signal to a distant DNA strand scission, where MMR excision begins (Acharya et al., 2003; Fishel, 1998).
There are several largely indistinguishable structures of MSH proteins bound to mismatched DNA (Lamers et al., 2000; Obmolova et al., 2000; Warren et al., 2007). No structures of the ATP-bound sliding clamp currently exist. When MSH is bound to a mismatch, five peptide domains may be identified that encircle and interrogate the mispaired nucleotides within an ~4 × 2 nm channel (Lamers et al., 2000; Obmolova et al., 2000). An adjacent ~3 × 2 nm channel is separated from the DNA binding channel by the domain I mismatch interrogation arms (Lamers et al., 2000; Obmolova et al., 2000). Recent chemical crosslink studies have suggested that ATP binding by MutS induces a conformational transition that moves the mismatch interrogation domain I toward an outside connector domain III (Winkler et al., 2011), consistent with a number of earlier predictions (Acharya et al., 2003; Gradia et al., 1999). Such movement could easily open a channel large enough to accommodate sliding by the 2 nm diameter of DNA.
Single-molecule studies have suggested that MSH proteins scan DNA for mismatched nucleotides by an implied rotation-coupled 1D-facilitated diffusion (Gorman et al., 2007; Halford and Marko, 2004; Jeong et al., 2011; von Hippel and Berg, 1989). Using single-molecule fluorescence resonance energy transfer (smFRET), Jeong et al. (2011) showed that Thermus aquaticus MutS (MutS) scans the DNA for ~1 s in physiological ionic strength, sampling perhaps 700 bp of naked DNA in each event. In the presence of ATP, MutS lingers on the mismatch for ~3 s before dissociating as an ATP-bound hydrolysis-independent sliding clamp that is stable on the DNA for nearly 600 s (Jeong et al., 2011). Diffusion by the searching MutS appears independent of ionic strength consistent with a sliding mechanism (Gorman et al., 2007; Jeong et al., 2011). However, the active diffusion mechanism of searching and sliding MSH proteins with mismatched DNA is unknown.
Here, we have visualized the real-time binding dynamics and diffusion of different forms of MutS on a single 15.3 kb DNA molecule containing a defined mismatch. We simultaneously probed for FRET interaction(s) between fluorophore-labeled MutS and the mismatch as well as the rotational dynamics of MutS by polarization-anisotropy (Ha et al., 1996). These studies have allowed us to provide additional biophysical insights into the nature and dynamics of multiple MutS DNA binding conformers that have not been illuminated by static structure analysis (Lamers et al., 2000; Obmolova et al., 2000; Warren et al., 2007).
RESULTS
Real-Time Tracking of MutS on Mismatched DNA
We constructed a 15.3 kb λ-based DNA containing a single Alexa647 fluorophore located 9 bp from a single mismatch and 5.2 kb from a 5′-biotinylated DNA-end that may be attached to a flow-cell surface (Figure 1A; Figure S1A available online). Once tethered, the remaining end was blocked by Digoxigenin-Antidigoxigenein (Dig-Antidig), and the DNA was stretched with defined hydrodynamic drag by applying a laminar flow (Figure 1B). More than 85% of the tethered DNAs were found to colocalize with the Alexa647 (Figure S1B). Wide-field prism-type total internal reflection fluorescence (TIRF) microscopy was used to combine single-particle tracking and FRET to follow the dynamics of individual MutS proteins on mismatched DNA.
Figure 1. Single-Molecule Tracking of MutS on DNA.
(A) Illustration of the 15.3 kb λ-base DNA used for smFlow-FRET (Supplemental Experimental Procedures).
(B) A schematic representation of smFlow-FRET using prism-type total internal reflection fluorescence (TIRF) microscopy.
(C) A representative kymograph that shows searching MutS (strong green signal), followed by mismatch binding (reduced green signal; increased red FRET) in the absence of ATP.
(D) Representative time trace of donor-acceptor intensity (top) and the resulting FRET efficiency (bottom).
(E) A histogram of the FRET efficiency obtained from MutS molecules (Emismatch = 0.71 ± 0.04; n = 46).
(F) A histogram of the dwell time for MutS at the unpaired dT in the absence of ATP (τmismatch = 32.2 ± 4.9 s; n = 48).
(G) The displacement of Cy3-MutS while it diffuses along the DNA (left, green) or bound to the mismatch exhibiting FRET indicated by Alexa647 emission (left, red). The mean square displacements (MSD) of Cy3-MutS (right, green) and Alexa647 FRET (right, red) were plotted versus time where the slope provides the diffusion coefficients of searching MutS (DMutS·searching = 0.035 ± 0.005 μm2 s–1, mean ± s.e.) and mismatch bound MutS (DMutS·mismatch = 0.002 ± 0.002 μm2 s–1, mean ± s.e.) in 50 mM NaCl.
(H) A representative kymograph of Cy3-MutS mismatch interaction(s) in the presence of ATP (200 μM). FRET emission by Alexa647 (red) indicates mismatch binding, followed by the formation of an ATP-bound MutS sliding clamp.
(I) Representative time trace of a donor Cy3-MutS and acceptor Alexa647 intensity (top) and any resulting FRET efficiency (bottom).
(J) The dwell time of Cy3-MutS bound to a mismatch in the presence of ATP (200 μM; τMutS·mismatch·ATP = 4.2 ± 0.9 s, mean ± s.e.; n = 49).
(K) The displacement of Cy3-MutS bound to the mismatch as indicated by FRET emission from Alexa647 (left, red) and ATP-bound Cy3-MutS (left, green). The MSD of Cy3-MutS (right, green) was plotted versus time where the slope indicates the diffusion coefficients of ATP-bound MutS (DMutS·ATP = 0.058 ± 0.001 μm2 s–1, mean ± s.e.) in 50 mM NaCl.
All error bars indicate the standard error (s.e.). See also Figure S1.
When bound to the unpaired dT mismatch, a Cy3-labled MutS will be 4–5 nm from the Alexa647 leading to an expected FRET efficiency of ~0.7 (Coban et al., 2006; Jeong et al., 2011). A representative kymograph of a single Cy3-MutS (donor) interacting with the Alexa647-mismatched DNA (acceptor) shows that MutS interacted with the DNA beyond the mismatch between 4–6 s, ultimately binding the mismatch at 6 s, at which time FRET was observed for the next ~30 s (Figure 1D). A histogram of several single-molecule observations (n = 46) allowed us to determine the FRET efficiency (Emismatch = 0.71 ± 0.04, mean ± s.d.; Figure 1E) and average dwell time (τmismatch = 32.2 ± 4.9 s, mean ± s.e.; Figure 1F) of MutS bound to a mismatch. These measures appear identical to our previous results using short oligonucleotides (Jeong et al., 2011).
We examined the diffusion of Cy3-MutS on single DNA molecules by resolving individual particles using two-dimensional Gaussian-fitted center-of-intensity profiles (Figure 1G, left; Thompson et al., 2002). We then determined the mean-square displacement (MSD) of the MutS trajectories that describes the distance traveled by a diffusive motion (Supplemental Experimental Procedures). The diffusion coefficient was calculated from the slope of the MSD versus time for MutS that was scanning the duplex DNA (searching MutS; DMutS·searching = 0.035 ± 0.005 μm2 s–1, mean ± s.e.; 50 mM NaCl) and MutS bound to the mismatch (DMutS·mismatch ≤ 0.002 ± 0.002 μm2 s–1, mean ± s.e.; 50 mM NaCl; Figure 1G, right). The diffusion analysis of MutS bound to the mismatch relied on Alexa647-DNA MSD fluctuation and therefore indicates the lower limit of motion detection with this system. However, the difference in diffusion between the searching MutS and MutS bound to a mismatch is at least 17-fold and is a further gauge that the laminar flow has a negligible effect on MutS diffusion. Taken together, these data are consistent with our previous conclusion that MutS diffuses along the DNA until it encounters and stably binds to a mismatch (Jeong et al., 2011).
In the presence of ATP the Cy3-MutS emission mostly disappears and then reappears, whereas the Alexa647 FRET signal is anticorrelated with the Cy3-MutS signal and ultimately disappears after significant FRET (Figures 1H and 1I). The high FRET state (E ~0.7) is consistent with MutS that is transiently bound to the mismatch (Jeong et al., 2011). The average dwell time for this transient MutS mismatch binding in the presence of ATP on the 15.3 kb DNA (τMutS·mismatch·ATP = 4.2 ± 0.9 s, mean ± s.e.; n = 49; Figure 1J) was nearly identical to that determined by smFRET on short oligonucleotides (Jeong et al., 2011). Once released from the mismatch, the Cy3-MutS diffuses along the DNA (DMutS·ATP = 0.058 ± 0.001 μm2 s–1, mean ± s.e.; 50 mM NaCl; Figure 1K, right). The diffusion dynamics of ATP-bound MutS appears to be at least 2-fold faster than searching MutS, a characteristic that is examined in detail below. The vast majority of ATP-bound MutS sliding clamps were extremely stable and continued to diffuse along the DNA during the observation period (100 s; Acharya et al., 2003; Gradia et al., 1999). Taken as a whole, these results suggest the MutS dynamics on the 15.3 kb DNA using single-molecule Flow-FRET (smFlow-FRET) was remarkably similar to our smFRET studies using short oligonucleotides (Jeong et al., 2011).
We examined whether hydrodynamic flow significantly biased diffusion by determining the net displacement of MutS on the 15.3 kb DNA in sequential camera frames (Figure S2; Blainey et al., 2006). The small and symmetric deviation from zero in the presence or absence of flow and in the presence or absence of ATP suggests that the hydrodynamic force used to stretch the immobilized DNA (0.15 ml/min) does not substantially bias the MutS motion on the DNA (Figure S2), a property that we examine in detail below.
Searching MutS and ATP-Bound MutS Display Distinct Diffusion Mechanics
To clarify the differences between searching MutS and ATP-bound MutS, we examined the effect of ionic strength on their diffusion properties. Using smFRET, we first confirmed that ionic strength reduced the dwell time of searching MutS on DNA (n = 104, 51, 127, and 31 at 25, 50, 100, and 150 mM, respectively; Figure 2A; Jeong et al., 2011). In contrast, the stability of ATP-bound MutS was insensitive to ionic strength (, mean ± s.e.; n = 76, 66, 138, and 167 at 25, 50, 100, and 150 mM, respectively; Figure 2A). Interestingly, the steady-state ATPase is affected by ionic strength (Acharya et al., 2003; Blackwell et al., 1998; Gradia et al., 2000). These results support previous conclusions that the formation and maintenance of ATP-bound MutS on mismatched DNA does not depend on ATP hydrolysis (Acharya et al., 2003; Gradia et al., 1997, 1999).
Figure 2. The Distinct Diffusion Mechanism of Searching MutS and ATP-Bound MutS.
(A) The dwell times of the Cy3-MutS on a 100 bp duplex DNA (blue) and ATP-bound MutS on a 100 bp DNA containing an unpaired dT mismatch (red; , mean ± s.e.) as a function of ionic strength. For the ATP-bound Cy3-MutS time-lapse smFRET was exploited (Jeong et al., 2011).
(B) The diffusion coefficients of searching Cy3-MutS (blue; D̄ MutS·searching = 0.032 ± 0.001 μm2 s–1, mean ± s.e.) and ATP-bound Cy3-MutS (red) on the 15.3 kb DNA containing a mismatch at various salt concentrations (25, 50, 100, and 150 mM NaCl).
(C) The drift rate of protein trajectories versus flow rate.
All error bars indicate s.e. See also Figure S2 and Movie S1.
The diffusion of searching MutS does not depend on ionic strength as previously reported (D̄ MutS·searching = 0.032 ± 0.001 μm2 s–1, mean ± s.e.; n = 26, 29, 30, and 31 at 25, 50, 100, and 150 mM, respectively; Figure 2B; Jeong et al., 2011). These results are consistent with a sliding diffusion mechanism in which searching MutS is in continuous contact with the DNA (Blainey et al., 2009; Jeong et al., 2011). In contrast, the diffusion of ATP-bound MutS increases dramatically with ionic strength (n = 58, 53, 56, 32, and 250 at 25, 50, 100, 150, and 300 mM, respectively; Figure 2B). These results are consistent with a diffusion mechanism in which ATP-bound MutS is in discontinuous contact with the DNA containing a mismatch.
The diffusion coefficient of ATP-bound MutS increases 3- to 4-fold over a 12-fold increase in ionic strength (Figure 2B). Whereas proteins, such as UL42 (Komazin-Meredith et al., 2008a), the core domain of p53 (Tafvizi et al., 2011) and PCNA (Kochaniak et al., 2009), have a relatively similar order-of-magnitude change in the ionic strength dependence, the diffusion coefficient of the yeast MMR MutL homologs Mlh1-Pms1 appeared to increase 38-fold over an 8-fold increase in ionic strength (Gorman et al., 2010). The significance of these wide-ranging salt-dependent diffusion coefficients may be understood from the quantitative relationship between the dissociation constant (KD) of a protein and the ionic strength because the diffusion coefficient of a hopping protein is directly associated with the microscopic KD (Winter et al., 1981). We estimated the number of charges involved in the electrostatic interactions between the protein and the DNA as described by Kochaniak et al. (2009). From the slope of log(D) versus log([NaCl]) the numbers of screened charges were estimated to be 0.23 ± 0.01 for MutS (Komazin-Meredith et al., 2008b; Record et al., 1976, 1978). We also estimate the numbers of screened charges for the yeast Mlh1-Pms1 to be 1.69 ± 0.47 from the data of Gorman et al. (2010). These results suggest that MutS displays stronger electrostatic contacts with the DNA while diffusing than does yeast Mlh1-Pms1.
A quantitative determination of the effect of flow rate on diffusion may distinguish sliding from hopping diffusion mechanisms even though both mechanisms will experience energetic barriers associated with the external drag force induced by flow (Blainey et al., 2006; Lin et al., 2009). For example, the competing drag induced by the viscous fluid flow with the frictional drag associated with a protein-DNA interaction may shift the probability density distribution of step sizes in the direction of flow-biased walk differently for proteins in continuous contact versus discontinuous with the DNA (Lin et al., 2009). The drift rate coupled to flow may be determined by measuring the total displacement of all protein trajectories divided by the total duration of all trajectories (Figure 2C; Lin et al., 2009; Tafvizi et al., 2008). We found that searching MutS displays little, if any, change in drift rate in the absence of flow compared to flow rates that were 10-fold greater than those generally used in our studies (n = 42, 80, 28, 28, and 18 at 0, 0.15, 0.3, 0.75, and 1.5 ml/min, respectively; Figure 2C; where the flow rates indicate infusion rates of the syringe pump). In contrast, ATP-bound MutS displays a substantial increase in drift rate at flow rates above 0.3 ml/min (n = 31, 40, 47, 47, and 187 at 0, 0.15, 0.3, 0.75, and 1.5 ml/min, respectively; Figure 2C and Movie S1). A weak flow bias suggests that searching MutS exhibits a sliding diffusion mechanism in which frictional contact with the DNA minimizes the drag force associated with viscous fluid flow, whereas the diffusion bias of ATP-bound MutS is consistent with a hopping mechanism in which discontinuous DNA contact reduces frictional drag enhancing the effects of viscous fluid flow (Blainey et al., 2006; Lin et al., 2009). To our knowledge, this is the first case in which an allosteric cofactor binding (ATP) alters a protein diffusion mechanism. These results also underline the accuracy of experiments using flow rates below 0.3 ml/min to determine the diffusion coefficient of ATP-bound MutS because no flow bias was observed (Figure 2B).
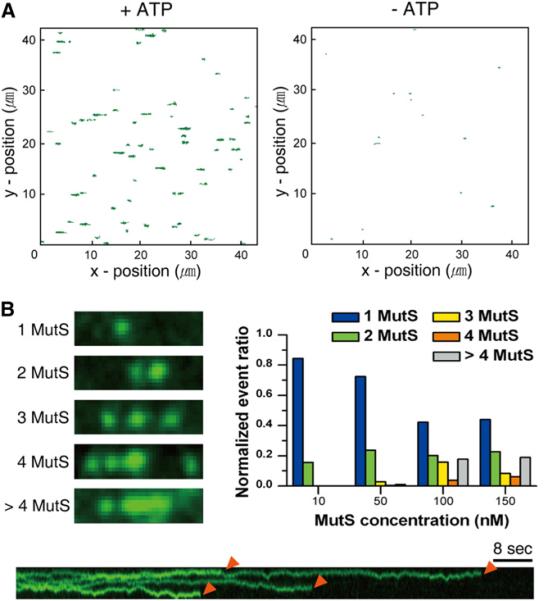
Visualizing Multiple ATP-Bound MutS Sliding Clamps on a Single Mismatched DNA
Multiple stable MutS particles appeared to be sequentially released from the mismatch in the presence of ATP (see Experimental Procedures; Movie S2), which results in the trajectories of the MutS proteins as shown in Figure S3 (left). MutS that is diffusing along DNA displays a random walk directional motion that is parallel to the flow because the DNA is stretched along the flow path (x-direction). Although the positions in the trajectory of MutS on the DNA are generally scattered asymmetrically, there will be a significant distribution of positions along the direction perpendicular to the laminar flow as a result of DNA fluctuation that may move perpendicular to the flow path. In the total MutS images, there will also exist trajectories of nonspecific surface bound proteins or abnormally moving proteins. These issues modestly increase the uncertainty of the calculation of the diffusion coefficient. To distinguish between MutS diffusing along DNA and MutS nonspecifically bound to the surface, we used an asymmetry test combined with a 1D directional test (Figure S3; Huet et al., 2006; Jaqaman et al., 2011). This algorithm eliminates particles with unnatural movement based on the degree of x-direction deviation from a symmetric x-y diffusion pattern produced by stretching the DNA in a laminar flow in the x-direction. The filtered trajectories of ATP-bound MutS are shown in Figure 3A (left. In the absence of ATP significant net displacement and occupancy is barely observed after the abnormal trajectories were filtered (Figure S3, right; Figure 3A, right). This is presumably a result of the shorter dwell times observed for these transient events (Jeong et al., 2011). Although some Cy3-MutS traces remain with the 15.3 kb DNA containing a mismatch in the absence of ATP (Figure 3A, right), we observe no significant diffusion events on a 48.5 kb λ DNA that does not contain a mismatch (data not shown). We note a broader distribution of the traverse positions along the longitudinal direction for the ATP-bound MutS (Figure 3A; Figure S3, left), which results from Brownian fluctuations of the free DNA end in the laminar flow (Blainey et al., 2006). Taken together, these results are consistent with the conclusion that ATP binding triggers the sequential release of multiple extremely stable MutS particles from the mismatch.
Figure 3. The Formation and Diffusion of Multiple ATP-Bound MutS Sliding Clamps.
(A) Comparison of single-molecule fields following binding of Cy3-MutS (green) in the presence of ATP (200 μM; left) and in the absence of ATP (right; 100 mM NaCl).
(B) Representative accounting of the number of Cy3-MutS particles on a single 15.3 kb DNA containing a mismatch (left; 100 mM NaCl). Normalized particle counts on a single 15.3 kb DNA containing a mismatch versus Cy3-MutS protein concentration (right). A representative kymograph that shows four ATP-bound MutS proteins are photobleached over time (bottom), which was used to determine the number of proteins diffusing on the DNA.
The theoretical diffraction limit for our smFlow-FRET system (~300 nm) combined with peak resolution inhibits a full accounting of the number of ATP-bound MutS particles on a single DNA substrate. We calculate that at best four MutS proteins could be fully resolved on the 5 μm DNA length (Figure 3B, left four panels). We counted the number of individual photobleaching events in a kymograph to determine the number of MutS particles on a single 15.3 kb DNA containing a mismatch (Figure 3B, bottom). An excess of four particles may be inferred from greater than 300 nm diameter particles as well as particles containing more than twice the expected intensity (Figure 3B, left bottom). The fraction of mismatched DNAs containing multiple MutS particles increased with increasing MutS protein concentration (Figure 3B, right), suggesting that at physiological concentrations (200–300 nM; Genschel et al., 1998), multiple MutS will be rapidly and sequentially loaded onto the DNA containing a mismatch. Together, these results visually confirm our previous indirect measures and demonstrate loading of multiple ATP-bound MutS that display quantitative diffusion characteristics (Jeong et al., 2011).
Searching MutS Displays Rotation-Coupled Diffusion
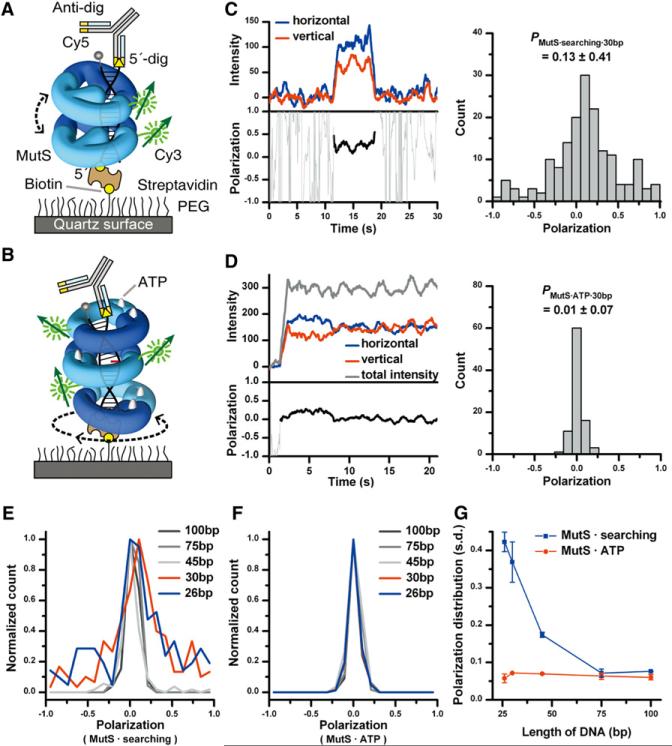
We exploited a single-molecule fluorescence polarization (smPolarization-TIRF) system to resolve the rotational dynamics of MutS diffusion on DNA (Supplemental Experimental Procedures). A steady-state polarization (P) is given by the ratio of (IH – IV)/(IH + IV), where IH and IV are the emission intensity horizontal and vertical to the microscope stage, respectively. The time-dependent depolarization of P may be used as a direct measure of rotation-coupled diffusion. For these measures to be accurate the rotational depolarization of the fluorophore bound to MutS must be significantly slower than the smPolarization-TIRF sampling time (50 ms). This can only occur if the MutS-bound fluorophore is relatively immobile, since free fluorophore will rotationally depolarize on the ns timescale (Gruber et al., 2000; Iqbal et al., 2008; Oiwa et al., 2003). To determine fluorophore mobility we examined the P of Cy3-MutS that is nonspecifically and randomly bound to the flow-cell surface. We found that the polarization of these randomly oriented Cy3-MutS was broadly distributed around zero (PCy3-MutS = 0.03 ± 0.43; Figure S4A). These results indicate that the rotational freedom of the Cy3 bound to MutS is significantly constrained during the smPolarization-TIRF measure (100 s). In addition, the Cy3-labeled DNA appears similarly though less efficiently constrained (PCy3-DNA = 0.15 ± 0.19; Figure S4B), presumably as a result of fluorophore stacking and a reduced rotational freedom of the biotin-streptavidin surface linkage (Iqbal et al., 2008).
We then constructed a DNA substrate, where the 5′-end was attached to the surface via biotin-streptavidin and the 3′-end was blocked with Dig-Antidig and labeled with Cy5 to identify co-localization and orientation with the Cy3-MutS (Figure 4A; Figure S4C; Jeong et al., 2011). Prior to Cy3-MutS binding the Cy5 was completely photobleached to eliminate any FRET (Figure S4C). A representative Cy3-MutS binding to a 100 bp duplex DNA results in identical horizontal and vertical emission signals that abruptly increase at ~6 s, remain steady for ~4 s and then abruptly disappear (Figure 4B, left). We did not observe any anti-correlation during the signal fluctuations and confirmed the dwell time (τduplex = 3.0 ± 0.2 s, mean ± s.d.; 100 mM NaCl; Figure S4D). During this dwell time the average polarization was near zero (PMutS·searching·100bp = 0.03 ± 0.08, mean ± s.d.; n = 101, Figure 4B, right). We interpret this pattern to indicate a time-averaged rotational polarization of Cy3-MutS during its interaction with duplex DNA, since Cy3-MutS may accomplish ~200 rotations on the duplex DNA during the 50 ms time resolution (based on the 24 bp MutS footprint, the effective DNA length of 76 bp and the diffusion coefficient; Figure 1G; Biswas and Hsieh, 1996). This large number of potential rotations would ultimately result in a largely identical IH and IV and p = 0.
Figure 4. The Rotational Diffusion of MutS along DNA Using smPolarization-TIRF.
(A) A schematic representation of the smPolarization-TIRF system (Supplemental Experimental Procedures). A circularly polarized beam, used to excite Cy3-MutS, was colocalized to the DNA via Cy5 emission. We define the emission polarization directions as a “horizontal” polarization (IH) in the plane parallel to the microscope stage and a “vertical” polarization (IV) in the plane perpendicular to the microscope stage.
(B) A representative trace of the IH (blue) and IV (red) fluorescence intensities (left panel, top) and resulting polarization (black, left panel, bottom) of Cy3-MutS binding to a 100 bp duplex DNA. The distribution of polarization was determined (right; PMutS·searching·100bp = 0.03 ± 0.08, mean ± s.d.; n = 101).
(C) A representative trace of the IH (blue) and IV (red) fluorescence intensities (left panel, top) and resulting polarization (black, left panel, bottom) of Cy3-MutS binding to a 100 bp DNA containing an unpaired dT mismatch in the absence of ATP. The distribution of polarization was determined (right; PMutS·mismatch·100bp = 0.05 ± 0.37, mean ± s.d.; n = 94).
(D) A representative trace of the IH (blue) and IV (red) fluorescence intensities (black, left panel, top) and resulting polarization (left panel, bottom) of Cy3-MutS binding to a 100 bp DNA containing an unpaired dT mismatch in the presence of 1mM ATP. The distribution of polarization was determined for the initial mismatch binding state (right; PMutS·mismatch·ATP1·100bp = 0.02 ± 0.26, mean ± s.d.; n = 111; Figure S4H) and the second ATP-bound Cy3-MutS state (right; PMutS·mismatch·ATP2·100bp = 0.01 ± 0.07, mean ± s.d.; n = 94).
All experiments were carried out at 100 mM NaCl. See also Table S1 and Figure S4.
Binding of Cy3-MutS to a 100 bp DNA containing a central mismatch resulted in an average dwell time similar to previous studies (τmismatch = 33.9 ± 3.3 s, mean ± s.d.; n = 90; Figure 4C, left; Figure S4E; Jeong et al., 2011). However, we observed a broad distribution of polarization with an average near zero (PMutS·mismatch·100bp = 0.05 ± 0.37, mean ± s.d.; n = 94; Figure 4C, right). Such a broad distribution can only occur if the DNA and the bound Cy3-MutS are rotationally constrained. Interestingly, there appears to be no correlation between the polarization of Cy3-MutS and the Cy5-DNA, suggesting multiple distinct binding interactions (Figure S4F). These observations appear contrary to the consistently overlapping MSH-mismatch cocrystal structures (Lamers et al., 2000; Obmolova et al., 2000; Warren et al., 2007). We conclude that the Cy3-MutS is bound to the mismatch in a variety of orientations that ultimately results in a wide distribution of polarization (Figure S4G).
We examined the polarization of the Cy3-MutS on mismatched DNA in the presence ATP (Figure 4D). A representative trace shows a polarized state (P~–0.4) that is depolarized at 5 s but remains associated with the mismatched DNA (Figure 4D, left). The initial state displays a broad distribution with an average polarization near zero (PMutS·mismatch·ATP1·100bp = 0.02 ± 0.26, mean ± s.d.; n = 111; Figure S4H), whereas the second state displays a narrow distribution with an average polarization near zero (PMutS·mismatch·ATP2·100bp = 0.01 ± 0.07, mean ± s.d.; n = 94; Figure 4D, right). Notably, the total intensity (IH + IV) remains constant during the entire Cy3-MutS interaction (Figure 4D and Figure S4I). The dwell time of the initial polarized state (τmismatch·ATP = 3.3 ± 0.2 s, mean ± s.d.; Figure S4J) appears identical to the transient dwell time of MutS bound to the mismatch prior to the formation of an ATP-bound sliding clamp (Jeong et al., 2011). Together these observations are consistent with the hypothesis that MutS transiently binds to the mismatch with a wide distribution of polarized orientations that ultimately resolve into an ATP-bound sliding clamp with no polarization. There are at least two possibilities for the lack of ATP-bound MutS sliding clamp polarization: (1) ATP induces a conformational transition that unlocks the fluorophore, which rapidly depolarized, or (2) ATP-bound MutS sliding clamp experiences rotational diffusion with a time averaged polarization near zero. We favor this latter hypothesis since the polarization decay appears to be approximately 4–5 orders-of-magnitude slower than free fluorophore depolarization (τpolarization·decay = 0.16 ± 0.02 s, mean ± s.d.; Figure S4K).
ATP-Bound MutS Rotates Freely around the Helical Axis of DNA
One method of directly examining the rotational diffusion mechanics of MutS is to constrain translational diffusion by varying the DNA length. For example, a 26 bp DNA allows 2 bp of effective diffusion length and a 30 bp DNA allows 6 bp of effective diffusion length to a MutS that occupies a 24 bp footprint (Biswas and Hsieh, 1996). If MutS tracks along the duplex DNA backbone it cannot execute a complete rotation around the helix on either of these short DNA lengths, predicting a limited depolarization and ultimately resulting in a broad distribution of P that depends on the range of random orientations of the DNA on the surface (Figure 5A). However, as the DNA length increases diffusion will not be constrained and depolarization will only be limited by the rate of diffusion, which on short DNAs is significantly faster than the sampling time. In contrast, a freely rotating clamp will not be constrained by any DNA length and depolarization will only be limited by rotational diffusion that is also significantly faster than the sampling time (Figure 5B). A representative trace of searching MutS on a 30 bp duplex DNA appears kinetically identical to binding with the 100 bp duplex DNA (compare Figure 4B, left, to Figure 5C, left). However, unlike the 100 bp duplex DNA, we observed a broad distribution of polarization with the 30 bp duplex DNA (PMutS·searching·30bp = 0.13 ± 0.41, mean ± s.d.; n = 170; Figure 5C, right). In contrast, both a representative trace and the polarization distribution of MutS on a 30 bp mismatched DNA in the presence of ATP appears identical to MutS binding with the 100 bp mismatched DNA in the presence of ATP (P MutS·mismatch·ATP2·30bp = 0.01 ± 0.07, mean ± s.d.; n = 91; compare Figure 4D to Figure 5D).
Figure 5. DNA Length-Dependent Polarization of Cy3-MutS.
(A and B) Illustration of Cy3-MutS binding and polarization that is restricted (A) and unrestricted (B) by the DNA length.
(C) A representative trace of the IH (blue) and IV (red) fluorescence intensities (left panel, top) and resulting polarization (black, left panel, bottom) of Cy3-MutS binding to a 30 bp duplex DNA. The distribution of polarization was determined (right; PMutS·searching·30bp = 0.13 ± 0.41, mean ± s.d.; n = 170).
(D) A representative trace of the IH (blue) and IV (red) fluorescence intensities (left panel, top) and resulting polarization (black, left panel, bottom) of Cy3-MutS binding to a 30 bp DNA containing an unpaired dT mismatch in the presence of ATP (200 mM). The distribution of polarization was determined (right; P MutS·mismatch·ATP2·30bp = 0.01 ± 0.07, mean ± s.d.; n = 91). The polarization distributions at various lengths (26, 30, 45, 75, and 100 bp) of (E) duplex DNA and (F) DNA containing an unpaired dT mismatch.
(G) The polarization distribution of searching Cy3-MutS and ATP-bound Cy3-MutS at different lengths. Error bars indicate s.e.
See also Table S1.
The polarization distribution at each DNA length (26–100 bp) for searching MutS on duplex DNA and ATP-bound MutS on mismatched DNA was normalized and plotted (Figures 5E and 5F, respectively). The standard of deviation is a quantitative measure of the polarization distribution and was plotted for each DNA length (Figure 5G). These results clearly show a broad polarization distribution of searching MutS on short DNAs that is largely eliminated with longer DNAs (Figures 5E and 5G). Together with an ionic strength-independent diffusion coefficient and a lack of flow-biased drift rate, these results strongly suggest that searching MutS rotationally tracks along the backbone while in continuous contact (sliding) with the DNA. In contrast, ATP-bound MutS does not exhibit any DNA length-dependent polarization distribution (Figures 5F and 5G). Together with a diffusion coefficient that is dependent of ionic strength, these results are consistent with the conclusion that ATP-bound MutS freely rotates, while in discontinuous contact (hopping) with the DNA.
DISCUSSION
We have visualized the dynamics and diffusion of MutS on single DNA molecules containing a mismatch in real time. In contrast to the static crystal structures we find that MutS undergoes several active transitions. These conformational transitions alter the diffusion characteristics of MutS, which may ultimately be projected onto the mechanism MMR recognition and downstream excision repair processes.
Here and in previous studies, we have observed searching MutS clamps that diffuse along duplex DNA for ~1 s while in continuous rotational contact with the backbone (Gorman et al., 2007; Jeong et al., 2011). When MutS encounters a mismatch, it momentary binds for 3–4 s. Our previous work has indicated that mismatched nucleotides introduce flexibility into an otherwise uniform worm-like DNA chain (Mazurek et al., 2009). Together, these results are consistent with the hypothesis that mismatch recognition involves stalling of translational diffusion by the searching MutS that may result from decreased backbone homogeneity. Gorman and colleagues visualized the diffusion of yeast MMR proteins Msh2-Msh6 and Mlh1-Pms1 on duplex λ DNA and λ DNA containing nucleosomes (Gorman et al., 2007, 2010). Our results indicate that there are significant operational differences between MSH proteins interacting with duplex DNA and mismatched DNA (Figure 2). In addition, we do not observe long-lived MutS bound to duplex DNA, suggesting that this species is rare (Gorman et al., 2007).
Transient mismatch binding by MutS provokes the release of ADP, left over from a previous mismatch encounter or following “priming” of nucleotide-free protein, followed by subsequent binding of ATP (ADP/ATP exchange; Acharya et al., 2003; Gradia et al., 1997). ATP binding results in the formation of a hydrolysis-independent sliding clamp that dissociates from the mismatch (Gradia et al., 1999; Jeong et al., 2011; Mendillo et al., 2005). The MSH subunits process ATP asymmetrically (Junop et al., 2001; Lamers et al., 2003). In addition, yeast MSH mutations have been discovered that appears to bind ATP but remain on the mismatch, where it assembles a ternary complex with the yeast MutL homologs (MLH) Mlh1-Pms1 (Hargreaves et al., 2010). Together, these observations have suggested the possibility that ADP→ATP exchange may result in combinatorial ADP/ATP-bound species on the mispair that is required for ternary complex formation with the MLH proteins (Kunkel and Erie, 2005). We regard this as unlikely because fully ATP-bound MSHs appear to be essential for both complex formation and mismatch repair (Acharya et al., 2003; Heinen et al., 2011; Mazur et al., 2006).
We have visualized the characteristics of ATP-bound MutS sequentially released from the mismatch. The diffusion of ATP-bound MutS is approximately 3-to 4-fold faster than searching MutS at physiological salt (Figure 2B). Once MutS forms a stable ATP-bound sliding clamp, its searching functions are no longer required. Asymmetric binding of ATP by MutS may alter the timing and/or symmetric opening of this central channel. An asymmetric geometry of the ATP-bound MutS might also retain some of the searching structure that could discontinuously track the DNA backbone and account for the relatively modest increase in diffusion. Interestingly, PCNA also forms a sliding clamp on DNA and appears to display a heterogeneous diffusion mechanism that combines random helical sliding along the DNA backbone with a nonhelical hopping diffusion (Kochaniak et al., 2009).
The smPolarization-TIRF demonstrated that searching MutS is in rotational contact with the DNA backbone during diffusion. In contrast, ATP-bound sliding clamps appear to rotate freely. There are several implications for MMR. First, free rotation by MutS increases the degrees-of-freedom for interactions with downstream DNA excision components that may be constrained by a specific protein-DNA interaction. Second, the conformational transition(s) required to form a freely diffusing sliding clamp suggest significantly more complexity to MutS than detailed in the MSH crystal structures (Lamers et al., 2000; Obmolova et al., 2000; Warren et al., 2007). Finally, although the complete MMR reaction has not been reconstituted on single DNA molecules, the biochemical and visual evidence appears to uniquely support the Molecular Switch Model for MMR (Acharya et al., 2003; Fishel, 1998; Gradia et al., 1997, 1999).
The observations presented here are consistent with an ATP-driven switch in the DNA diffusion dynamics of MutS. The concept of Rectified Brownian Motion (RBM) suggests that allostery may introduce barriers to directional diffusion (Fox, 1998). However, to our knowledge, this is the first case in which an allosteric conformational transition alters the fundamental diffusion mechanism.
EXPERIMENTAL PROCEDURES
MutS Protein
Thermus aquaticus MutS (C42A,T469C) was expressed, purified, and labeled with Cy3 as previously described (Jeong et al., 2011). The Cy3-labeling efficiency per a dimer MutS was 55%. Photobleaching analysis of single Cy3-MutS molecules demonstrated that 83% of the MutS homodimers contained a single Cy3.
DNA Substrate for Single-Particle Tracking with FRET
We constructed 15.3 kb DNA containing an unpaired dT and an Alexa647 fluorophore that does not interfere with MutS binding and may be used as a FRET acceptor from a Cy3 donor. l DNA was digested with BsrGI or ApaI, which results in 5,204 and 10,076 bp fragments that contain a 4 nt single-stranded DNA (ssDNA) 5′-overhang and the 12 nt ssDNA left cohesive end (cosL) of λ, respectively. The cosL tail and 4 nt BsrGI tail of 5.2 kb fragment were annealed and ligated with a 47 nt ssDNA containing Alexa647, 21 nt 5′-biotin ssDNA, and 19 nt complementary ssDNA linker, respectively. The cosL tail and 4 nt ApaI tail of a 10.1 kb fragment were annealed and ligated with a 48 nt ssDNA containing an unpaired dT and a 29 bp 5′-digoxigenin dsDNA, respectively. The two modified l fragments were PAGE purified, annealed, and ligated via the 35 and 36 nt ssDNA overhanging ends. The resulting 15.3-kb-long DNA was isolated from a 0.5% agarose gel and eluted by a QIAEX II Gel Extraction Kit (Qiagen). More detailed information is available in the Supplemental Experimental Procedures.
Single-Particle Tracking with FRET
The 15.3 kb DNA (10 pM) in the blocking buffer (20 mM Tris-HCl [pH 7.5], 2 mM EDTA, 0.0025% [v/v] Tween 2.0, 100 mg/ml BSA and NaCl concentrations as described in the text) was introduced into the flow chamber at the flow rate 0.04 ml/min by a syringe pump (Harvard apparatus). Following anchoring of the 5′-biotin DNA to the PEG-biotin-streptavidin quartz surface, the unattached DNA molecules were removed by extensive washing. Anti-digoxigenin (Roche, Indianapolis, IN, USA) was linked to Dig-end of the DNA by the incubation in the flow chamber in the blocking buffer. A reaction buffer that includes 10 nM Cy3-MutS at a flow rate of 0.15 ml/min generates a drag force on the DNA, which results in stretching of the immobilized DNA. We followed individual Cy3-MutS on the stretched DNA for 100 s.
For the visualization of multiple sliding clamps we incubated 150 nM Cy3-MutS with immobilized 15.3 kb DNA in 1 mM ATP and 100 mM NaCl for 5 min. Stringent washing of the flow chamber with 2 ml reaction buffer for 4 min removed Cy3-MutS that was not stably bound to DNA as well as free Cy3-MutS in solution. We imaged Cy3-MutS proteins that still dwell on the 15.3 kb DNA at the flow rate of 0.15 ml/min. For diffusion coefficient analysis, three Cy3-MutS on a single DNA were compared to a single Cy3-MutS on a single DNA.
To visualize donor and acceptor signals simultaneously, the field of view from an electron multiplying charge-coupled device (EMCCD) camera (Andor iXonEM+897) was divided into two channels for Cy3 and Alexa647 emission signals. After correction for the donor (ID) and the acceptor (IA) intensity background and the leakage of the donor emission into the acceptor channel, the FRET efficiency was computed as a ratio of IA / (ID + IA). The experiments were performed in the presence of an oxygen scavenging system (0.8% [w/v] D-glucose [Sigma-Aldrich, St. Louis, MO, USA], 165 U/ml glucose oxidase [Sigma-Aldrich], and 2,170 U/ml catalase [Sigma-Aldrich]) and binding buffer (20 mM Tris-HCl [pH 7.6], 25–150 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 0.1 mM DTT) to minimize photobleaching, and 143 mM 2-Mercaptoethanol (Sigma-Aldrich) to suppress photoblinking of Cy3 fluorophores.
DNA Substrates for Single-Molecule Polarization
Oligonulceotide DNA substrates (Table S1; IDT, Coralville, IA, USA) were constructed by annealing paired PAGE-purified oligos at a molar ratio of 1:1.3 in an annealing buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, and 1 mM EDTA) for a final concentration of 4 mM. The solution containing oligos was incubated at 95°C for five minutes and was then slowly cooled to room temperature over three hours. The annealed DNA substrates were stored at 4°C.
Single-Molecule Fluorescence Polarization
A 532-nm DPSS laser (Cobolt Samba, 100 mW) was used to excite the fluorescence polarization probe Cy3. The circular polarization of the excitation beam was achieved by shifting the phase of the linearly polarized laser using a quarter-wave plate (Thorlabs, Newton, NJ, USA) in front of the sample. The linearity of the beam with the polarization ratio of 100:1 was improved by placing a polarizer (Thorlabs) with the polarization ratio of 10,000:1 before the quarter-wave plate. The resulting circular polarization beam shows 10% deviation between vertical and horizontal components. The fluorescent emission signals were imaged in a prism-type TIRF microscope (Olympus IX-71, water-type 603 objective, numerical aperture = 1.2) using an EMCCD (Hamamatsu ImagEM C9100-13) and the imaging software MetaMorph 7.6 (Molecular Devices, Sunnyvale, CA, USA). The image focused by the 603 objective lens was enlarged with a 1.63 magnifier. The polarized signals from each Cy3-MutS molecule were separated to vertical and horizontal polarized components (IV and IH) using the DV2 fluorescence polarization imaging system (Photometrics, DV2-36pol).
The polarization data were analyzed using IDL (ITT VIS) and MATLAB (The MathWorks) scripts. The experiments were performed in the presence of an oxygen scavenging system. Polarization was calculated as the ratio of (IH IV) / (IH + IV). A 10-point adjacent averaging smoothed all traces of Cy3 intensity and polarization. The histograms of polarization were taken from the averaged single binding traces.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Samir Hamdan, Yong Woon Kim, and Michael Poirier for helpful discussions. This work was supported by the National Research Foundation (NRF) of Korea and was funded by the Ministry of Education, Science, and Technology (MEST; grants no. 2010-0027576 and 2011-0013901 to J.-B.L. and no. 2011-0007166 to C.B.) and National Institutes of Health (NIH; CA67007 to R.F.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures, one table, Supplemental Experimental Procedures, and two movies and can be found with this article online at doi:10.1016/j.str.2012.04.017.
REFERENCES
- Acharya S, Foster PL, Brooks P, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol. Cell. 2003;12:233–246. doi: 10.1016/s1097-2765(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Berg OG, Winter RB, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- Biswas I, Hsieh P. Identification and characterization of a thermo-stable MutS homolog from Thermus aquaticus. J. Biol. Chem. 1996;271:5040–5048. doi: 10.1074/jbc.271.9.5040. [DOI] [PubMed] [Google Scholar]
- Blackwell LJ, Bjornson KP, Modrich P. DNA-dependent activation of the hMutSalpha ATPase. J. Biol. Chem. 1998;273:32049–32054. doi: 10.1074/jbc.273.48.32049. [DOI] [PubMed] [Google Scholar]
- Blainey PC, van Oijen AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc. Natl. Acad. Sci. USA. 2006;103:5752–5757. doi: 10.1073/pnas.0509723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blainey PC, Luo G, Kou SC, Mangel WF, Verdine GL, Bagchi B, Xie XS. Nonspecifically bound proteins spin while diffusing along DNA. Nat. Struct. Mol. Biol. 2009;16:1224–1229. doi: 10.1038/nsmb.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban O, Lamb DC, Zaychikov E, Heumann H, Nienhaus GU. Conformational heterogeneity in RNA polymerase observed by single-pair FRET microscopy. Biophys. J. 2006;90:4605–4617. doi: 10.1529/biophysj.105.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R. Mismatch repair, molecular switches, and signal transduction. Genes Dev. 1998;12:2096–2101. doi: 10.1101/gad.12.14.2096. [DOI] [PubMed] [Google Scholar]
- Fox RF. Rectified Brownian movement in molecular and cell biology. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 1998;57:2177–2203. [Google Scholar]
- Fukui K. DNA mismatch repair in eukaryotes and bacteria. J. Nucleic Acids 2010 Jul. 2010;27:2010. doi: 10.4061/2010/260512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschel J, Littman SJ, Drummond JT, Modrich P. Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J. Biol. Chem. 1998;273:19895–19901. doi: 10.1074/jbc.273.31.19895. [DOI] [PubMed] [Google Scholar]
- Gorman J, Chowdhury A, Surtees JA, Shimada J, Reichman DR, Alani E, Greene EC. Dynamic basis for one-dimensional DNA scanning by the mismatch repair complex Msh2-Msh6. Mol. Cell. 2007;28:359–370. doi: 10.1016/j.molcel.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J, Plys AJ, Visnapuu ML, Alani E, Greene EC. Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat. Struct. Mol. Biol. 2010;17:932–938. doi: 10.1038/nsmb.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers DM, Halford SE. Protein motion from non-specific to specific DNA by three-dimensional routes aided by supercoiling. EMBO J. 2003;22:1410–1418. doi: 10.1093/emboj/cdg125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradia S, Acharya S, Fishel R. The human mismatch recognition complex hMSH2-hMSH6 functions as a novel molecular switch. Cell. 1997;91:995–1005. doi: 10.1016/s0092-8674(00)80490-0. [DOI] [PubMed] [Google Scholar]
- Gradia S, Subramanian D, Wilson T, Acharya S, Makhov A, Griffith J, Fishel R. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol. Cell. 1999;3:255–261. doi: 10.1016/s1097-2765(00)80316-0. [DOI] [PubMed] [Google Scholar]
- Gradia S, Acharya S, Fishel R. The role of mismatched nucleotides in activating the hMSH2-hMSH6 molecular switch. J. Biol. Chem. 2000;275:3922–3930. doi: 10.1074/jbc.275.6.3922. [DOI] [PubMed] [Google Scholar]
- Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus HG. Anomalous fluorescence enhancement of Cy3 and cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjug. Chem. 2000;11:696–704. doi: 10.1021/bc000015m. [DOI] [PubMed] [Google Scholar]
- Ha T, Enderle T, Chemla S, Selvin R, Weiss S. Single Molecule Dynamics Studied by Polarization Modulation. Phys. Rev. Lett. 1996;77:3979–3982. doi: 10.1103/PhysRevLett.77.3979. [DOI] [PubMed] [Google Scholar]
- Halford SE, Szczelkun MD. How to get from A to B: strategies for analysing protein motion on DNA. Eur. Biophys. J. 2002;31:257–267. doi: 10.1007/s00249-002-0224-4. [DOI] [PubMed] [Google Scholar]
- Halford SE, Marko JF. How do site-specific DNA-binding proteins find their targets? Nucleic Acids Res. 2004;32:3040–3052. doi: 10.1093/nar/gkh624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves VV, Shell SS, Mazur DJ, Hess MT, Kolodner RD. Interaction between the Msh2 and Msh6 nucleotide-binding sites in the Saccharomyces cerevisiae Msh2-Msh6 complex. J. Biol. Chem. 2010;285:9301–9310. doi: 10.1074/jbc.M109.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen CD, Cyr JL, Cook C, Punja N, Sakato M, Forties RA, Lopez JM, Hingorani MM, Fishel R. Human MSH2 (hMSH2) protein controls ATP processing by hMSH2-hMSH6. J. Biol. Chem. 2011;286:40287–40295. doi: 10.1074/jbc.M111.297523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S, Karatekin E, Tran VS, Fanget I, Cribier S, Henry JP. Analysis of transient behavior in complex trajectories: application to secretory vesicle dynamics. Biophys. J. 2006;91:3542–3559. doi: 10.1529/biophysj.105.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal A, Arslan S, Okumus B, Wilson TJ, Giraud G, Norman DG, Ha T, Lilley DM. Orientation dependence in fluorescent energy transfer between Cy3 and Cy5 terminally attached to double-stranded nucleic acids. Proc. Natl. Acad. Sci. USA. 2008;105:11176–11181. doi: 10.1073/pnas.0801707105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaqaman K, Kuwata H, Touret N, Collins R, Trimble WS, Danuser G, Grinstein S. Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell. 2011;146:593–606. doi: 10.1016/j.cell.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C, Cho WK, Song KM, Cook C, Yoon TY, Ban C, Fishel R, Lee JB. MutS switches between two fundamentally distinct clamps during mismatch repair. Nat. Struct. Mol. Biol. 2011;18:379–385. doi: 10.1038/nsmb.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W. Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair. Mol. Cell. 2001;7:1–12. doi: 10.1016/s1097-2765(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Kochaniak AB, Habuchi S, Loparo JJ, Chang DJ, Cimprich KA, Walter JC, van Oijen AM. Proliferating cell nuclear antigen uses two distinct modes to move along DNA. J. Biol. Chem. 2009;284:17700–17710. doi: 10.1074/jbc.M109.008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazin-Meredith G, Mirchev R, Golan DE, van Oijen AM, Coen DM. Hopping of a processivity factor on DNA revealed by single-molecule assays of diffusion. Proc. Natl. Acad. Sci. USA. 2008a;105:10721–10726. doi: 10.1073/pnas.0802676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazin-Meredith G, Santos WL, Filman DJ, Hogle JM, Verdine GL, Coen DM. The positively charged surface of herpes simplex virus UL42 mediates DNA binding. J. Biol. Chem. 2008b;283:6154–6161. doi: 10.1074/jbc.M708691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Erie DA. DNA mismatch repair. Annu. Rev. Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK. The crystal structure of DNA mismatch repair protein MutS binding to a G × T mismatch. Nature. 2000;407:711–717. doi: 10.1038/35037523. [DOI] [PubMed] [Google Scholar]
- Lamers MH, Winterwerp HH, Sixma TK. The alternating ATPase domains of MutS control DNA mismatch repair. EMBO J. 2003;22:746–756. doi: 10.1093/emboj/cdg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhao T, Jian X, Farooqui Z, Qu X, He C, Dinner AR, Scherer NF. Using the bias from flow to elucidate single DNA repair protein sliding and interactions with DNA. Biophys. J. 2009;96:1911–1917. doi: 10.1016/j.bpj.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur DJ, Mendillo ML, Kolodner RD. Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol. Cell. 2006;22:39–49. doi: 10.1016/j.molcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Mazurek A, Johnson CN, Germann MW, Fishel R. Sequence context effect for hMSH2-hMSH6 mismatch-dependent activation. Proc. Natl. Acad. Sci. USA. 2009;106:4177–4182. doi: 10.1073/pnas.0808572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendillo ML, Mazur DJ, Kolodner RD. Analysis of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 and MLH1-PMS1 complexes with DNA using a reversible DNA end-blocking system. J. Biol. Chem. 2005;280:22245–22257. doi: 10.1074/jbc.M407545200. [DOI] [PubMed] [Google Scholar]
- Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407:703–710. doi: 10.1038/35037509. [DOI] [PubMed] [Google Scholar]
- Oiwa K, Jameson DM, Croney JC, Davis CT, Eccleston JF, Anson M. The 2′-O- and 3′-O-Cy3-EDA-ATP(ADP) complexes with myosin subfragment-1 are spectroscopically distinct. Biophys. J. 2003;84:634–642. doi: 10.1016/S0006-3495(03)74883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record MT, Jr., Lohman ML, De Haseth P. Ion effects on ligand-nucleic acid interactions. J. Mol. Biol. 1976;107:145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Record MT, Jr., Anderson CF, Lohman TM. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q. Rev. Biophys. 1978;11:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Schurr JM. The one-dimensional diffusion coefficient of proteins absorbed on DNA. Hydrodynamic considerations. Biophys. Chem. 1979;9:413–414. [PubMed] [Google Scholar]
- Tafvizi A, Huang F, Leith JS, Fersht AR, Mirny LA, van Oijen AM. Tumor suppressor p53 slides on DNA with low friction and high stability. Biophys. J. 2008;95:L01–L03. doi: 10.1529/biophysj.108.134122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafvizi A, Huang F, Fersht AR, Mirny LA, van Oijen AM. A single-molecule characterization of p53 search on DNA. Proc. Natl. Acad. Sci. USA. 2011;108:563–568. doi: 10.1073/pnas.1016020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Larson DR, Webb WW. Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 2002;82:2775–2783. doi: 10.1016/S0006-3495(02)75618-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PH, Berg OG. Facilitated target location in biological systems. J. Biol. Chem. 1989;264:675–678. [PubMed] [Google Scholar]
- Warren JJ, Pohlhaus TJ, Changela A, Iyer RR, Modrich PL, Beese LS. Structure of the human MutSalpha DNA lesion recognition complex. Mol. Cell. 2007;26:579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Winkler I, Marx AD, Lariviere D, Heinze RJ, Cristovao M, Reumer A, Curth U, Sixma TK, Friedhoff P. Chemical trapping of the dynamic MutS-MutL complex formed in DNA mismatch repair in Escherichia coli. J. Biol. Chem. 2011;286:17326–17337. doi: 10.1074/jbc.M110.187641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter RB, Berg OG, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor—operator interaction: kinetic measurements and conclusions. Biochemistry. 1981;20:6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.